Abstract
One of the morphological correlates of septo-optic dysplasia is hypoplasia of the optic nerves. As of now, it remains unknown, in how far this disorder also affects the organization of the optic radiation. Using diffusion- tensor imaging (DTI), the non-invasive evaluation of large fiber tracts including the optic radiation has become possible. We have compared DTI- data from a patient suffering from septo-optic dysplasia with those of a group of eleven healthy control subjects. The anisotropy showed statistically significant reduction in the patient with septo-optic dysplasia within the visual fiber tracts and an unordered eigenvector map. A comparison of the anisotropy in the pyramidal tract showed no significant difference. Since the patient was congenitally blind, it remains unclear whether the findings are the results of the underlying disorder or occur in all congenitally blind patients. One might presume, that, in order for the optic radiation to fully develop, an afferent input to the lateral geniculate body is necessarry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Septo-optic dysplasia (SOD) is considered as a mild form of holoprosencephaly and refers to a heterogenous group of disorders that include absence or dysplasia of the septum pellucidum and optic nerve hypoplasia to a variable degree. Clinical hallmarks of this disorder include impairment of vision that might range from very mild forms to congenitally blind patients, nystagmus, and disorders of the hypothalamic-pituitary axis [1]. Only rarely, all symptoms are present in a single patient. In addition to the absence of the septum pellucidum and the optic nerve hypoplasia, neuroradiological findings might include a wide variety of cerebral anomalies, the most common being schizencephaly, and other malformations of cortical development such as polymicrogyria.
As of now, it remains unknown, in how far this disorder also affects the organization of the white- matter tracts, especially those of the visual pathways. This is mainly due to the fact that until recently, there were no non-invasive in-vivo means to study the course and the organization of white- matter tracts. However, with the advent of diffusion tensor imaging (DTI), the evaluation of fiber bundles within the brain has become possible [2, 3].
In this manusscript, we describe the DTI findings in a patient with septo-optic dysplasia and compare them to a group of eleven healthy control subjects. To the best of our knowledge, this is the first report of a patient with SOD to be studied by diffusion tensor imaging.
Methods
This 23 -year old, right-handed female patient was referred to our department for the evaluation of recurrent headaches. Septo-optic dysplasia was diagnosed in the congenitally blind patient in early youth. The patient suffered from a lack of ADH resulting in diabetes insipidus that was present since childhood and controlled by desmopressin. Apart from her blindness, the patient was neurologically intact and had no neuropsychological deficits. There was no history of seizures or nystagmus. Her childhood development concerning speech, motoric, and intellectual abilities was unremarkable. The ophthalmological examinations revealed bilateral amaurosis and pale papillae.
Written informed consent to perform additional diffusion- weighted MR sequences was obtained before the investigation. Imaging was performed on a 1.5T scanner (Philips Intera, Best, The Netherlands) using a standard headcoil. After the routine MR sequences (T1, T2, FLAIR in different sections, whole-head, thin-sectioned T1- weighted gradient echo sequence), diffusion-weighted images [4] were obtained employing a pulse-triggered diffusion-weighted multishot spin-echo EPI sequence with two b values (0 s/mm2, 1000 s/mm2 ) along each of nine different gradient axes with the following parameters: Repetition time (TR): 1 beat, echo time (TE): 22 ms, flip angle (FA): 90°, field of view (FOV): 200×200 mm, matrix: 128×128, slice thickness: 2 mm, no interslice gap.
All images were reoriented to a magnet coordinate system with iso-voxels of 2 mm. After computation of the ADC map, the components of the tensor (eigenvectors and eigenvalues) were computed in a least squares approach. Measuring 9 nine different gradient axes, the symmetric diffusion tensor with 6 degrees of freedom was overdetermined. By this approach, the tensor with minimimal squared errors was choosen .
The anisotropy as the relation of the two largest eigenvalues and the main eigenvector was calculated for every voxel. Using the high-resolution, thin-sectioned, T1-weighted gradient echo scan and the software package SPM 99 (Wellcome Department, London, UK), the resulting data was normalized to the MNI (Montreal Neurological Institute) coordinate system. After this procedure, the anisotropy map and the principal eigenvector map were available in MNI-coordinates.
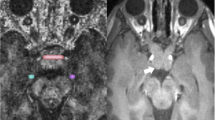
For comparison, we investigated 11 healthy control subjects using the same imaging parameters for diffusion tensor imaging to compare these results to those of our patient. We compared the mean anisotropy inside the optical radiation and the pyramidal tracts, that were defined from regions of interests (ROI) in MNI -coordinates (Table 1). These ROIs resulted from myelin-stained histological serial sections of ten human brains in which the pyramidal tract and the optic radiation were identified histologically [5]. The identified voxels were transformed into a reference brain so that a probability map of the course of these long fiber tracts in MNI- coordinates became availiable.
The ROI comprised all voxels that were part of the optical radiation in more than 70% of the histological samples (figure Fig. 1). A second ROI comprised all voxels that were part of the pyramidal tract in more than 90% of the histological samples. All voxels within one of the ROIs, that additionally were situated within the white matter due to a threshhold in the T1 gradient echo scan, were statisticially assessed with regard to the anisotropy. We employed a two- sample t -test to compare the anisotropy of the healthy control subjects to the anisotropy of the patient for both the optical radiation and the pyramidal tracts.
The regions of interest (ROI) of the geniculo-calcarine tract (A) and pyramidal tract (B) in Montreal Neurological Institute (MNI) -Coordiantes. These ROI’s are determined from myelin- stained histological sections of 10 ten human brains that were transformed to a reference brain [5]
Results
In all control subjects, the optic radiation could readily be identified (figure Fig. 2). As presumed from anatomical studies, the fibers arising from the lateral geniculate body of the thalamus could be separated in those that ran directly backward within the inferior parietal lobes lateral to the superior part of both lateral ventricles to reach the primary visual cortex and those fibers that were first bend forward to the temporal lobe in the so-called loop of Meyer.
Axial eigenvector map (A and B) and anisotropy map (C and D) of the geniculo-calcarine tract in a control subject (A and C) and the patient suffering from septo-optic-dysplasia (B and D). In the upper row, eigenvectors for anisotropies greater than 1.3 are presented as lines, whereas the lower row represents the anisotropy ranging from 1.2 to 3.0 color-coded on a thermal scale. In the control subject, the regular anatomy of the visual fiber tract can be visualized as arising from the lateral geniculate body, bending forward around the lateral ventricles, and runnning along the posterior horns to the calcarine fissure in the so-called loop of Meyer. There is a high anisotropy in these fiber tracts, nearly comparable to the pyramidal tracts. In the patient, on the other hand, there is a markedly decreased anisotropy of the optic radiation and a not orderly arranged tensor map. This indicates, that, in order for the optic radiation to fully develop, there has to be an afferent input from the retina and the geniculate body. If this input is missing, one might presume, that the fibers from the geniculate body are not initiated to sprout the way they would do under normal circumstances. Although on the selected images the forceps minor and the internal capsule appear more disorganized, the statistical evaluation revealed no significant differences of the anisotropy between the patient and the controls
This loop traversed lateral and superior to the temporal horns of the lateral ventricles and then ran parallel to the posterior horns of the lateral ventricles from which it was separated in all subjects by a thin layer of fibers of the posterior forceps of the corpus callosum, the so-called tapetum. Posterior to the posterior horns, the optic radiation bent medially and diverged fan-like to project on the striate cortex.
In the patient with SOD, MR imaging demonstrated an absent septum pellucidum (Fig. 2B; 3B);, furthermore, a severe hypoplasia of both optic nerves and the optic chiasm was found. No other associated abnormalities such as schizencephaly, cortical dysplasia, or hypoplasia of the corpus callosum were noted. Diffusion tensor imaging demonstrated a markedly reduced anisotropy of the optic radiation and a disorderly arranged tensor map of the optic radiation as compared to control subjects (figure Fig. 2). While the diffusion tensor map of other white-matter tracts, such as the pyramidal tracts, showed no statistically significant differences between the patient and the group of volunteers, the optic radiation, identified as described above, showed statistically significant ( p< 0.05, T = 2.2, F = 12 ) differences with a reduced anisotropy in the patient with septo-optic dysplasia. The mean anisotropy of the optic radiation was 1.61± +-0.07 in the healthy subjects and 1.45 in the patient. In the pyramidal tracts (figure Fig. 3), we measured an anisotropy of 1.67± +-0.07 for the controls and 1.6 for the patient. We did not find any significant differences between the healthy subjects and the patient concerning other fiber systems, e.g., within the corpus callosum or the forceps major and minor.
Eigenvector map in a coronal section in a healthy subject (A) and the patient (B) for depiction of the pyramidal tract. Note the absence of the septum pellucidum, the hallmark of neuroimaging in septo-optic dysplasia (SOD) and the normal depiction of the pyramidal tracts compared to the control subject [15]
Discussion
In the white matter, the diffusion of free water molecules is not the same in all directions, a property called anisotropy [6]. This anisotropy is caused by the course of fiber tracts and is dependent on the intra-axonal organization (axonal membrane, neurofilamentary cytoskeleton), the density of fiber packing, the degree of myelination, and the fiber diameter [7]. The variability in the orientation of all white- matter tracts in a macroscopically imaged voxel determines the overall degree of anisotropy designated to this specific voxel. Along the course of the fiber tracts, an unrestricted diffusivity is present, whereas perpendicular to the long axis of the predominant fiber tracts, a reduced diffusivity can be appreciated. To fully characterize the molecular diffusion, it is necessary to calculate the diffusion tensor.
Several measures of diffusion anisotropy, including fractional anisotropy, relative anisotropy, and volume ratio, can be calculated on the basis of formulas that incorporate the tensor elements to generate quantitative brain maps [6]. We have chosen both eigenvector maps and anisotropy maps to evaluate the orientation and organization of the postthalamic visual pathways in our patient. Anisotropy indexes have previously been used to evaluate white-matter disease in Krabbe’s disease, AIDS, multiple sclerosis, hypertensive encephalopathy, age-related changes [8], schizophrenia, Alzheimer’s disease, ischemic leukoaraiosis, intoxication [9], and epilepsy and are regarded as a sensitive and specific indicator for abnormalities of the white matter that may even be invisible on routine MR sequences [2].
It is known that blind atrophic human eyes at least reveal persisting retinal ganglion cell axons [10]. Up to now, there had been no study concerning the postthalamic course of the fiber tracts in patients with septo-optic dysplasia. Nor are any studies present in the literature that investigate congenitally blind patients using this relatively new technique of white- matter visualization. Therefore, it is not possible to discern in our single case study between the effects of the missing afferent input from the lateral geniculate bodies to the calcarine fissure and the effects of septo-optic dysplasia on the course of the white matter tracts.
We presume, that the reduced anisotropy and the disorderly arranged tensor map indicate that the normal white-matter tracts were not fully developed and therefore more loosely arranged. This indicates, that, in order for the optic radiation to fully develop, there has to be an afferent input from the retina to the lateral geniculate body. If this input is missing, one might presume that the fibers arising from the lateral geniculate body are not initiated to sprout the way they would do under normal circumstances. It is known that afferent input is needed to maintain the segregation of the lateral geniculate body in the ferret [11]. Not only the lack of electric input [12] but also the decrease of anterogradely transported neurotransmitters [13] can cause atrophy of the axons in the lateral geniculate body that project into the primary visual cortex.
In spite of this, the ontogenetic organizsation of the visual cortex seems to be independent of neuronal input [14]. Here, DTI as an non-invasive method can therefore obviously help to shed light on the normal and disturbed development of the optic radiation in specific pathological conditions. This warrants further research in this evolving field of neuroimaging.
References
Stangel M, Vogeley KT, Jandeck C, Boeger F, Marx P, Koch HC (1998) Septooptische Dysplasie (de-Morsier-Syndrom). Nervenarzt 69:352–356
Melhem ER, Mori S, Mukundan G, Kraut MA, Pomper MG, van Zijl PC (1996) Diffusion tensor MR imaging of the human brain. Radiology 201:637–648
Ching-Po Lin, Wen-Yih Isaac Tseng, Hui-Cheng Cheng Jyh-Horng Chen (2001) Validation of diffusion tensor magnetic resonances axonal fiber imaging with registered manganese-enhanced optic tracts. Neuroimage 14:1035–1047
Stejskal EO, Tanner JE (1965) Spin diffusion measurements: spin echos in the presence of a time dependent field gradient. J Chem Phys 42:2808–2929
Buergel U, Schormann T, Schleicher A, Zilles K (1999) Mapping of histologically identified long fiber tracts in human cerebral hemispheres to the MRI-volume of a reference brain: Position and spatial variability of the optic radiation. Neuroimage 10:489–499
Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996) Diffusion tensor MR imaging of the human brain. Radiology 201:637–648
Beaulieu C, Allen PS (1994) Determinations of anisotropic water diffusion in nervers. Magn Reson Med 31:394–400
Hanyu H, Asano T, Ogawa K, Takasaki M, Shindo H, Kakizaki D, Abe K (1997) Age-related changes of diffusional anisotropy in the cerebral white matter in normal subjects. No To Shinkei. 49:331–336
Kinoshita Y, Ohnishi A, Kohshi K, Yokota A (1999) Apparent diffusion coefficient on rat brain and nerves intoxicated with methylmercury. Environ Res. 80:348–354
Cursiefen C, Holbach LM, Schlotzer-Schrehardt U, Naumann GO (2001) Persisting retinal ganglion cell axons in blind atrophic human eyes. Graefes Arch Clin Exp Ophthalmol. 239:158–164
Chapman B (2000) Necessity for afferent activity to maintain eye-specific segregation in ferret lateral geniculate nucleus. Science 287:2479–2482
Yu-Xi F, Djupsund K, Hongfeng G, Hayden B, Kai S, Yang D (2002) Temporal specificity on the cortical plasticity of visual space representation. Science 296:1999–2003
Wahle P, Di Cristo G, Schwerdtfeger G, Engelhardt M, Berardi N, Maffei L (2003) Differential effects on cortical neurotropic factors on development of lateral geniculate nucleus and superior colliculus neurons: anterograde and retrograde actions. Development 130:611–622
Crowley JC, Katz LC (1999) Development of ocular dominance columns in absence of retinal input. Nat Neurosci. 2:1043–1045
Krings T, Coenen VA, Axer H, Moeller-Hartmann W, Mayfrank L, Weidemann J, et al (2001) Three-dimensional visualization of motor cortex and pyramidal tracts employing functional and diffusion weighted MRI. Methods, applications and limitations. Klin Neurorad 11:105–121
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schoth, F., Krings, T. Diffusion- tensor imaging in septo-optic dysplasia. Neuroradiology 46, 759–763 (2004). https://doi.org/10.1007/s00234-004-1207-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-004-1207-1