Abstract
Our objective was to review the frequency and pattern of signal abnormalities seen on conventional MRI in patients with suspected neuropsychiatric systemic lupus erythematosus (NP-SLE). We reviewed 116 MRI examinations of the brain performed on 85 patients with SLE, (81 women, four men, aged 21–78 years, mean 40.6 years) presenting with neurological disturbances. MRI was normal or nearly normal in 34%. In 60% high-signal lesions were observed on T2-weighted images, frequently in the frontal and parietal subcortical white matter. Infarct-like lesions involving gray and white matter were demonstrated in 21 of cases. Areas of restricted diffusion were seen in 12 of the 67 patients who underwent diffusion-weighted imaging. Other abnormalities included loss of brain volume, hemorrhage, meningeal enhancement, and bilateral high signal in occipital white-matter. The MRI findings alone did not allow us to distinguish between thromboembolic and inflammatory events in many patients. Some patients with normal MRI improved clinically while on immunosuppressive therapy. More sensitive and/or specific imaging methods, such as spectroscopy and perfusion-weighted imaging, should be investigated in these subgroups of patients with suspected NP-SLE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neuropsychiatric systemic lupus erythematosus (NP-SLE) is a serious and potentially life-threatening manifestation of lupus, occurring in 25–70% of cases, and associated with an increased risk of death [1]. Patients present with a wide range of central (CNS) and peripheral nervous system deficits. Patients who also have antiphospholipid antibodies (APL-Ab) are at additional risk for neuropsychiatric events. Patients with lupus are also at increased risk for a wide range of CNS events related to immunosuppressive therapy, including infection and drug toxicity. Unrelated but common neurologic disorders, such as migraine or multiple sclerosis, may be difficult to distinguish from NP-SLE.
CNS vasculitis, an important, potentially severe form of NP-SLE, may present with seizures, movement disorders, altered consciousness, stroke, or coma [2]. While a true inflammatory vasculitis is uncommon, the clinician must entertain this diagnosis when a patient with lupus has CNS signs or symptoms. Diagnosis of CNS vasculitis can be difficult, as the definitive test, brain biopsy, is infrequently performed. Empiric treatment, based on the serologic and imaging data, is therefore common.
In addition to the history and examination, other diagnostic approaches include serologic tests for active lupus (C-reactive protein, complement, anti-DS DNA) and analysis of cerebrospinal fluid [3, 4]. However, NP-SLE may occur in the absence of markers for active lupus, and angiography may be abnormal in as few as 5–10% of patients because of the small size of the vessels involved [5]. Diagnosis strongly relies on MRI; while the MRI manifestations of NP-SLE have been the subject of numerous reports [6, 7, 8, 9, 10, 11, 12, 13, 14], the impact of the findings on management is not clear. Our purpose was to characterize the MRI findings in patients presenting with a wide spectrum of neurologic disease, classified according to the new SLE case definitions, and to correlate them with the clinical course to evaluate the role of MRI in diagnosis and management.
Materials and methods
We reviewed 116 MRI studies of 85 patients enrolled in the University of Michigan SLE cohort. All research was performed in accordance with our institutional review board and with the informed consent of the participants. Patients were included if they fulfilled the 1982 Revised Criteria for SLE set forth by the American Rheumatism Association [15] and had complete MRI of the brain for neurologic symptoms or signs. During the period 1993–2001, 23 patients were imaged twice and five were examined three or more times.
We defined complete MRI of the brain as a minimum of T1- and T2-weighted images, and either proton density (PD)-weighted or fluid-attenuated inversion-recovery (FLAIR) images, including at least two orthogonal planes. All examinations were performed at 1.5 Tesla. Contrast medium was given in 93 of the 116 studies, followed by axial/sagittal/coronal T1-weighted images. In 67 studies we obtained echoplanar diffusion-weighted imaging (DWI: b=1000 s/mm2, TE 89 TR 10 000ms, filed of view 22 cm, one excitation, matrix 128×128). We carried out time-of-flight MRA of the cerebral vessels on 16 patients and MR venography of the dural venous sinuses on four. We performed catheter angiography on three patients, to exclude SLE-related CNS vasculitis.
All MRI studies were reviewed in consensus by two neuroradiologists (PCS, PM) and one radiology resident (JJ). Images were scored for loss of brain volume (none, mild, moderate, severe), abnormal signal, abnormal contrast enhancement, abnormal diffusion, hemorrhage or mineralization, and any other abnormalities. Brain lesions were classified as infarct-like (moderate-size to large, roughly wedge-shaped areas of abnormal high signal on T2-weighted images and/or encephalomalacia involving gray and white matter) or white-matter lesions (subcortical, deep, or periventricular, punctate or patchy). The site and number of the lesions were also noted. When patients had more than one examination, we classified lesions as stable, resolving, or progressing. DWI was examined for areas of restricted or increased diffusion, using apparent diffusion coefficient (ADC) where available. Subsequent comparison with the initial reports revealed no significant discrepancies. The consensus panel did not review the catheter angiograms.
Clinical data extracted were age, sex, and race, disease characteristics (primary and secondary diagnoses, duration of diagnosis, medications, and presence of APL-Ab) (Table 1), symptoms and signs, neurologic and pathologic diagnoses, therapy, and clinical course. Presenting symptoms or signs were classified according to the 1999 American College of Rheumatology Nomenclature and Case Definitions for Neuropsychiatric Lupus Syndromes [2] (Table 2). Presentations were divided into one of the following groups: global disease (including acute confusional state, cognitive decline, and psychosis), focal disease (stroke, cranial neuropathies), headache, seizures, and complex presentations (multiple deficits or difficult to place into any of the other categories). Statistical analyses of these subgroups were performed using Fisher’s exact test.
Results
Imaging findings are summarized in Table 3. About one third (34%) of the patients had normal MRI. Of these, 41% presented with headache as a major symptom, and patients presenting with only headaches were significantly more likely to have normal MRI than those with all other presentations ( P =0.03). Patients with normal MRI were less than half as likely to present with seizures than those with abnormal MRI (7% vs 17%), although the difference was not significant ( P =0.27). Parenchymal volume loss was found in 43% of patients, predominantly mild and diffuse.
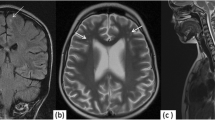
Abnormal signal was typically high on FLAIR and other T2-weighted images, most frequently in the frontal and parietal subcortical white matter, although it could appear anywhere in the brain, including the brain stem. Most (32) of the patients with high-signal foci had five or fewer lesions, and smaller groups had 6–20 (24 patients) or more 20 lesions (15 patients). Abnormal signal was seen in the white matter with all types of clinical presentation and was frequently nonspecific, interpreted as being consistent with focal ischemia, demyelination, vasculitis, or other conditions. In 14 patients (16%), the lesions had an extent or time-course which led the initial readers to suggest the possibility of NP-SLE (Fig. 1).
Typical white-matter lesions in neuropsychiatric systemic lupus erythematosus (NP-SLE). Fluid-attenuated inversion-recovery (FLAIR) image of a 48-year-old woman with an acute confusional state. There are numerous foci of abnormal signal in the white-matter of the frontal and parietal lobes. In the clinical context, NP-SLE is the most likely diagnosis, although these lesions could have many other causes
Infarct-like lesions were seen in 21% of studies (Fig. 2). Blood products, in the form of subarachnoid or parenchymal hemorrhage, subdural hematoma, petechial hemorrhage, or a hemorrhagic infarct, were detected in six studies (5%). In some instances, gyriform hemorrhage could not be reliably distinguished from mineralizing laminar necrosis. Of the 93 examinations with contrast-enhanced images, only 10 (11%) showed abnormal intracranial enhancement, mainly focal and heterogeneous. Other findings with a low frequency included cystic encephalomalacia, meningeal contrast enhancement, internal carotid artery occlusion, venous angioma, iron deposition, vestibular schwannoma, and deep venous sinus thrombosis. In three patients (4%), MRI yielded alternative diagnoses not related to collagen vascular disease, such as one vestibular schwannoma and two cases of cervical spondylosis, accounting for the symptoms.
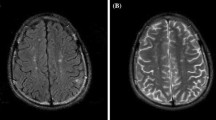
Areas of restricted diffusion were seen in addition to abnormalities on T2-weighted images in 12 of the 67 DWI studies (Fig. 3). Increased diffusion indicative of vasogenic edema was observed in one study, the other examinations demonstrating areas of restricted diffusion indicative of ischemia or an infarct. MRA was positive in five of 16 patients; in three it showed stenosis of the left internal carotid artery, stenoses of the great vessels and left internal carotid artery, and irregularity of the carotid bulbs, but in the other two there were false-positive findings: one equivocal aneurysm and one area of diminished flow-related enhancement, not confirmed on catheter angiography. One of the four patients who underwent MRV had dural venous sinus thrombosis. Catheter angiography, predictably, did not shown changes of CNS vasculitis in any of the three patients.
Abnormal diffusion. A 38-year-old woman with SLE presenting with acute confusion and focal neurologic deficits. Axial a FLAIR and b diffusion-weighted imaging demonstrate multiple areas of restricted diffusion, consistent with ischemia. These are nonspecific, and can be seen with embolic disease, microangiopathy, or inflammatory vasculitis
All but two patients lost to clinical follow-up had at least one follow-up rheumatology clinic note. Virtually all improved or remained stable on therapy; two patients failed treatment. These were the only two to undergo pathologic examination: one patient had a brain biopsy revealing changes of CNS vasculitis and progressive multifocal leukoencephalopathy, and in the other, the only patient who died, post-mortem examination showed no evidence of vasculitis.
The results of MRI by symptom category (Table 4a) and by APL-Ab status (Table 4b) show a general trend toward fewer normal studies and increasingly severe imaging abnormalities as the severity and complexity of symptoms and signs increase. A significantly higher percentage of patients presenting with headaches had normal or nearly normal MRI studies than patients with complex symptoms (64 vs 19%) ( P =0.04). White-matter lesions were less frequent in patients with only headaches (34%) than in patients with focal or complex presentations (64 and 71%, respectively), although these differences did not reach statistical significance ( P =0.14 and P =0.11). Infarcts were most frequent in patients with severe symptoms (seizures, focal, or combined) and were observed in significantly more patients with focal or complex symptoms (26 and 35%, respectively) than those without ( P =0.05 and P =0.004). Infarcts were found in a higher percentage of patients with APL-Ab than without (27 vs 15%), but this also did not attain statistical significance ( P =0.20). Intracranial hemorrhage and diffusion abnormalities were not seen in patients presenting with headaches only but were observed in those with seizures, focal or global symptoms.
There were 14 patients with acute stroke-like deficits (Table 5). These occurred in a higher percentage of patients with APL-Ab than in those without (23 vs 13%) ( P =0.22), although not a statistically significant difference. Abnormalities were seen on MRI in 11 (79%) of the 14 patients, including eight with infarcts of varying acuteness. DWI in three of these showed restricted diffusion, consistent with acute infarcts. MRA showed abnormalities in two of the patients, a left internal carotid artery occlusion and an atherosclerotic plaque at the carotid bulb. All patients in this group received treatment on presentation: 11 received had antithrombotic therapy, and 10 immunosuppression for suspected lupus-related CNS vasculitis (Table 5).
We treated 36 patients medically for suspected NP-SLE, of whom 20 had intravenous methylprednisolone and/or cyclophosphamide. Only two patients had concomitant systemically active lupus, such as nephritis, that also required intravenous therapy. Of the treated patients 12 had MRI studies initially interpreted as consistent with CNS lupus, based on the extent of white-matter lesions and/or evidence of rapid progression on short-term follow-up. The abnormalities were nonspecific in 15, and in the other nine there was no active lesion or no significant change from previous studies. Treatment was discontinued in two patients after their MRI was found to be normal, however three received a full course of intravenous therapy despite normal MRI.
Follow-up studies were available for 26 patients, ranging from 4 days to 5 years. In eight of these patients abnormalities progressed on follow-up MRI, three showed regression, and 15 no change. All but one of the patients eventually improved clinically, even in cases in which the imaging abnormalities progressed.
Discussion
The underlying pathologic basis of NP-SLE has been the subject of several investigations. Multiple microinfarcts, noninflammatory thickening of small vessels with intimal proliferation, small-vessel occlusion, and intracranial embolism or hemorrhage have been observed in pathologic specimens [16, 17, 18]. True vasculitis, with inflammatory infiltrate and fibrinoid necrosis is relatively rare, occurring in 6–9% of cases [17]. A bland vasculopathy is most common, consisting of vascular hyalinization, perivascular lymphocytosis, and endothelial proliferation [3, 16, 17, 18]. Although the underlying mechanisms are not understood and are probably multifactorial, suggested mechanisms include autoantibody-mediated activation of the thrombotic system (as with APL-Ab); immune-complex mediated vascular damage; and direct CNS attack by autoantibodies, including antineuronal, antiribosomal P, and antilymphocytotoxic antibodies [3, 4, 19].
Given that these mechanisms seem to have a final common pathway involving the cerebral microvasculature, it is not unexpected that MR has been shown to be neither very sensitive nor specific in the diagnosis of NP-SLE, estimates of sensitivity and specificity being in the 30 and 40%, respectively. Routine low-resolution (256 matrix) MRA, as used in our patients, is unlikely to be sensitive enough to reveal caliber change in the small and medium-sized vessels typically affected by SLE. However, the clinical ramifications of this lack of specificity and sensitivity appear not to have been explored. Previous studies have found MRI more likely to show abnormalities in patients with focal rather than diffuse deficits, showing a higher proportion of focal lesions and infarcts [4, 9]. We also demonstrated increased frequency of lesions in patients with focal and complex rather than diffuse or global problems.
Perhaps the most important role of imaging in NP-SLE is assessment of acute focal (stroke-like) neurologic deficits. The differential diagnosis typically includes of lupus-related CNS vasculitis, thromboembolic events due to vasculopathy, APL-Ab- mediated thrombosis, microangiopathy (including thrombotic thrombocytopenic purpura), Libman-Sacks endocarditis, and accelerated atherosclerosis; the pathogenesis in many patients is probably multifactorial. Accurate assessment is crucial, as treatment for these alternative diagnoses differs. Immunosuppressive agents are typically used for suspected vasculitis, while lifelong anticoagulation is the mainstay of therapy for APL-Ab-mediated thromboembolic events [20, 21]. Patients with APL-Ab have been found to be at higher risk for unfavorable long-term prognosis than other patients with SLE [22].
All 14 patients with acute focal neurologic deficits were treated: 11 with antithrombotics (aspirin, coumadin) and 10 with immunosuppression (steroids, cyclophosphamide). Two patients with normal MRI and a presumed transient ischemic attack and two with cerebrovascular occlusion on MRA were appropriately triaged to antithrombotic therapy. One patient with increasing numbers of small lesions in gray and white matter was treated for suspected CNS vasculitis with steroids and cyclophosphamide. The seven patients who were treated with both types of therapy tended to be APL-Ab positive (five) and to have multiple gray and white matter abnormalities (Fig. 2). DWI in two such patients showed restricted diffusion suggestive of ischemic foci, but DWI did not seem to further differentiate inflammatory vasculitis, thromboembolic disease, or bland vasculopathy. These patients’ notes reflected clinical uncertainty as to the underlying pathophysiology, especially the presence of vasculitis. The fact that they were treated with both anti-SLE and antithrombotic agents (typically prednisone and aspirin) suggests that it is not necessarily possible to distinguish thrombotic or inflammatory events, or both, using clinical and MRI data.
Most of our patients with normal or nearly normal MRI imaging did not undergo specific therapy for SLE. They tended to have minor deficits or symptoms or signs not necessarily expected to improve with therapy (hearing loss, cognitive decline). However, three received a full course of high-dose intravenous steroids or pulsed cyclophosphamide based on clinical and laboratory findings alone, without imaging evidence of active disease, suggesting that in the clinicians’ view, MRI lacked sensitivity in this subgroup. One such patient presented with fever, headaches, and myalgia with increased cerebrospinal fluid protein, elevated sedimentation rate and C-reactive protein. Another presented with severe headaches, serositis, and normal laboratory tests, and the third with refractory hallucinations and normal laboratory values. Although the possibility of spontaneous remission cannot be excluded, the fact that these patients improved clinically having received anti-SLE therapy suggests that a small number of patients with normal MRI may nevertheless have treatable NP-SLE.
Recent investigations have shown a correlation between antineuronal autoantibodies and diffuse CNS disease in SLE [1, 4, 19]. The imaging features of such autoantibody-mediated disease are not known, but it is plausible that diffuse CNS attack at a molecular level may escape detection on conventional MRI. While serologic tests and clinical judgment remain the mainstay of diagnosis and management of these cases, more sensitive imaging techniques such as MR spectroscopy hold promise in detection of subclinical metabolic brain abnormalities in SLE [23, 24, 25, 26, 27, 28]. Perfusion MRI shown promise for detecting ischemic areas in CNS vasculitis that had escaped detection by conventional MRI [29].
DWI and diffusion tensor imaging also may be useful. A recent investigation of DWI in SLE [30] suggests two major patterns of abnormality: restricted diffusion, suggestive of cytotoxic edema seen with vascular occlusion; and increased diffusion suggestive of vasogenic edema, seen with vasculopathy. We had ten patients with areas of abnormal diffusion; one patient had small areas of restricted diffusion on a background of increased diffusion (vasogenic edema), while the others had restricted diffusion, indicative of an infarct. Notably, six patients whose serial MRI showed improvement or worsening, but none of those with stable appearances, had positive DWI. This suggests that DWI may be a marker of “active” disease, and diffusion abnormalities should at least be taken as an indication for follow-up MRI.
Potential pitfalls in these patients include, for example, an SLE/multiple sclerosis overlap syndrome, described previously [31, 32]. In such patients it can be difficult or impossible to ascribe inflammatory-type signal abnormalities to NP-SLE or demyelinating disease with certainty. Opportunistic infection related to immunosuppression is another potential pitfall, as in one of our patients (Fig. 4) who failed to respond to therapy and was found to have progressive multifocal leukoencephalopathy on brain biopsy [33]. This underscores the lack of specificity of conventional MRI in the face of concomitant infection, an important consideration given that infection may be exacerbated by immunosuppressive anti-SLE therapy.
We have attempted to examine the current role of MRI in the management of neuropsychiatric SLE. Although it is useful in the majority of patients with suspected NP-SLE, we had several in whom the abnormalities did not serve to triage patients appropriately to antithrombotic or immunosuppressive therapy. On the other hand, patients with normal MRI may have deficits which warrant treatment on clinical grounds alone, suggesting a lack of sensitivity of our imaging strategy. The general reluctance to proceed to biopsy, and reliance on empiric therapy underscores the need for more sensitive noninvasive imaging. Our findings make the case for the investigation of newer techniques in the investigation of suspected NP-SLE.
References
Kovacs J, Urowitz M, Gladman D (1993) Dilemmas in neuropsychiatric lupus. Rheum Dis Clin North Am 19: 795–819
ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature (1999) The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 42: 599–608
Schumacher HR (ed) (1993) Primer on the rheumatic diseases, 10th ed. Arthritis Foundation, Atlanta, pp 100–117
West SG, Emlen W, Wener MH, Kotzin BL (1995) Neuropsychiatric lupus erythematosus: a 10-year prospective study on the value of diagnostic tests. Am J Med 99: 153–163
Pomper M, Miller T, Stone J, Tidmore W, Hellman D (1999) CNS vasculitis in autoimmune disease: MR imaging findings and correlation with angiography. AJNR 20: 75–85
McCune J, MacGuire A, Aisen A, Gebarski S (1988) Identification of brain lesions in neuropsychiatric systemic lupus erythematosus by magnetic resonance scanning. Arthritis Rheum 31: 159–166
Jacobs L, Kinkel P, Costello PB, Alukal MK, Kinkel WR, Green FA (1988) Central nervous system lupus erythematosus: the value of magnetic resonance imaging. J Rheumatol 15: 601–606
Sibbitt WL Jr, Sibbitt RR, Griffey RH, Eckel C, Bankhurst AD (1989) Magnetic resonance and computed tomographic imaging in the evaluation of acute neuropsychiatric disease in systemic lupus erythematosus. Ann Rheum Dis 12: 1014–1022
Bell CL, Partington C, Robbins M, Graciano F, Turski P, Kornguth S (1991) Magnetic resonance imaging of central nervous system lesions in patients with lupus erythematosus. Correlation with clinical remission and antineurofilament and anticardiolipin antibody titers. Arthritis Rheum 34: 432–441
Baum KA Hopf U, Nehrig C, Stover M, Schorner W (1993) Systemic lupus erythematosus: Neuropsychiatric signs and symptoms related to cerebral MRI findings. Clin Neurol Neurosurg 95: 29–34
Stimmler MM, Coletti PM, Quismorio FP Jr (1993) Magnetic resonance imaging of the brain in neuropsychiatric systemic lupus erythematosus. Semin Arthritis Rheum 22: 335–349
González-Crespo MR, Blanco FJ, Ciruelo E, Mateo I, López Pino MA, Gómez-Reino JJ (1995) Magnetic resonance imaging of the brain in systemic lupus erythematosus. Br J Rheumatol 34: 1055–1060
Tanabe J, Weiner MW (1997) MRI-MRS of the brain in systemic lupus erythematosus. How do we use it to understand causes of clinical signs? Ann N Y Acad Sci 823: 169–184
Sibbitt WL Jr, Sibbitt RR, Brooks WM (1999) Neuroimaging in neuropsychiatric systemic lupus erythematosus. Arthritis Rheum 42: 2026–2038
Tan E, Cohen A, Fries J, et al (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25: 1271–1277
Ellis SG, Verity MA (1979) Central nervous system involvement in systemic lupus erythematosus: a review of neuropathologic findings in 57 cases, 1955–1977. Semin Arthritis Rheum 8: 212–221
Van Dam AP (1991) Diagnosis and pathogenesis of CNS lupus. Rheumatol Intl 11: 1–11
Belmont MH, Abramson SB, Lie JT (1996) Pathology and pathogenesis of vascular injury in systemic lupus erythematosus. Arthritis Rheum 39: 9–22
Sanna G, Piga M, Terryberry JW, et al (2000) Central nervous system involvement in systemic lupus erythematosus: cerebral imaging and serological profile in patients with and without overt neuropsychiatric manifestations. Lupus 9: 573–583
Provenzale JM, Barboriak DP, Allen NB, Ortel TL (1996) Patients with antiphospholipid antibodies: CT and MR findings of the brain. Am J Roentgenol 167: 1573–1578
Provenzale JM, Barboriak DP, Allen NB, Ortel TL (1998) Antiphospholipid antibodies: findings at arteriography. AJNR 19: 611–616
Karassa FB, Ioannidis JP, Boki KA, et al (2000) Predictors of clinical outcome and radiologic progression in patients with neuropsychiatric manifestations of systemic lupus erythematosus. Am J Med 109: 628–634
Sibbitt WL Jr, Haseler LJ, Griffey RH, Hart BL, Sibbitt RR, Matwiyoff NA (1994) Analysis of cerebral structural changes in systemic lupus erythematosus by proton MR spectroscopy. AJNR 15: 923–928
Chinn RJ, Wilkinson ID, Hall-Craggs MA, et al (1997) Magnetic resonance imaging of the brain and cerebral proton spectroscopy in patients with systemic lupus erythematosus. Arthritis Rheum 40: 36–46
Sibbitt WL Jr, Haseler LJ, Griffey RR, Friedman SD, Brooks WM (1997) Neurometabolism of active neuropsychiatric lupus determined with proton MR spectroscopy. AJNR 18: 1271–1277
Friedman SD, Stidley CA, Brooks WM, Hart BL, Sibbitt WL Jr (1998) Brain injury and neurometabolic abnormalities in systemic lupus erythematosus. Radiology 209: 79–84
Lim MK, Suh CH, Kim HJ, et al (2000) Systemic lupus erythematosus: brain MR imaging and single-voxel1H MR spectroscopy. Radiology 217: 43–49
Axford JS, Howe FA, Heron C, Griffiths JR (2001) Sensitivity of quantitative1H magnetic resonance spectroscopy of the brain in detecting early neuronal damage in systemic lupus erythematosus. Ann Rheum Dis 60: 106–111
Yuh W, Ueda T, Maley J (1998) Perfusion and diffusion imaging: a potential tool for improved diagnosis of CNS vasculitis. AJNR 20: 87–89
Moritani T, Shrier DA, Numaguchi Y, et al (2001) Diffusion-weighted echo-planar MR imaging of CNS involvement in systemic lupus erythematosus. Acad Radiol 8: 741–753
Marullo S, Clauvel JP, Intrator L, Danon F, Brouet JC, Oksenhendler E (1993) Lupoid sclerosis with antiphospholipid antibodies. J Rheumatol 20: 747–749
Scott TF, Brillman J (1994) Antiphospholipid antibody syndrome mimicking multiple sclerosis and systemic lupus erythematosus clinically and by magnetic resonance imaging. Arch Intern Med 154: 917–920
Attwood J, Sundgren PC, West A, Cinti S, McKeever P (2002) Progressive multifocal leukoencephalopathy (PML) in a patient with systemic lupus erythematosus. Co-existence of PML and lupus central nervous system disease, or PML masquerading as lupus CNS disease? Case report and review of the literature. In press
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jennings, J.E., Sundgren, P.C., Attwood, J. et al. Value of MRI of the brain in patients with systemic lupus erythematosus and neurologic disturbance. Neuroradiology 46, 15–21 (2004). https://doi.org/10.1007/s00234-003-1049-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-003-1049-2