Abstract
We carried out baseline and short-term follow-up MRI, including perfusion-weighted imaging (PWI) and tests of neurologic and cognitive function on 15 consecutive patients with large-vessel ischemic stroke who showed a persistent large perfusion-diffusion mismatch at enrollment up to seven days after the onset of symptoms. Of these, ten underwent induced blood pressure elevation with phenylephrine and oral medications (in eight) or intravenous fluids (in two) with the goal of improving perfusion; five had no such treatment. Significant functional improvement was defined by a reduction of 3 or more points on the NIH stroke scale (NIHSS). Significant improvement in perfusion was defined by a reduction in the volume of hypoperfused brain by 30 cc on PWI using time-to-peak (TTP) maps, without enlargement of the infarct. There was a strong, statistically significant association between improved function and improved perfusion: six (75%) of eight patients who improved in function, but none of the seven who did not, showed a reduction in volume of hypoperfused brain. All six patients who met the perfusion goal, and only two (22%) of nine who did not showed significant functional improvement (Fisher’s exact: P <0.01). There were no differences between patients who improved functionally and those who did not with respect to age, initial volume of abnormality on DWI or PWI, initial NIHSS, or changes on DWI. These findings indicate that reduction in volume of hypoperfused brain on PWI is a marker of response to treatment to improve perfusion even in subacute stroke and that partial reperfusion of regions of salvageable but dysfunctional tissue is a mechanism of improved function associated with induced blood pressure elevation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diffusion (DWI) and perfusion-weighted imaging (PWI) have been advocated for guiding treatment of acute stroke [1, 2, 3]. It has been hypothesized that the area of hypoperfusion shown on PWI, minus the area of dense ischemia or infarct seen on DWI (the “diffusion/perfusion mismatch” [4]) represents the “ischemic penumbra”, a potentially salvageable region of tissue surrounding the core infarct in which there is enough blood flow to survive, but not enough to function [5, 6, 7]. The ischemic penumbra has been described as the target of all acute stroke therapies [8]. Thus, a mismatch has been proposed as a criterion for intra-arterial thrombolysis, extension of the window for i.v. thrombolysis, or use of neuroprotective agents [9, 10]. DWI and PWI are also being used to evaluate the effects of intervention in trials of pharmacological treatment of acute stroke (<6 h after the onset of symptoms). However, no studies have as yet demonstrated conclusively that the imaging techniques effectively identify candidates for treatment or reliably indicate response to it.
“Ischemic penumbra” is used in two ways. One meaning is the tissue at risk of infarction [11], the other the brain in which blood flow is diminished to the point at which function is impaired [8]. These might be measured by different techniques or correspond to different thresholds of hypoperfusion. Several studies have shown that volume of hypoperfused brain measured by using one PWI parameter, time-to-peak (TTP), correlates with the degree of dysfunction in acute stroke [12, 13]. However, depending on the threshold, TTP or mean transit time (MTT) maps, which are closely correlated [14], may overestimate the area at risk of infarction [15]. These findings are not contradictory, since tissue can be dysfunctional due to hypoperfusion without being irrevocably destined to undergo infarction.
For either definition, the time limits of the ischemic penumbra are unknown. It is often assumed that the penumbra tissue can survive up to about 36 h without restoration of perfusion [2]. However, patients with large vessel stenosis may have prolonged, symptomatic ischemia without progression to an infarct [16]. Patients with diffusion/perfusion mismatch up to seven days after the onset of symptoms have demonstrated improved function, and a reduced volume of hypoperfused brain on TTP maps, when blood flow was restored by endarterectomy [13] or induced blood-pressure elevation [17]. This indicates that PWI might be useful as a marker of response to treatment aimed at improving perfusion in acute or subacute stroke, i.e., up to 1 week after the onset of symptoms.
The only FDA-approved intervention for improving perfusion demonstrated to improve function in acute stroke is i.v. thrombolysis with tissue plasminogen activator (tPA). However, benefit from tPA has been demonstrated only when its use was restricted to a 3 h window after the onset of symptoms. This very narrow window of opportunity for re-establishing cerebral perfusion has limited this intervention to fewer than 5% of all patients with acute ischemic stroke [17, 18]. It is plausible that patients who present after 3 h and have persistently dysfunctional, but salvageable, brain tissue would benefit from treatment that re-establishes blood flow to that area. Case reports and small series have indicated that induced blood pressure elevation is relatively safe and associated with improved function in acute to subacute stroke [19, 20, 21]. The hypothesized mechanism is improved blood flow, although direct evidence for this is lacking. Animal studies using microelectrodes have demonstrated that within the ischemic penumbra (defined as an area of tissue where blood flow interferes with electrical activity of neurons without cell death), there is a linear relationship between systemic mean arterial pressure (MAP) and regional cerebral blood flow [22]. Xenon-133 single-photon emission tomography studies of human stroke have also shown that the ischemic penumbra shows impaired autoregulation, so that raising MAP can restore perfusion [23]. A recent prospective study of induced hypertension in patients with stroke indicated that a response to treatment was more frequent in patients with large vessel stenosis than in patients with other causes [21]. Since these patients are also most likely to have persistent penumbral tissue, it is plausible that treatment effects were due to improved perfusion, although no direct evidence was provided.
Our aim of this investigation was to demonstrate that improvements in function achieved with blood pressure elevation are associated with improvements in regional perfusion on PWI. We hypothesized that patients who showed functional gains would show reduced volume of hypoperfused brain on PWI and that those who showed no functional response to treatment or had no treatment to increase blood pressure would show no significant reduction in hypoperfusion.
Materials and methods
We prospectively studied 15 consecutive patients who met the following criteria: 1. quantifiable, stable or worsening aphasia, hemispatial neglect, and/or hemiparesis; 2. within 7 days from the onset of stroke symptoms; 3. >20% and >30 cc diffusion/perfusion mismatch. Exclusion criteria were: 1. a contraindication to MRI; 2. cardiac ejection fraction <25%; 3. congestive heart failure or cardiac ischemia; 4. hemorrhage on initial CT; and 5. impaired arousal or agitation requiring sedation. Informed consent was obtained using a process and forms approved by the local Institutional Review Board. In patients with impaired language comprehension due to aphasia or cognitive deficits, informed assent was provided by identified decision makers (closest living relative).
Treatment was determined by the attending neurologist. In eight patients, where there was no rationale for selecting intervention to increase blood pressure or conventional management, they were randomly assigned (2:1) to induced blood pressure elevation versus conventional management, which included cessation of antihypertensive medication. Of the remaining seven patients, five had induced blood pressure elevation because they were deteriorating with conventional management; and two had only conventional management because of a relative contraindication to raising their blood pressure. Intervention was started within 2 h of the initial MRI (Table 1).
We gave 10 patients (the “treated” patients) treatment to raise their blood pressure: two received only i.v. saline (200 cc or more/h) to raise MAP, because of relative contraindications to phenylephrine (e.g. bradycardia). The other eight treated patients received i.v. phenylephrine and fluids. We managed five patients (the “untreated” patients) conventionally, without specific intervention to increase blood pressure. They received maintenance i.v. normal saline when not taking fluids by mouth. In both treated and untreated patients, any antihypertensive medication was discontinued prior to the initiation of the study. All patients received aspirin 81–325 mg/day.
Our purpose was not to evaluate the effects of treatment, but to assess changes on MR which might provide markers of improvement with specific treatment or conventional management. The treated and untreated patients were very similar with respect to a number of features (Table 1): there were no significant differences between them as regards age, baseline NIHSS, volume of baseline DWI abnormality or of hypoperfusion on PWI, or diffusion/perfusion mismatch (Wilcoxon rank sum test: df13; z=-0.67–).80; P =0.21–0.40). All patients had large-vessel stenosis or occlusion, affecting the middle cerebral artery in six of ten treated and four of five untreated patients; the remainder had internal carotid artery stenosis or occlusion, with or without vertebrobasilar disease. Women accounted for seven of the ten treated and three of the five untreated patients. Treated and untreated patients underwent the same tests of neurologic and cognitive function and MRI studies.
The NIHSS was administered at the start of the study (baseline, before any intervention) and at 7 am on the third day (follow-up). Treated patients were tested prior to weaning off phenylephrine. The tests were administered by a certified research technician blinded to the hypotheses of the study. Significant improvement in function was defined as improvement by 3 points. We selected this level because it was twice the average improvement in scores from day 1 to day 3 reported in 50 patients with ischemic stroke who received “conventional supportive therapy” [24].
DWI and PWI were performed at 1.5 tesla, with echo-planar imaging (EPI) on day 1 of the study (Table 1). The same sequences were used for short-term follow-up on days 2–6, mean day 3.0). DWI trace images were obtained using a multislice, isotropic, single-shot EPI sequence, with bmax 1000 s/mm2: TR 10 000 TE 120 ms. We also obtained bmin 0 images for generating maps of apparent diffusion coefficient (ADC) to confirm the acuteness of lesions seen on DWI. For PWI, we obtained single-shot gradient-echo EPI images (TR 2000 TE 60 ms with a 20 cc GdDTPA bolus injected automatically at 5 cc/s followed by 15 cc normal saline at 5 cc/s. This sequence generated 17 slices with 2 s temporal resolution.
All volumes were measured by a trained technician blinded to the results of functional testing. Total volumes of hypoperfused brain were delineated by analysis of TTP maps, displayed on a 20-color scale in which each color change corresponds to 2.5 s difference in delay in TTP concentration of tracer in each pixel. Hypoperfusion was defined as >2.5 s delay compared to the homologous region in the other cerebral hemisphere. Volumes of brain giving high signal on DWI and hypoperfusion on PWI, respectively, were measured by a technician supervised by a neuroradiologist, both blinded to treatment group and clinical response. The borders of abnormality on each 5 mm slice of DWI or PWI were traced by hand using the “Scion Image” program. The areas of abnormality in cm2 on each slice were summed, and the sum was multiplied by the slice thickness to determine the volume in cm3.
Improvement in hypoperfusion was defined as a reduction in volume of hypoperfused tissue of >30 cc, since in our experience this corresponds to about 30% of the average total volume of hypoperfused brain in ischemic stroke due to large vessel stenosis or occlusion. We used an absolute value rather than a percentage because we thought a 30 cc improvement would be likely to have functional consequences irrespective of the initial volume of hypoperfusion.
Our protocol for phenylephrine-induced blood pressure elevation is described elsewhere [25]. MAP is initially increased by increments of 10% with i.v. phenylephrine, until improvement in neurologic function is observed or a MAP of 130 mm Hg is reached. When the goal MAP is reached, phenylephrine is replaced with oral medication (fludrocortisone, midodrine, and salt tablets) to maintain it. The oral medication is gradually tapered after discharge, as long as no functional deterioration is observed. In the two treated patients, in whom blood pressure was elevated with i.v. fluids (200 cc/h) alone we used the same goals.
The results of daily tests were used to determine the MAP at which function improved; untreated patients underwent the same daily tests. These included any of the following on which the patient was impaired at day 1: 1. oral naming; 2. comprehension of spoken words and 3. sentences; 4. line cancellation, 5. copying a scene; 6. a gap-detection task [13, 25]; and 7. strength of grip (measured with dynamometer). Different forms of the cognitive tests, with stimuli matched in level of difficulty, were presented each day. They were not used as outcome measures.
Patients were divided into those who improved by at least 3 points on the NIHSS by day 3 (“functional gainers”) and those who did not (“functional nongainers”). Between-group comparisons in potential factors that might influence recovery (age, initial volume of DWI or PWI abnormality, initial NIHSS score, and changes in volume of high signal on DWI, and abnormality on PWI) were evaluated with the Wilcoxon ranked sum test, because of the small number of subjects. Fisher’s exact analyses were used to determine if the functional gainers included a higher proportion than the functional nongainers of patients who: 1. reached the goal reduction in the volume of hypoperfused brain by 30 cc; 2. showed an increase in infarct size of 4 cc (30% of the mean initial infarct size on DWI); or 3. received treatment. These analyses were used to determine the imaging and functional characteristics associated with improvement in function.
Within the groups of treated and untreated patients, changes in outcome measures (NIHSS, volume of infarct, and volume of hypoperfused tissue) were evaluated with the nonparametric, paired Signs test. Differences between treated and untreated patients with respect to NIHSS score, initial volume and change in volume of abnormality on DWI and PWI at day 3, were evaluated with the Wilcoxon ranked sum test.
Results
There were no significant differences between the functional gainers and nongainers in: age (64.9±12.6 vs 67.6±14.2 years); initial NIHSS score (12.1±4.6 vs 8.0±5.4; z=-1.39); initial volume of infarct/densely ischemic tissue on DWI (11.9±12.2 vs 7.9±3.8 cc); initial volume of hypoperfused tissue on PWI (147±104 vs 119±70.0 cc; z=-0.29); or increase in volume of infarct on DWI (6.41±12.8 vs 1.51±5.36 cc; z=-1.0). Functional gainers showed a larger (but nonsignificant) increase in DWI abnormality than nongainers, but also a significantly larger reduction in the volume of hypoperfused tissue on PWI (96.1± 64.9 vs 18.1±18.3 c; z=2.1; P <0.02).
Fisher’s exact analyses confirmed that improvement in hypoperfusion was the only imaging factor significantly associated with improved function. As shown in Table 2, 75% of the functional gainers showed reperfusion by 30 cc, whereas none of the nongainers showed reperfusion to this degree. All six patients whose volume of hypoperfused brain decreased by at least 30 cc reached the functional goal, whereas only two (22%) of nine patients who failed to reach the goal reduction in hypoperfusion reached the functional goal (Fisher’s exact test: P <0.01). In contrast, the proportion of patients who showed an increase in infarct volume by 4 cc was no different in functional gainers and nongainers (Fisher’s exact test; P =0.28). The proportion of patients who received treatment specifically to increase blood pressure was not significantly different in functional gainers and nongainers (87.5 vs 42.9%; Fisher’s exact test: P =0.11).
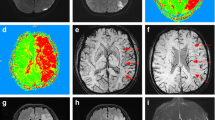
Although some untreated patients improved (some of whom showed spontaneous increases in blood pressure), and some treated patients did not, the treated patients did show greater functional gains overall than untreated patients who received conventional management: their mean NIHSS score improved from 9.3 before treatment to 4.8 on day 3 of treatment (Signs test: P <0.001). Treated patients also showed a significant reduction in the mean volume of hypoperfused tissue from pretreatment (147 cc) to immediate follow-up PWI (58.3 cc; P <0.001), consistent with at least partial reperfusion of the initially hypoperfused territory. Pretreatment and follow-up images are shown in Fig. 1.
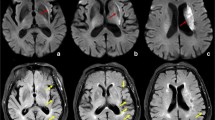
In contrast, untreated patients showed no difference in mean NIHSS scores between days 1 and 3 (12 vs 11.8; Signs test: P =0.5); the mean change was 0.6 (worse), range -4 to +4 points. They also showed no significant change, from baseline to immediate follow-up, in mean volume of hypoperfused brain on PWI (105 vs 94; df 4; P =0.19). Only patient 11 showed a visually apparent decrease in the volume of hypoperfused tissue, but also had hemorrhagic conversion of the infarct (Fig. 2).
Treated patients had significantly better (lower) mean NIHSS scores on day 3 than untreated patients (4.8 vs 11.8; Wilcoxon rank sum test: z=2.3; df 13; P <0.01), although there was no difference in scores on day 1. They also showed significantly more improvement than the untreated patients (mean change −4.5 vs 0.6; z= -2.3; df 13; P =0.01). They showed significantly greater reduction in volume of hypoperfused territory between day 1 and immediate follow-up PWI (mean 88.9 vs 11.2 cc; z=-2.2; df 13; P =0.01). There was no difference between the groups in change in high signal on DWI from days 1 to 3: the mean increase was 3.2±8.1 cc in treated and 11.0±21 cc in the untreated patients (z=0.31; df 13; P =0.38), although there was a trend toward smaller increases in the treated patients.
In most patients, baseline imaging showed relatively small subcortical areas of high signal on DWI and large cortical abnormalities on PWI (Figs 1, 2). This was probably due to the fact that we selected patients with substantial diffusion/perfusion mismatch (who all had large-vessel intracranial stenoses). At short-term follow-up, there was no extension of the high signal on DWI cortical regions that appeared to be reperfused on PWI. Cortical regions that failed to show reperfusion did sometimes evolve on DWI (patients 5, 11 and 12); in these cases, persistently hypoperfused regions indicated by PWI probably progressed to infarcts.
Discussion
We demonstrated an association between improved perfusion of peri-infarct regions and improved function in patients with induced blood pressure elevation. Patients who showed reduction in the volume of hypoperfused tissue (often following induced blood pressure elevation) showed significant improvement on the NIHSS, whereas those who did not show reperfusion generally did not show functional gains. This provides support for the hypothesis that the observed effects of induced hypertension in treating acute stroke (reported in several studies) are due to reperfusion of previously hypoperfused but viable tissue.
Although our results do not provide evidence that PWI and DWI can identify optimal candidates for blood pressure elevation, they are consistent with the hypothesis that the diffusion/perfusion mismatch represents functionally salvageable tissue, or ischemic penumbra [3, 12]. Heiss [26] defined the “ischemic penumbra” as a region of tissue receiving blood flow above the threshold for loss of morphological integrity and below the threshold for functional integrity. These thresholds of regional cerebral flow (rCBF) have been identified using blood flow studies with xenon-133 [27] or technetium 99m compounds [28, 29], or positron-emission tomography (PET) [7, 30]. The suggestion that the diffusion/perfusion mismatch can also define the extent and site of the ischemic penumbra receives preliminary support by our finding of a strong correspondence between reversal of hypoperfusion in penumbral regions identified on PWI and DWI, and partial resolution of the clinical deficit.
Other investigators have operationally defined the ischemic penumbra on DWI as the region of mismatch between the initial lesion and the infarct 1–3 days later [11, 15]. It was found that measures of relative cerebral blood flow (rCBF), rather than MTT or TTP maps, calculated from initial PWI, best predicted extension of the infarct. However, assuming that some patients reperfused spontaneously, the regions of diminished rCBF apparently corresponded to the region of tissue that progressed to infarction, irrespective of reperfusion. Thus, rCBF calculated from PWI may be a better estimate of the region destined to die, rather than of the entire region of potentially salvageable tissue. It has also been argued that MTT maps, while excellent indicators of abnormal cerebral perfusion, may include areas of “benign oligemia.” While this is likely to be true in some cases (especially in patients with extracranial carotid artery stenosis), our study provides evidence that a >2.5 s delay in TTP on the symptomatic side was an excellent indicator of dysfunctional tissue. Diffusion/perfusion mismatch defined in this way corresponded to both reversible clinical deficits and salvageable tissue.
While it has been argued that PWI and DWI have a potentially important role in assessing the need for intervention (e.g., thrombolysis) in acute stroke, the role of these studies in the subacute period has not been addressed [10, 11, 31, 32]. This paper indicates that PWI may be useful in delineating blood pressure-sensitive perfusion deficits even in subacute stroke. Our results suggest that reperfusion may be effective in patients with acute or subacute ischemic stroke up to 7 days or more after the onset of symptoms if there is a still a substantial area of salvageable tissue identified by diffusion/perfusion mismatch that corresponds to the clinical deficit. Thus, these imaging techniques may enable extension of the window of opportunity for intervention from the currently accepted 3–6 h to several days, and may be useful in monitoring the need for ongoing intervention.
Our results are consistent with observations from PET [33, 34, 35] studies demonstrating persistently ischemic tissue surrounding the infarct for many hours or even days after stroke, which can progress to infarction or recover if blood flow is restored. PET studies demonstrate that patients who showed a large area of hypoperfusion with increased oxygen extraction fraction (OEF) 5–18 h after stroke had an outcome ranging from death to full recovery, whereas all patients with early reperfusion (before 5 h) recovered [36, 37].
Our results are also consistent with PET studies showing that regions of hypoperfusion are associated with ischemic symptoms [38] and with worsening symptoms with drops in systemic blood pressure (e.g., orthostatic transient ischemic attacks) [34]. These findings have led to the conclusion that the finding of persistently hypoperfused regions (particularly with increased OEF) should warrant consideration of endarterectomy in cases of carotid stenosis, extracranial-intracranial bypass, or at least strict avoidance of systemic hypotension [7]. Although OEF, measured with PET, may be a more quantitative measure of penumbral tissue, PWI is much more rapid and logistically more accessible in the setting of acute stroke. Serial images over several days can be obtained, without the concern of ionizing radiation. Our results indicate that PWI might therefore be particularly useful in monitoring, as well as selecting, intervention. The risks and benefits of pharmacological blood pressure elevation, relative to conventional management, warrant investigation with larger numbers of patients in randomized, controlled trials.
References
Lutsep HL, Albers GW, DeCrespigny A, Kamat GN, Marks MP, Moseley ME (1997) Clinical utility of diffusion-weighted magnetic imaging in the assessment of ischemic stroke. Ann Neurol 41: 574–580
Beauchamp N, Bryan RN (1998) Acute cerebral ischemic infarction: a pathophysiologic review and radiologic perspective. Am J Roentgenol 171: 73–84
Fisher M, Albers GW (1999) Applications of diffusion-perfusion magnetic resonance imaging in acute ischemic stroke. Neurol 52: 1750–1756
Sorensen AG, Buonanno FS, Gonzalez RG, et al (1996) Hyperacute stroke: evaluation with combined multisection diffusion-weighted imaging and hemodynamically-weighted echo-planar MR imaging. Radiology 199: 391–401
Skyhøj Olsen T, Bruhn P, Oberg RG (1986) Cortical hypoperfusion as a possible cause of “subcortical aphasia”. Brain 106: 393–410
Powers, WJ (1993) Acute hypertension after stroke: the scientific basis for treatment decisions. Neurology 43: 461–467
Baron JC, Marchal G (2000) Functional imaging in vascular disorders. In: Mazziotta JC, Toga AW, Frackowiak RSJ (eds) Brain mapping: the disorders. Academic Press, San Diego, pp 299–311
Hakim AM (1998) Ischemic penumbra: the therapeutic window. Neurology 51: S44-S46
Schellinger PD, Fiebach JB, Jansen O, et al (2001) Stroke magnetic resonance imaging within 6 hours after onset of hyperacute cerebral ischemia. Ann Neurol 49: 460–469
Sunshine, JL, Tarr RW, Lanzieri CF, Landis DMD, Selman WR, Lewin JS (1999) Hyperacute stroke: ultrafast MR imaging to triage patients prior to therapy. Radiology 212: 325–332
Schlaug G, Benfield A, Baird AS, et al (1999) The ischemic penumbra operationally defined by diffusion and perfusion MRI. Neurology 53: 1528–1537
Barber PA, Darby DG, Desmond PM, et al (1998) Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology 51: 418–426
Hillis AE, Barker PB, Beauchamp NJ, Gordon, B, Wityk RJ (2000) MR perfusion imaging reveals regions of hypoperfusion associated with aphasia and neglect. Neurology 55: 782–788
Jacobs MA, Simpson C, Giugni E, et al (in press) MR perfusion imaging in acute stroke: comparison of time to peak and mean transit time. Stroke 33: 367 [abstract]
Parsons MW, Yang, Q, Barber PA, et al (2000) MR perfusion imaging: acute rCBF is more accurate than rMTT or rCBV in prediction of infarct size. Stroke 31: 275
Wityk RJ, Hillis AE, Beauchamp N, Aldrich E, Barker P (2000) Mismatch of diffusion-perfusion weighted MRI in stroke patients beyond 48 hours. Neurology 54 [Suppl 3]: A474 [abstract]
Hillis AE, Barker PB, Beauchamp NJ, Winters BD, Mirski M, Wityk RJ (2001) Restoring blood pressure reperfused Wernicke’s area and improved language. Neurology 56: 670–672
Alberts MJ (1999) tPA in acute ischemic stroke. Neurology 51 [Suppl 3]: S53–S55
Wise G, Sutter R, Burkholder J (1972) The treatment of brain ischemia with vasopressor drugs. Stroke 3: 135–140
Rordorf G, Cramer SC, Efird JT, Schwamm LH, Buonanno F, Koroshetz W (1997) Pharmacological elevation of blood pressure in acute stroke. Stroke 28: 2133–2138
Rordorf G, Koroshetz W., Ezzeddine MA, Segal AZ, Buonanno FS (2001) A pilot study of drug-induced hypertension for treatment of acute stroke. Neurology 56: 1210–1213
Astrup J, Symon L, Branston NM, Lassen NA (1977) Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke 8: 51–57
Skyhøj Olsen T, Larsen B, Herning M, Skriver EB, Lassen N (1983) Blood flow and vascular reactivity in collaterally perfused brain tissue. Stroke 14: 332–401
Wityk RJ, Pessin MS, Kaplan RF, Caplan LR (1994) Serial assessment of acute stroke using the NIH stroke scale. Stroke 25: 362–365
Hillis AE, Kane A, Tuffiash E, et al (in press) Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke. Brain Lang 79: 495–510
Heiss WD (1983) Flow thresholds for functional and morphological damage of brain tissue. Stroke 14: 329–331
Nakano S, Kinoshita K, Jinnouchi S, Hoshi H, Watanabe K (1989) Critical cerebral blood flow thresholds studied by SPECT using xenon-133 iodoamphetamine. J Nucl Med 30: 337–342
Buell U, Braun H, Ferert A, Stirner H, Weiller C, Ringlestein EB (1988) Combined SPECT imaging of regional cerebral blood flow (99mTc-hexemthyl-propyleneamine oxime, HMPAO) and blood volume (99mTc-RBC) to assess regional cerebral perfusion reserve in patients with cerebrovascular disease. Nuklearmedizin 27: 51–56
Lassen NA, Andersen AR (1988) Technetium-99 compounds for measurement of cerebral blood flow. J Nucl Med 29: 1464–1465
Hossmann KA (1994) Viability thresholds and the penumbra of focal ischemia. Ann Neurol 36: 557–565
Marks MP, Tong DC, Beaulieu C, Albers GW, de Crespigny A, Moseley ME (1999) Evaluation of early reperfusion and IV tPA therapy using diffusion- and perfusion-weighted MRI. Neurology 52: 1792–1798
Rordorf G, Koroshetz WJ, Copen WA, et al (1998) Regional ischemia and ischemic injury in patients with acute middle cerebral artery stroke as defined by early diffusion-weighted and perfusion-weighted MRI. Stroke 29: 939–943
Ackerman RH, Correia JA, Alpert NM, et al (1981) Positron imaging in ischemic stroke disease using compounds labeled with oxygen-15. Arch Neurol 38: 537–543
Baron JC, Bousser MG, Comar D, Castaigne P (1981) Reversal of focal “misery-perfusion syndrome” by extra-intracranial arterial bypass in hemodynamic cerebral ischemia: a case study with15O positron tomography. Stroke 12: 454–459
Wise RJS, Bernardi S, Frackowiak RSJ, Legg NJ, Jones T (1983) Serial Observations on the pathophysiology of acute stroke. The transition from ischaemia to infarction as reflected in regional oxygen extraction. Brain 106: 197–222
Marchal G, Serrati C, Rioux P, et al (1993) PET imaging of cerebral perfusion and oxygen consumption in acute ischemic stroke: Relation to outcome. Lancet 341: 925–927
Marchal G, Rioux P, Serrati C, et al (1995) Value of acute-stage PET in predicting neurological outcome after ischemic stroke: Further assessment. Stroke 26: 524–525
Derdeyn CP, Yundt KD, Videen TO, Carpenter DA, Grubb RL, Powers WJ (1998) Increased oxygen extraction fraction is associated with prior ischemic events in patients with carotid occlusion. Stroke 29: 754–758
Acknowledgements
The research reported in this paper was supported by an NIH grant, K23 DC00174-01 (to A.H.), the Charles A. Dana Foundation (grant to A.H.), and the Rodgers-Wilbur Foundation (gift to R.W.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hillis, A.E., Wityk, R.J., Beauchamp, N.J. et al. Perfusion-weighted MRI as a marker of response to treatment in acute and subacute stroke. Neuroradiology 46, 31–39 (2004). https://doi.org/10.1007/s00234-002-0918-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-002-0918-4