Abstract
The phloretin-induced reduction in the dipole potential of planar lipid bilayers containing cholesterol, ergosterol, stigmasterol, 7-dehydrocholesterol and 5α-androstan-3β-ol was investigated. It is shown that effects depend on the type and concentration of membrane sterol. It is supposed that the effectiveness of phloretin in reducing the dipole potential of the bilayers that contain cholesterol, ergosterol and 7-dehydrocholesterol correlates with the ordering and condensing effects. The role of the concentration-dependent ability of different sterols to promote lateral heterogeneity in membranes is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sterols are ubiquitous in the plasma membranes of eukaryotic cells, whereas they are universally absent from the membranes of prokaryotes. Cholesterol is one of the most important lipid species in eukaryotic cells and constitutes 25–50 % of membrane lipids, depending on the cell type (Sackmann 1995). Cholesterol is predicted to be important for several plasma membrane-based properties, including protein sorting and cell signaling; e.g., cholesterol acts as precursor to mammalian steroid hormones. Any significant decrease in the concentration of cholesterol in the body can result in severe health problems. For example, this decrease occurs in Smith-Lemli-Opitz syndrome. It is due to a deficiency of the 3β-hydroxysterol Δ7-reductase, which catalyzes the conversion of 7-dehydrocholesterol to cholesterol by removing one double bond from the ring system between atoms C7 and C8. Smith-Lemli-Opitz syndrome is characterized by a variety of features, including microcephaly, mental retardation and second and third toe syndactyly; the severity of these symptoms depends on the magnitude of the decrease in cholesterol concentration (Porter 2000). Ergosterol is the main sterol in fungi as well as in some protozoa and insects. Ergosterol differs from cholesterol by the presence of two additional double bonds, the first of which is in the ring system between C7 and C8 and the second of which is in the tail between atoms C22 and C23. There is also an additional methyl group, which is attached to C24 in the tail. Phytosterols, such as sitosterol, campesterol and stigmasterol, are found in plant plasma membranes. Ingested phytosterols are recognized by the body and decomposed in the liver. There is a disease, known as sitosterolemia (Bhattacharyya and Connor 1974), in which these three phytosterols are not decomposed. Under these circumstances, the phytosterols remain in the body and have been shown to be detrimental to human health. If not treated, sitosterolemia can lead to severe coronary problems, resulting in premature death.
In the absence of sterols, the membrane is in a liquid (fluid) state and is characterized by translational disorder, rapid lateral diffusion and a significant degree of lipid chain disorder. This bilayer phase is hence termed a “liquid-disordered phase.” As the temperature decreases, the lipid bilayer undergoes a phase transition in which the bilayer enters a state with in-plane translational order (as in a crystal), slow lateral diffusion and a significant degree of lipid-chain order. This phase is therefore termed the “solid-ordered phase.” The presence of cholesterol complicates this picture. In addition to its ability to increase lipid-chain order in fluid membranes, cholesterol can promote a special membrane phase, the liquid-ordered phase (Mouritsen and Jorgensen 1994, 1997; Bergelson et al. 1995; Simons and Ikonen 1997; Edidin 2003; McMullen et al. 2004; Maxfield 2002). These specialized lipid domains are known as “rafts.” The liquid-ordered phase is a liquid due to translational disorder and rapid diffusion in the plane of the bilayer, but at the same time, this phase has high lipid-chain conformational order. Because of the high order in the liquid-ordered phase, the bilayer is almost as thick as in the solid-ordered phase and, hence, has many of the desirable mechanical properties of a solid membrane without actually being crystalline. The liquid-ordered phase is unique to membranes containing cholesterol and other higher sterols, such as ergosterol, and absent in membranes containing cholesterol precursors, for example, 7-dehydrocholesterol (Aittoniemi et al. 2006). Xu et al. (2001) showed that the extent of domain (raft) formation is greatest for ergosterol, followed by stigmasterol, sitosterol and cholesterol.
The presence of rafts in both model and cellular membranes and their importance for biological activity have received increasing attention in the past few years. Cell membranes are now appreciated as exceedingly well-organized structures containing localized “microdomains”; these microdomains are deployed laterally along the plane of the membrane and appear to confer added levels of biological control. In the last few years, a hypothesis has been developed in which rafts also modulate membrane receptor activity by virtue of their elevated (or decreased) membrane dipole potential (O’Shea 2005; O’Shea et al. 2008).
The membrane dipole potential originates from the specific orientations of lipid and water dipoles at the membrane–solution interface. As a consequence, the membrane interior is several hundred millivolts positive with respect to the external aqueous phases. The adsorption of some electroneutral molecules, dipole modifiers, may lead to significant changes in the magnitude of the potential drop (Tsybulskaya et al. 1984; Malkov and Sokolov 1996). Some plant polyphenols, flavonoids, can cause a significant decrease in the dipole potential of the membrane. The quantitative characterization of the effects of flavonoids on the membrane dipole potential of target cells is necessary for pharmacological applications because of the antioxidant, antibacterial and anticancer activity of flavonoids (Cowan 1999; Havsteen 2002; Middleton et al. 2000). Recently, we showed that chalcones (phloretin and phloridzin) and flavonols (quercetin and myricetin) significantly decrease the dipole potential of phospholipid- and sterol-containing membranes (Efimova and Ostroumova 2012; Ostroumova et al. 2013). This article focuses on how the sterol structure and its modifications influence on phloretin–lipid bilayer interactions. The role of sterols in the formation of special membrane domains and the partitioning of dipole modifiers between different lipid phases are discussed.
Materials and Methods
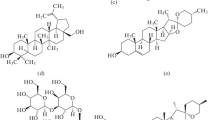
All chemicals were of reagent grade. Synthetic 1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC), cholesterol (Chol), ergosterol (Erg), stigmasterol (Stigm), 7-dehydrocholesterol (DhChol) and 5α-androstan-3β-ol (Andr) were obtained from Avanti Polar Lipids (Pelham, AL, USA). Phloretin (3-[4-hydroxyphenyl]-1-[2,4,6-trihydroxyphenyl]-1-propanone), quercetin (2-[3,4-dihydroxyphenyl]-3,5,7-trihydroxy-4H-1-benzopyran-4-one) and DMSO were purchased from Sigma Chemical (St. Louis, MO, USA). Water was distilled twice and deionized. Solutions of 0.1 M KCl were buffered using 5 mM HEPES at pH 7.4. The ionophore nonactin (NonA) was purchased from Sigma Chemical. The chemical structures of the sterols are shown in Fig. 1.
Virtually solvent-free planar lipid bilayers were prepared using a monolayer-opposition technique (Montal and Muller 1972) on a 50-μm-diameter aperture in a 10-μm-thick Teflon film that separated two (cis and trans) compartments of a Teflon chamber. The aperture was pretreated with hexadecane. Lipid bilayers were made from DPhPC and 5, 33 or 67 mol% sterol (Chol, Erg, DhChol, Stigm or Andr). After the membrane was completely formed and stabilized, NonA from a stock solution (7 μg/ml in ethanol) was added to both compartments to obtain a final concentration ranging from 10−7 to 10−6 M. Ag/AgCl electrodes with agarose/2 M KCl bridges were used to apply the transmembrane voltage (V) and measure the transmembrane current (I). “Positive voltage” refers to cases in which the cis side compartment was positive with respect to the trans side. All experiments were performed at room temperature.
Ion currents were measured using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) in the voltage-clamp mode. Data were digitized using a Digidata 1440A and analyzed using pCLAMP 10 (Axon Instruments) and Origin 7.0 (OriginLab, Northampton, MA). Current traces were filtered using an 8-pole Bessel filter at 30 kHz. The conductance (G) of the lipid bilayer was determined from I at a constant V = 50 mV.
The steady-state conductance of K+–NonA was modulated via the two-sided addition of phloretin or quercetin (from millimolar stock solutions in ethanol) to the membrane-bathing solution at final concentrations ranging from 2.5 to 100 μM. The final concentration of ethanol in the chamber did not exceed 0.8 %. Changes in φ d (\(\Updelta \varphi\) d) were calculated by assuming that the membrane conductance was related to φ d by the Boltzmann distribution as follows (Andersen et al. 1976):
where G m and G 0m are the steady-state membrane conductance induced by NonA in the presence and absence of phloretin (quercetin), respectively, and q e, k and T have their usual meanings. The changes in φ d for defined experimental conditions were averaged from three to five bilayers (mean ± SD). Applicability of Eq. 1 is determined by the fact that the shape of the I–V characteristic of K+–NonA-treated membranes remains superlinear at all flavonoid concentrations (Andersen et al. 1976; see Supplementary Material, Fig. 1S). This means that translocation of the charged K+–NonA complex through the membrane interior which is dependent on φ d retains the rate-limiting step at all flavonoid concentrations.
A Langmuir adsorption isotherm was used to describe the adsorption of flavonoids to lipid bilayers in a first-order approximation as follows (De Levie et al. 1979; Reyes et al. 1983; Cseh et al. 2000; Efimova and Ostroumova 2012; Ostroumova et al. 2013):
where \(\Updelta \varphi_{\text{d}} (C)\) is the dipole potential change at the C concentration of dipole modifiers, \(\Updelta \varphi_{\text{d}} (\infty )\) is the maximum potential change and K is the dissociation constant, which provides a meaningful measure of the affinity between the modifier and the lipid. The dissociation constant can be determined as the slope of a linear dependence of [Δφ d(∞)]/[Δφ d(C)] on 1/C. The linear approximation of indicated dependences was made using Origin 7.0.
Results and Discussion
Bilayers were made using DPhPC with various sterol contents, and the reductions in dipole potential for these bilayers at various phloretin concentrations are shown in Fig. 2. Figure 2a shows the effect of phloretin on \(\left| {\Updelta \varphi_{\text{d}} } \right|\) in membranes with 0, 5, 33, and 67 mol% Chol. One can see that the presence of 33 mol% Chol in the membrane leads to significant inhibition of the phloretin effect on the membrane dipole potential compared with 0, 5 and 67 mol% Chol in the membrane-forming solution. Figure 2b presents the dependences of the \(\left| {\Updelta \varphi_{\text{d}} } \right|\) of ergosterol-containing bilayers on the dipole modifier concentration. It is evident that the concentration of Erg in the membrane determines the phloretin-induced \(\left| {\Updelta \varphi_{\text{d}} } \right|\): the maximum is observed at 5 mol% Erg, and the \(\left| {\Updelta \varphi_{\text{d}} } \right|\) value slows down for lower (down to 0 mol%) as well as for higher (up to 67 mol%) Erg concentrations. For 7-DhChol, the presence of large amounts of sterol in the membrane (67 mol%) leads to a significant decrease in \(\left| {\Updelta \varphi_{\text{d}} } \right|\) (Fig. 2c). The phloretin-induced \(\left| {\Updelta \varphi_{\text{d}} } \right|\) of stigmasterol- and 5α-androstan-3β-ol-containing bilayers (Fig. 2d, e, respectively) weakly depends on the sterol amount.
Changes in the membrane dipole potential (Δφ d) for various concentrations of phloretin in solution bathing membranes containing Chol (a), Erg (b), 7-DhChol (c), Stigm (d) and Andr (e). The membranes were constructed using DPhPC (●), DPhPC:sterol (95:5 mol%) (□), DPhPC:sterol (67:33 mol%) (∆) and DPhPC:sterol (33:67 mol%) (►) and bathed in 0.1 M KCl at pH 7.4. V = 50 mV. The results for the DPhPC, DPhPC:Chol (67:33 mol%) and DPhPC:Erg (67:33 mol%) bilayers are from Efimova and Ostroumova (2012)
All the curves presented in Fig. 2 are practically linear at low concentrations of phloretin and tend to saturate at high ones. A Langmuir adsorption isotherm can be used to describe the adsorption of flavonoids to lipid bilayers in a first-order approximation (De Levie et al. 1979; Reyes et al. 1983; Cseh et al. 2000; Efimova and Ostroumova 2012; Ostroumova et al. 2013). The applicability of Langmuir adsorption isotherm for the description of adsorption of phloretin to lipid bilayers was discussed in Cseh and Benz (1998) and Cseh et al. (2000). The authors suggested that the concentration-dependent changes in phloretin dipole moment due to dipole–dipole interactions on the lipid surface may affect the phloretin-induced dipole potential decrease. Also, phloretin might affect some nonelectrostatic parameters of lipid bilayer, in particular its mechanical properties (Andersen et al. 1976; Valenta et al. 2004; Auner et al. 2005; Tarahovsky et al. 2008). However, the fact that the Langmuir adsorption isotherm is a good approximation for the data obtained (see Supplementary Material, Fig. 2S) indicates that the contributions of other components such as changes in phloretin dipole moment or surface density may be neglected. Table 1 shows the following characteristic parameters of the Langmuir adsorption isotherm: the maximum potential change, \(\Updelta \varphi_{\text{d}} (\infty )\), and the dissociation constant, K, both of which were calculated using approximations of the experimental data.
The present results allow the following conclusions: (1) phloretin is most effective for ergosterol-containing bilayers and least effective for 7-DhChol-containing bilayers, (2) the dependence of \(\Updelta \varphi_{\text{d}} (\infty )\) on the Chol and Erg concentrations is nonmonotonic and (3) the dissociation constants, K, which characterize the inverse affinity of phloretin for the membranes are within the same order of magnitude.
Chol produces ordering and condensing effects in PC membranes. Therefore, the area per lipid molecule decreases as the Chol concentration increases and reaches a plateau for concentrations above 35 mol% (Róg et al. 2009). The ordering effect of Erg is greater than that of Chol (Cournia et al. 2007), whereas the effects of 7-DhChol on membrane order and condensation are smaller than those of Chol (Serfis et al. 2001; Berring et al. 2005). One can suppose that the ordering effect of these three sterols correlates with the effectiveness of phloretin in reducing the dipole potential of the bilayers that contain these sterols. Two facts might be useful to rationalize this assumption. The first is that the dipole potential (φ d) is directly proportional to the dipole surface density according to the equation of a parallel plate capacitor (Flewelling and Hubbell 1986; Simon et al. 1992; Starke-Peterkovic and Clarke 2009). Thus, the dipole surface density should be a function of membrane composition, especially due to the different condensing effects of various sterols. The second one might be a preference for defined conformations and orientations of the phloretin molecule, which produces a larger projection of dipole moment to the normal to the plane of the membrane in more condensed and ordered bilayers.
The dependences of \(\left| {\Updelta \varphi_{\text{d}} } \right|\) on the sterol concentrations may be also related to the lateral organization of these membranes, more precisely to the different partitioning of phloretin or NonA between liquid-ordered and liquid-disordered domains. One feature of the Chol phase diagram is the occurrence of a liquid-ordered phase for a wide temperature range at bilayer concentrations >25 mol% (Thewalt and Bloom 1992). According to the phase diagram (Goñi et al. 2008) POPC membranes are in a liquid-disordered state at 5 mol% Chol. The coexistence of liquid-ordered and liquid-disordered domains is observed at 33 mol% Chol. Furthermore, at 67 mol% Chol, the membrane is in a liquid-ordered state. Erg is the most efficient relative to Chol at promoting the liquid-ordered phase (Cournia et al. 2007). Gao et al. (2008) found that Andr is less efficient at promoting the formation of an ordered phase compared to Chol, Stigm and Erg. 7-DhChol does not promote raft formation (Aittoniemi et al. 2006). The formation of hydrogen bonds between one hydroxyl of a dipole modifier and the P=O group of the phospholipids is believed to cause the phloretin-induced changes in the dipole potential of lipid bilayers (Tarahovsky et al. 2008). One can assume that the possibility for hydrogen bond formation depends on the lipid phase. Supporting this idea, lipid P=O groups are not available to phloretin in the gel state (Disalvo et al. 2004).
Pure PC membranes and PC bilayers containing a small amount of sterol are in a liquid-disordered state but are characterized by various surface densities of lipid dipoles due to the ordering and condensing effects of sterol (Aittoniemi et al. 2006). Therefore, the effect of phloretin on membranes containing 5 mol% of Chol or Erg might be greater than that on pure PC membranes. An increase in sterol concentration led to the appearance and growth of area of liquid-ordered domains. Differences in the partitioning of phloretin and/or NonA between liquid-ordered and liquid-disordered domains might decrease the measured \(\left| {\Updelta \varphi_{\text{d}} } \right|\). An independent confirmation of the assumption might be the dependence of the changes in the dipole potential of the cholesterol-containing membranes on the concentration of a phloretin analogue, quercetin, which can also alter the dipole potential (Fig. 3). Table 2 presents characteristic parameters of the Langmuir adsorption isotherm for quercetin with cholesterol-containing bilayers. One can see that the dependence is very similar to that observed for phloretin-treated, Erg-containing bilayers.
Changes in the membrane dipole potential, |Δφ d|, for various concentrations of quercetin in the membrane bathing solution. The membranes were constructed using DPhPC (●), DPhPC:Chol (95:5 mol%) (□), DPhPC:Chol (67:33 mol%) (∆) and DPhPC:Chol (33:67 mol%) (►) and bathed in 0.1 M KCl at pH 7.4. V = 50 mV. The results for DPhPC and DPhPC:Chol (67:33 mol%) bilayers are from Efimova and Ostroumova (2012)
The obtained results extend the understanding of the mechanisms underlying the influence of various sterols on flavonoid-induced changes in the membrane dipole potential. The effects might be attributed to the increase of dipole surface density in the membrane at low concentration of sterols and a phase separation in the bilayer at high concentration of sterols. Quantitative characterization of the effects of flavonoids on the dipole potential of target cell membranes should be taken into account when predicting and evaluating pharmacological activity.
References
Aittoniemi J, Róg T, Niemelä P, Pasenkiewicz-Gierula M, Karttunen M, Vattulainen I (2006) Tilt: major factor in sterols’ ordering capability in membranes. J Phys Chem B 110:25562–25564
Andersen OS, Finkelstein A, Katz I, Cass A (1976) Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol 67:749–771
Auner BG, O’Neill MA, Valenta C, Hadgraft J (2005) Interaction of phloretin and 6-ketocholestanol with DPPC-liposomes as phospholipid model membranes. Int J Pharm 294:149–155
Bergelson LO, Gawrisch K, Feretti JA, Blumenthal R (1995) Domain organization in biological membranes. Mol Membr Biol 12:1–162
Berring EE, Borrenpohl K, Fliesler SJ, Serfis AB (2005) A comparison of the behavior of cholesterol and selected derivatives in mixed sterol-phospholipid Langmuir monolayers: a fluorescence microscopy study. Chem Phys Lipids 136:1–12
Bhattacharyya AK, Connor WE (1974) Beta-sitosterolemia and xanthomatosis: a newly described lipid storage disease in two sisters. J Clin Invest 53:1033–1043
Cournia Z, Ullmann GM, Smith JC (2007) Differential effects of cholesterol, ergosterol and lanosterol on a dipalmitoyl phosphatidylcholine membrane: a molecular dynamics simulation study. J Phys Chem 111:1786–1801
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
Cseh R, Benz R (1998) The adsorption of phloretin to lipid monolayers and bilayers cannot be explained by Langmuir adsorption isotherms alone. Biophys J 74:1399–1408
Cseh R, Hetzer M, Wolf K, Kraus J, Bringmann G, Benz R (2000) Interaction of phloretin with membranes: on the mode of action of phloretin at the water–lipid interface. Eur Biophys J 29:172–183
De Levie R, Rangarajan SK, Seelig PF, Andersen OS (1979) On the adsorption of phloretin onto a black lipid membrane. Biophys J 25:295–300
Disalvo EA, Lairion F, Martini F, Almaleck H (2004) Water in biological membranes at interfaces: does it play a functional role? J Argent Chem Soc 92:1–22
Edidin M (2003) The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct 32:257–283
Efimova SS, Ostroumova OS (2012) Effect of dipole modifiers on the magnitude of the dipole potential of sterol-containing bilayers. Langmuir 28:9908–9914
Flewelling RF, Hubbell WL (1986) The membrane dipole potential in a total membrane potential model: applications to hydrophobic ion interactions with membranes. Biophys J 49:541–552
Gao W, Chen L, Wu R, Yu Z, Quinn PJ (2008) Phase diagram of androsterol-dipalmitoylphosphatidylcholine mixtures dispersed in excess water. J Phys Chem B 112:8375–8382
Goñi FM, Alonso A, Bagatolli LA, Brown RE, Marsh D, Prieto M, Thewalt JL (2008) Phase diagrams of lipid mixtures relevant to the study of membrane rafts. Biochim Biophys Acta 1781:665–684
Havsteen BH (2002) The biochemistry and medical significance of the flavonoids. Pharmacol Ther 96:67–202
Malkov DY, Sokolov VS (1996) Fluorescent styryl dyes of the RH series affect a potential drop on the membrane/solution boundary. Biochim Biophys Acta 1278:197–204
Maxfield FR (2002) Plasma membrane microdomains. Curr Opin Cell Biol 14:483–487
McMullen TPW, Lewis RNAH, McElhaney RN (2004) Cholesterol–phospholipid interactions, the liquid-ordered phase in model and biological membranes. Curr Opin Colloid Interface Sci 8:459–468
Middleton E, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:673–751
Montal M, Muller P (1972) Formation of bimolecular membranes from lipid monolayers and study of their electrical properties. Proc Natl Acad Sci USA 65:3561–3566
Mouritsen OG, Jorgensen K (1994) Dynamical order and disorder in lipid bilayers. Chem Phys Lipids 73:3–26
Mouritsen OG, Jorgensen K (1997) Small-scale lipid membrane structure: simulation vs. experiment. Curr Opin Struct Biol 7:518–527
O’Shea P (2005) Physical landscapes in biological membranes: physico-chemical terrains for spatio-temporal control of biomolecular interactions and behaviour. Philos Trans R Soc A 363:575–588
O’Shea P, Somekh MG, Barnes WL (2008) Shedding light on life: visualizing nature’s complexity. Phys World 21:29–34
Ostroumova OS, Efimova SS, Schagina LV (2013) Changes in the dipole potential of phospholipid membranes induced by the adsorption of flavonoids (in Russian). Biophysics 58:474–480
Porter FD (2000) RSH/Smith-Lemli-Opitz syndrome: a multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol Genet Metab 71:163–174
Reyes J, Greco F, Motais R, Latorre R (1983) Phloretin and phloretin analogs: mode of action in planar lipid bilayers and monolayers. J Membr Biol 72:93–103
Róg T, Pasenkiewicz-Gierula M, Vattulainen I, Karttunen M (2009) Ordering effects of cholesterol and its analogues. Biochim Biophys Acta 1788:97–121
Sackmann E (1995) Biological membranes architecture and function. In: Lipowsky R, Sackmann E (eds) Structure and dynamics of membranes. Elsevier, Amsterdam, pp 1–64
Serfis AB, Brancato S, Fliesler SJ (2001) Comparative behavior of sterols in phosphatidylcholine-sterol monolayer films. Biochim Biophys Acta 1511:341–348
Simon SA, McIntosh TJ, Magid AD, Needham D (1992) Modulation of the interbilayer hydration pressure by the addition of dipoles at the hydrocarbon/water interface. Biophys J 61:786–799
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572
Starke-Peterkovic T, Clarke RJ (2009) Effect of headgroup on the dipole potential of phospholipid vesicles. Eur Biophys J 39:103–110
Tarahovsky YS, Muzafarov EN, Kim YA (2008) Raft making and rafts braking: how plant flavonoids may control membrane heterogeneity. Mol Cell Biochem 314:65–71
Thewalt JL, Bloom M (1992) Phosphatidylcholine: cholesterol phase diagrams. Biophys J 63:1176–1181
Tsybulskaya MV, Antonenko YN, Tropsch AE, Yaguzhinskii LS (1984) Iodine-containing hormones—dipole modifiers of phospholipid membranes [in Russian]. Biophysics 29:801–805
Valenta C, Steininger A, Auner BG (2004) Phloretin and 6-ketocholestanol: membrane interactions studied by a phospholipid/polydiacetylene colorimetric assay and differential scanning calorimetry. Eur J Pharm Biopharm 57:329–336
Xu X, Bittman R, Duportail G, Heissler D, Vilcheze C, London E (2001) Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem 276:33540–33546
Acknowledgments
We are grateful to Prof. Valery V. Malev for fruitful discussions. This work was partly supported by the Russian Foundation for Basic Research (Grants 12-04-00948, 12-04-31332, 12-04-33121), the Program “Molecular and Cell Biology” of the Russian Academy of Sciences, a Grant from the president of RF (MK-1813.2012.4) and Russian state contract 8119 (MES, FTP, SSEPIR).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ostroumova, O.S., Efimova, S.S. & Schagina, L.V. Phloretin-Induced Reduction in Dipole Potential of Sterol-Containing Bilayers. J Membrane Biol 246, 985–991 (2013). https://doi.org/10.1007/s00232-013-9603-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-013-9603-2