Abstract
α-Hemolysin (HlyA) from Escherichia coli lyses mammalian erythrocytes by creating nonselective cation pores in the membrane. Pore insertion triggers ATP release and subsequent P2X receptor and pannexin channel activation. Blockage of either P2X receptors or pannexin channels reduces HlyA-induced hemolysis. We found that erythrocytes from Python regius and Python molurus are remarkably resistant to HlyA-induced hemolysis compared to human and Trachemys scripta erythrocytes. HlyA concentrations that induced maximal hemolysis of human erythrocytes did not affect python erythrocytes, but increasing the HlyA concentration 40-fold did induce hemolysis. Python erythrocytes were more resistant to osmotic stress than human erythrocytes, but osmotic stress tolerance per se did not confer HlyA resistance. Erythrocytes from T. scripta, which showed higher osmotic resistance than python erythrocytes, were as susceptible to HlyA as human erythrocytes. Therefore, we tested whether python erythrocytes lack the purinergic signalling known to amplify HlyA-induced hemolysis in human erythrocytes. P. regius erythrocytes increased intracellular Ca2+ concentration and reduced cell volume when exposed to 3 mM ATP, indicating the presence of a P2X7-like receptor. In addition, scavenging extracellular ATP or blocking P2 receptors or pannexin channels reduced the HlyA-induced hemolysis. We tested whether the low HlyA sensitivity resulted from low affinity of HlyA to the python erythrocyte membrane. We found comparable incorporation of HlyA into human and python erythrocyte membranes. Taken together, the remarkable HlyA resistance of python erythrocytes was not explained by increased osmotic resistance, lack of purinergic hemolysis amplification, or differences in HlyA affinity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The gram-negative bacterium Escherichia coli plays a dual role in most mammals. E. coli is the most prevalent gram-negative bacterium in the human intestine (Cavalieri et al. 1984; Johnson and Stell 2000), where it contributes to normal intestinal function and protects against intestinal infection by pathogenic bacteria (Hudault et al. 2001) and is possibly involved in vitamin K production (Conly and Stein 1992). On the other hand, E. coli also gives rise to serious extraintestinal infections, such as urinary tract infections, neonatal meningitis, and peritonitis (Cavalieri et al. 1984; Johnson and Stell 2000). E. coli strains isolated from patients often produce virulence factors, which contribute to the severity of the infection and are rarely produced by the commensal, noninvasive strains (Johnson and Stell 2000; Cavalieri et al. 1984). One such virulence factor is α-hemolysin (HlyA), a pore-forming toxin commonly produced by E. coli strains isolated from extraintestinal infections (Cavalieri et al. 1984; Johnson and Stell 2000).
HlyA belongs to the repeat-in-toxin (RTX) family. It is a protein of ~107 kDa (Felmlee et al. 1985) that can insert itself into plasma membranes to form a nonselective pore with a diameter of ~2 nm (Bhakdi et al. 1988). HlyA inserts as a monomer in a receptor-independent fashion (Bhakdi et al. 1986, 1988; Menestrina et al. 1987; Valeva et al. 2005), although HlyA insertion may be aided by binding to membrane glycophorins before insertion (Cortajarena et al. 2001, 2003). HlyA has been reported to cause reversible oscillations in the intracellular Ca2+ concentration, [Ca2+]i, in some cell types (Koschinski et al. 2006; Soderblom et al. 2002), whereas other cell types, including erythrocytes, lyse when subjected to the toxin (Bhakdi et al. 1988; Gadeberg et al. 1983).
Previous studies on HlyA have focused on the effect on mammalian cells, and to our knowledge, the effect of HlyA has not been studied in reptilian cells. Reptile erythrocytes have, however, been reported to be more resistant to α-toxin from Staphylococcus aureus (a different type of pore-forming toxin) than mammals (Slifkin 1965). Integration of α-toxin into the erythrocyte membrane is, contrary to HlyA, receptor dependent, which can readily explain the discrepancy in α-toxin sensitivity between the species.
The mechanism of HlyA-induced cell damage was classically thought to result from the pore itself (Bhakdi et al. 1986, 1988). It was recently shown that ATP release and P2X receptor signaling amplifies the effect of HlyA in a process involving pannexin channels (Skals et al. 2009). P2X7 receptors are known to be pro-apoptotic when stimulated continuously (Le Stunff et al. 2004; Costa-Junior et al. 2009) and have been shown to induce apoptosis by interaction with pannexin-1 channels (Locovei et al. 2007). Inhibition of P2 receptors or pannexin channels completely prevents HlyA-induced lysis of mammalian erythrocytes (Skals et al. 2009, 2010). These findings fit well with the one-hit model that states that a single HlyA molecule is sufficient to lyse an erythrocyte (Jorgensen et al. 1980).
The above-mentioned studies indicate that the mechanism of HlyA-induced hemolysis is not quite as simple as previously thought, and that the full biologic effect of HlyA requires the intrinsic capacity of erythrocytes for local purinergic signaling. Thus, the properties of the erythrocyte to some degree determine the outcome of insertion of a pore-forming toxin in the membrane. As reptilian erythrocytes in many physiological respects are distinct from mammalian ones, we tested whether the response to HlyA is qualitatively different in python erythrocytes. To our surprise, we found that erythrocytes from Python regius and Python molurus are remarkably resistant to the effects of HlyA. Reptilian erythrocytes in general were not resistant to HlyA because erythrocytes from the turtle Trachemys scripta were as sensitive to HlyA as human erythrocytes. On the basis of these observations, we hypothesized that the response to HlyA differs significantly between human and python erythrocytes. The explanation for the HlyA resistance of python erythrocytes could provide additional insight into the mechanism of HlyA, a mechanism that remains incompletely resolved.
Materials and Methods
Toxin Purification
HlyA was purified from the supernatant of liquid cultures of the uropathogenic E. coli strain ARD6 (serotype O6:K13:H1) in Lysogeny broth (LB) medium in a process modified from the method described by Hyland et al. (2001). A colony of E. coli ARD-6 was transferred to 4 ml sterile LB medium and incubated overnight in a shaker (37°C, 200 rpm). The next morning, 1 ml of the overnight culture was transferred to 1 l sterile LB medium supplemented with 10 mM CaCl2 and incubated 4.5 h in a shaker (37°C, 200 rpm). After incubation, cultures were centrifuged twice (2943×g, 15 min, 4°C) to pellet bacteria and the supernatant was sterile filtered to remove any remaining bacteria (pore size 0.22 μm, Millipore, Bedford, MA). The supernatant pH was adjusted to 4.5 (1 M malonic acid) and HlyA was precipitated overnight with ethanol (25% vol, 4°C). The precipitate was centrifuged (17,300×g, 30 min, 4°C, Sorvall RC-5C, Thermo Scientific) and the pellet resuspended in 6 M guanidine–HCl, precipitated for 60 min with ethanol (90 %vol, −20°C), and centrifuged at 12,960×g. The final precipitate containing HlyA was resuspended in a Tris-buffered 8 M guanidine–HCl solution with 10 mM DTT (pH 6.0). The hemolytic strength of the HlyA suspensions varied between purifications. HlyA concentrations in Figs. 1 and 2 are not comparable because these experiments were performed with different HlyA purifications, which resulted in different strengths of the toxin solutions. Each experimental series was always completed with the same HlyA purification.
HlyA- and osmotically induced lysis of human and P. regius erythrocytes. a Time course of the effect of HlyA on human and python erythrocytes. HlyA (0.25 μl ml−1) caused ~100% lysis of human erythrocytes (n = 6) over 60 min at 37°C but had no effect on python erythrocytes (n = 5). b Dose–response curve for HlyA on human and python erythrocytes. Human and python erythrocytes were incubated at various concentrations of HlyA for 60 min at 37°C. Complete hemolysis of human erythrocytes (n = 6) was induced by 0.25 μl ml−1 HlyA, whereas 10 μl ml−1 HlyA was required to induce ~100% hemolysis of P. regius (n = 5). c Osmotic resistance of human (n = 6) and python erythrocytes (n = 5). The erythrocytes were incubated 60 min at 37°C at solutions of varying ionic strength. Values are presented as mean ± SEM, n = number of animals or volunteers in each experiment. *Statistical difference between the hemolysis of human and python erythrocytes (P < 0.05)
HlyA- and osmotically induced lysis of T. scripta and P. molurus erythrocytes. a Osmotic resistance of T. scripta and P. molurus erythrocytes. The erythrocytes were incubated for 60 min at 25°C at solutions of varying ionic strength (n = 6). b Concentration-response effects of HlyA on T. scripta and P. molurus erythrocytes. The erythrocytes were incubated at various concentrations of HlyA for 60 min at 25°C. EC50 of HlyA was ~0.2 μl ml−1 for T. scripta and ~15 μl ml−1 for P. molurus (n = 6). Values are presented as mean ± SEM, n = number of animals in each experiment. *Statistical difference between the hemolysis of turtle and python erythrocytes (P < 0.05)
Isolation of Erythrocytes
Blood samples were obtained from healthy P. regius, P. molurus, and T. scripta specimens, either by venipuncture of the inferior vena cava on terminally anesthetized animals (pentobarbital, 50 mg kg−1) or drawn from arterial catheters of conscious animals. Blood was drawn into a syringe containing heparin and immediately transferred to Venosafe K2EDTA tubes (Terumo Europe, Leuven, Belgium) to avoid coagulation. Human blood samples were obtained from volunteers by venipuncture (Venosafe K2EDTA tubes) according to approval from the Danish Scientific Ethics Committee (license M20110217). Erythrocytes were washed twice in 0.9% NaCl (w/v), the buffy coat was removed, and the erythrocytes were washed twice in HEPES-buffered saline (HBS) (centrifugation at 2325×g, 3 min for human and 582×g, 2 min for python and turtle). Finally, erythrocytes were centrifuged at 2325×g and supernatant was removed, leaving behind a concentrated preparation of erythrocytes. Python and turtle erythrocytes were stored for use on subsequent days in Alsever’s solution at 4°C and used within 14 days of isolation. Human erythrocytes were always used on the same day.
Hemolysis
Erythrocytes and HlyA were diluted in HBS and mixed in 96-well plates to yield 1.25% (v/v) erythrocyte suspensions with the desired HlyA concentrations. The suspensions were incubated at 37°C for 60 min (unless otherwise stated) in an absorbance reader (Powerwave 340, Biotek) while being shaken at 1140 rpm. Upon incubation, plates were centrifuged (2325×g, 3 min) and absorbance of the supernatant was detected at 540 nm as a measure of released hemoglobin. Control samples for 0% (no HlyA) and 100% hemolysis (lysis buffer) were included in each experiment and the level of hemolysis was calculated by the following equation:
For experiments with colored compounds (pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid tetrasodium salt [PPADS] and Brilliant Blue G [BBG]), controls samples were prepared for each concentration of the compound as these compounds themselves absorbed light at 540 nm. Comparisons of HlyA sensitivity and osmotic fragility between T. scripta and P. molurus erythrocytes were carried out at 25°C as T. scripta erythrocytes did not tolerate 37°C.
Hematocrit
Hematocrit was used as a measure of cell volume in python erythrocytes. Erythrocytes were incubated in HBS with ATP (3 mM, 5 min, 37°C) or regular HBS, after which the suspensions were transferred to hematocrit tubes and centrifuged (8050×g, 3 min) in a hematocrit centrifuge (Heraeus Sepatech GmbH, Osterode am Harz, Germany). Erythrocyte swelling results in an increase in hematocrit, shrinkage a decrease. Thus, hematocrit can be used as a measure of changes in cell volume.
Live Cell Imaging
Python erythrocytes were attached to coverslips with BD Cell-Tak (BD Bioscience, Franklin Lakes, NJ, USA), placed in a perfusion chamber (Warner Instruments, Hamden, CT, USA), and inspected on an inverted microscope (TE-2000, Nikon) equipped with differential interference contrast (DIC, 63X, NA1.2). Images were captured at 1 Hz using an intensified SVGA charge-coupled device (CCD) camera and imaging software (VoxCell, VisiTech International, Sunderland, UK). Experiments lasted for up to 90 min. Images were converted to movies with the ImageJ software (National Institutes of Health, Bethesda, MD, USA). The same setup was used for fluorescence microscopy to estimate changes in [Ca2+]i with fluo-4. Excitation light (488 nm) was provided by a monochromator (Visitech International, Sunderland, UK), fluorescence was detected above 520 nm. Erythrocytes were loaded with fluo-4 in the presence of 500 μM probenicid (5 μM fluo-4, 120 min, 20°C) to prevent removal of probe from the cytoplasm.
Fluorescence Labeling of HlyA
Labeling of HlyA with commercially available amine reactive fluorescent probes was not possible because of incompatibilities between the HlyA buffer and such labeling kits. The thiol-reactive probe 6-bromoacetyl-2-dimethylaminonaphthalene (BADAN) has previously been used to label HlyA (Schindel et al. 2001); however, this required production of a cysteine-containing version of HlyA by mutagenesis. The HlyA gene was modified to encode a cysteine in the C-terminal and ligated into the PrSET plasmid (Invitrogen). E. coli ARD6 bacteria were transformed with the HlyA-cys-PrSET plasmid and cysteine containing HlyA (HlyA-cys) was purified from these bacteria. HlyA-cys was purified as described above, except for the final resuspension. HlyA-cys was suspended in a labeling buffer (100 mM Tris, 8 M guanidine–HCl, 100 mM NaCl, pH 8.5) and BADAN was added to a final concentration of 1 mM. BADAN labeling of HlyA-cys was performed at 4°C overnight. The next morning, DTT (5 mM) was added to stop the labeling reaction and labeled HlyA-cys was precipitated with ethanol (90% vol, −20°C) and spun at 12,960×g. Precipitates were washed in ethanol repeatedly to remove the ethanol soluble unreacted BADAN. After each wash, the supernatant was tested for fluorescence at 312 nm. When fluorescence could no longer be detected, the precipitates were washed one final time to ensure efficient removal of unreacted BADAN. As a control for unspecific binding of BADAN to HlyA, native HlyA was subjected to BADAN labeling (sham labeling). Protein concentration of labeled HlyA-cys and sham-labeled HlyA was determined with the Pierce BCA Protein Assay Kit (ThermoScientific).
Binding of BADAN-HlyA-cys to Erythrocyte Ghosts
The ability of BADAN-labeled HlyA to bind to python and human erythrocyte membranes was studied on erythrocyte ghosts. Ghosts were produced as described by Gasbjerg and Brahm (1991). To ensure complete removal of hemoglobin, the ghost purification procedure was repeated twice. Suspensions of human and python erythrocyte ghosts were adjusted to the same hematocrit, diluted 1:10 and incubated with BADAN-labeled HlyA-cys (10 mg ml−1) or sham-labeled HlyA (10 mg ml−1) at 37°C. Samples (1 ml) were removed at 20-min intervals, washed twice to remove unbound fluorescent toxin, and resuspended in 1 ml HBS. Fluorescence of the erythrocyte ghosts was measured in a monochromator based spectrofluorometer (Quantum Master UV-visual QM-4, Photon Technologies International). The ghosts were excited at 390 nm and emission was detected from 450–600 nm. Fluorescence of ghost membranes incubated with HBS only was subtracted from the fluorescence of ghosts with fluorescent toxin bound to yield the increase in fluorescence, a measure of the amount of HlyA binding.
Solutions and Materials
The HEPES-buffered saline (HBS) consisted of (in mM): [Na+] 138.0, [Cl−] 132.9, [K+] 5.3, [Ca2+] 1.8, [Mg2+] 0.8, [SO4 2−] 0.8, [HEPES] 14.0, [glucose] 5.6, pH 7.4 at 37°C. The osmolality of the HBS was 299 mOsm. For hemolysis experiments with hexokinase, [Mg2+] and [SO4 2−] was raised to 2.5 mM and [glucose] raised to 10 mM to accommodate the requirements of hexokinase, while [Na+] was lowered to 134.1 mM and [Cl−] lowered to 129.0 mM to maintain the osmolality. For Fluo-4-AM loading, probenicid (500 μM) was included in the buffer. Probenicid was dissolved in NaOH (500 μl, 1 M) and added to the buffer; pH was then titrated to 7.4 at 37°C with 5 M HCl.
Alsever solution consisted of (in mM): [C6H8O7] (lactic acid) 2.6, [C6H5O7 3−] (lactate) 27.2, [Na+] 153.5, [Cl−] 71.9, [Glucose] 114.
Lysis buffer consisted of(in mM): [NH4 +] 170, [Cl−] 170, [EDTA] 0.11, [Na+] 0.22, [K+] 1, [HCO3 −] 1.
PPADS, carbenoxolone, probenecid, hexokinase, suramin, and ATP were purchased from Sigma-Aldrich, fluo-4-AM from Invitrogen and BBG from ICN Biomedicals (Aurora, OH, USA). ATP was dissolved in HBS, pH adjusted to 7.4 at 37°C. Fluo-4 and BBG was dissolved in dimethyl sulfoxide (DMSO); DMSO never exceeded 0.2% (v/v) in final solutions. PPADS, carbenoxolone, probenicid, hexokinase, and suramin were dissolved in water.
Statistical Analysis
Data are presented as mean ± SEM. Indicated are the number of animals or volunteers. Data were tested for normal distribution by the Kolmogorov-Smirnov test. Significant differences were determined by paired or unpaired Student’s t-test or one-way ANOVA (Tukey post hoc test) for multiple comparisons as appropriate. In both cases, a P value of less than 0.05 was considered statistically significant.
Results
Osmotic and HlyA-induced Hemolysis
P. regius erythrocytes were unaffected by concentrations of HlyA that resulted in 100% lysis of human erythrocytes (Fig. 1a), although very high concentrations of HlyA lysed the python erythrocytes. Whereas 0.25 μl ml−1 HlyA caused ~100% lysis of human erythrocytes, 10 μl ml−1 HlyA was needed to induce ~100% hemolysis of python erythrocytes (Fig. 1b). Python erythrocytes were more resistant to osmotically induced hemolysis than human erythrocytes (Fig. 1c). Interestingly, hemolysis of python erythrocytes was maximal 60 mOsm and became submaximal at lower osmolalities. We do not have any obvious explanation for this consistent finding, but consequently, we had to use a lysis buffer to produce the 100% hemolytic control. To investigate whether high tolerance to osmotic stress resistance could explain the remarkable resistance to HlyA, we investigated the HlyA sensitivity of erythrocytes from another reptile, the turtle Trachemys scripta, previously reported to have similar osmotic stress tolerance as snake erythrocytes (Aldrich and Saunders 2001). The turtle erythrocytes had a slightly higher osmotic resistance than python erythrocytes (Fig. 2a). Because they were as sensitive to HlyA as human erythrocytes (Fig. 2b), it does not seem that osmotic stress resistance per se confers resistance to HlyA.
Purinergic Signaling in P. regius Erythrocytes
The HlyA-induced hemolysis of mammalian erythrocytes involves ATP release and activation of ATP-sensitive P2 receptors (Skals et al. 2009). To investigate whether the remarkable resistance of the python erythrocytes to HlyA could be ascribed to lack of P2 receptor-dependent amplification of the HlyA-induced hemolysis, we exposed python erythrocytes to 3 mM ATP to stimulate P2 receptors. This treatment resulted in a rise in [Ca2+]i (Fig. 3a) and shrinkage as shown by a decrease in hematocrit (Fig. 3b) and by direct visualization in the microscope (Fig. 3c; Suppl. Movie 1). Shrinkage was not detectable with lower ATP concentrations (1 mM; data not shown). The shrunken python erythrocytes were wrinkled and appeared different from human erythrocytes that normally have a decreased diameter and appear spiky (crenated) when shrunken (Skals et al. 2010). Like all nucleated erythrocytes, python erythrocytes possess a cytoskeletal marginal band (Cohen 1991; Joseph-Silverstein and Cohen 1984). The marginal bands were intact upon stimulation with 3 mM ATP for 5 min (Suppl. Fig. 1), and are likely responsible for maintaining python erythrocyte diameter during shrinkage.
ATP induced increments in [Ca2+]i and shrinkage of P. regius erythrocytes. a Relative fluorescence from 5 fluo-4 loaded python erythrocytes from one experiment. Upon addition of 3 mM ATP, fluorescence increased, indicating that [Ca2+]i increased. b Reduction in hematocrit of 5 erythrocyte suspensions induced by 3 mM ATP. Shrinkage of individual cells results in a lowering of the hematocrit of the sample. Data are given as the mean ± SEM. *Statistical differences in the hematocrit of control samples and samples incubated with ATP (P < 0.05). c DIC images of P. regius erythrocytes before and after addition of 3 mM ATP. (Full movie can be seen as Suppl. Movie 1)
Involvement of Purinergic Receptors and Pannexin Channels in HlyA-induced Hemolysis
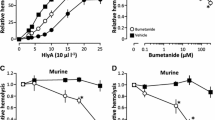
Python erythrocytes incubated with HlyA at EC50 displayed increments in [Ca2+]i (Fig. 4). The increase in cytosolic [Ca2+] was rather modest compared to what has previously been shown in human erythrocytes (Skals et al. 2010). Interestingly, the subtle increment in [Ca2+]i was followed by an intense fluorescence increase in a subset of erythrocyte organelles localized close to the nucleus (Suppl. Fig. 2). It is reasonable to assume that HlyA triggers a Ca2+ influx over the plasma membrane through the pore itself and/or in combination with P2X receptor activation, and that this ongoing influx results in an increased sequestration of Ca2+ in the organelles in question. Time-lapse microscopy of the python erythrocytes revealed that HlyA triggered an initial shrinkage of the erythrocytes that preceded the eventual swelling and lysis (Fig. 5a, Suppl. Movie 2), similarly to what has been reported on human erythrocytes (Skals et al. 2010). Involvement of P2 receptors in the HlyA-induced shrinkage and hemolysis was supported by the fact that the unspecific P2 receptor antagonist PPADS abolished both swelling (Fig. 5b, Suppl. Movie 3) and hemolysis (Fig. 6a). Hemolysis was also abolished by the general P2 receptor antagonist suramin (Fig. 6b), the P2X7 selective antagonist BBG (Fig. 6c) and by scavenging of extracellular ATP by hexokinase and glucose (Fig. 6d). In addition, the unspecific blocker of pannexin channels, carbenoxolone, which is also known to inhibit HlyA-induced hemolysis in human erythrocytes, also abolished the HlyA-induced lysis of python erythrocytes (Fig. 6e). These results do not support that the resistance of python erythrocytes to HlyA results from a lack of P2 receptor signaling or pannexin channels.
HlyA-induced shrinkage and lysis can be blocked by PPADS. a DIC images of P. regius erythrocytes before and after addition of HlyA at EC50. The erythrocytes initially shrink and subsequently swell and lyse. The white arrows indicate nuclei of lysed erythrocytes. b DIC images of P. regius erythrocytes before and after addition of HlyA at EC50 and 500 μM PPADS. PPADS blocks both the initial shrinkage and the eventual lysis induced by HlyA. Pictures shown are representative for 5 experiments. Full movies can be seen as Suppl. Movies 2 and 3
HlyA-induced hemolysis can be blocked by PPADS and carbenoxolone. a Dose–inhibition curves for PPADS at different HlyA concentrations leading to 25% hemolysis (EC25), 50% hemolysis (EC50), 75% hemolysis (EC75) and 100% hemolysis (EC100) after 60 min (n = 7). b Dose–inhibition curve for suramin at EC50 for HlyA after 60 min (n = 5). c Dose–inhibition curve for BBG at EC50 for HlyA after 60 min (n = 5). d Dose–inhibition curve for hexokinase at EC50 for HlyA (n = 5). e Dose–inhibition curve for carbenoxolone at EC50 for HlyA (n = 7). *Hemolysis statistically different from the hemolysis without antagonists or enzyme (P < 0.05). Values are presented as mean ± SEM; n = number of animals in each experiment
Binding of Fluorescent HlyA-cys to Human and Python Erythrocyte Membranes
A third and equally likely explanation for the python erythrocyte resistance toward HlyA could be a reduced insertion of HlyA in the membrane of python erythrocytes. Therefore, we made a plasmid construct for a cystein containing version of HlyA and expressed it in our original HlyA expressing E. coli (O6:K13:H1), which has the molecular machinery necessary for HlyA secretion. The HlyA-cys was collected from the E. coli suspension and labeled with BADAN (see Materials and Methods). Human erythrocyte ghosts were incubated with BADAN-labeled HlyA-cys, which resulted in an increase in membrane fluorescence (Fig. 7a). Specificity of BADAN labeling was confirmed by the fact that incubating erythrocyte ghosts with sham-labeled HlyA did not result in any increase in membrane fluorescence (Fig. 7a). Hereafter, we compared the degree of membrane binding between python and human erythrocyte membranes. The fluorescently labeled HlyA-cys bound equally well to both human and python and python erythrocytes (Fig. 7b), indicating that the HlyA resistance of python erythrocytes is not a consequence of low HlyA affinity to the python erythrocyte membrane.
Binding of fluorescent HlyA to erythrocyte membranes. a Increases in fluorescence of human erythrocyte ghosts upon incubation with either BADAN-labeled HlyA-cys or sham-labeled native HlyA. Only labeled HlyA-cys leads to increased fluorescence, confirming the specificity of labeling to the C-terminal cysteine residue. b Increases in fluorescence (excitation at 390 nm, emission at 460 nm) of human (n = 4) or P. regius (n = 4) erythrocyte ghosts upon incubation with BADAN-labeled HlyA-cys. The fluorescence of both human and python erythrocyte ghosts increased, indicating that HlyA binds equally well to both types of erythrocyte ghosts. Values are presented as mean ± SEM, n = number of animals or volunteers in each experiment. At no time were the fluorescence increase of human and python erythrocyte ghosts statistically different (P < 0.05)
Discussion
We show that python erythrocytes are remarkably resistant to the toxin HlyA. Concentrations of HlyA that cause complete lysis of human erythrocytes do not have any effect on P. regius erythrocytes. Hemolysis of python erythrocytes could, nevertheless, be induced at very high concentrations of HlyA. A similar observation has been made with regards to the effect of α-toxin from Staphylococcus aureus on erythrocytes from squamate and crocodylid reptiles (Slifkin 1965). α-Toxin from S. aureus is known to interact with specific receptors for incorporation into the erythrocyte membrane (Cassidy and Harshman 1973) and human erythrocytes, which lack these receptors, are resistant to the toxin (Cassidy and Harshman 1973). HlyA, on the other hand, is believed to insert receptor independently into biologic membranes (Bhakdi et al. 1988), which in principle should reduce the interspecies variation in the susceptibility to the toxin. With this in mind, we were surprised to find the large difference in HlyA susceptibility between human and python erythrocytes.
We sought to explain the remarkable HlyA resistance of python erythrocytes. HlyA is thought to cause hemolysis by pore-formation, thus increasing the conductance of the erythrocyte membrane. The increased ion-fluxes across the membrane overwhelms the ability of the Na+/K+ pump to compensate for the Gibbs-Donnan forces and the erythrocyte swells and bursts (Bhakdi et al. 1986). Therefore, the most straightforward explanation for the python erythrocyte resistance to HlyA would be a general resistance to osmotically induced lysis. Turtle erythrocytes were slightly more resistant to osmotic stress than python erythrocytes; both were more resistant to osmotic hemolysis than human erythrocytes. This is not surprising as the osmotic fragility of erythrocytes correlates inversely with cell volume (Peinado et al. 1992). The turtle erythrocyte susceptibility to HlyA was similar to that of human erythrocytes. Thus, there was no simple correlation between the osmotic resistance and the responsiveness to HlyA.
In human, murine and equine erythrocytes, the HlyA pore in itself is not sufficient to short-circuit the Na+/K+ pumps and cause lysis. It has been shown previously that in these species, insertion of HlyA triggers ATP release and subsequent activation of P2X receptors, which are ligand-gated, nonselective cation channels (Skals et al. 2009). These channels must contribute significantly to the overall conductance, either by themselves or by subsequent activation of pannexin channels, as inhibition of these channels completely abolished the HlyA-induced hemolysis. Because the HlyA effects are amplified through the P2-receptor dependent pathway, one possible explanation for the python erythrocyte resistance could be that they lack the capacity for purinergic signaling.
We found, however, that the python erythrocytes functionally express P2 receptors. This is consistent with the literature where P2 receptors have been found in erythrocytes from numerous reptiles like American alligators (Wormser et al. 2011), Necturus maculosus (Light et al. 2001), Tropidurus torquatus (Beraldo and Garcia 2007), and the teiid lizard Ameiva ameiva (Beraldo et al. 2002). We could show that ATP on its own caused increments in [Ca2+]i and an associated volume reduction. The need for millimolar ATP concentrations suggests that shrinkage was mediated by a P2X7-like receptor, as this receptor requires high ATP concentrations for activation relative to the other P2X receptors. Scavenging extracellular ATP with hexokinase or inhibiting P2 receptors with the nonselective P2-receptor antagonists PPADS and suramin or the P2X7 selective antagonist BBG all reduced the HlyA-induced hemolysis of P. regius erythrocytes. This indicates that P2 receptors, possibly P2X7-like receptors, are involved in HlyA-induced hemolysis of python erythrocytes. Activation of P2 receptors by addition of ATP in the absence of HlyA was not sufficient to lyse python erythrocytes. This is in agreement with what has been reported for mammalian erythrocytes (with the exception of canine erythrocytes, in which hemolysis can be induced through P2X7 receptor activation; Sluyter et al. 2007). P2X-receptor-dependent hemolysis occurs only in combination with the pore-forming toxin, i.e., an increase in baseline membrane conductance is required before P2X channel activation becomes pro-hemolytic. In human, murine and equine erythrocytes inhibition by carbenoxolone abolished HlyA-induced hemolysis (Skals et al. 2009). These results suggest that pannexin channels might be required for the full hemolytic process. Similarly, we were able to inhibit HlyA-induced hemolysis with the unspecific pannexin channel blocker carbenoxolone. Thus, the resistance to HlyA cannot be explained by the lack of P2 receptors or pannexin channels required for the normal hemolytic process.
We have previously shown that in mammalian erythrocytes, HlyA induces a significant volume reduction before the eventual swelling and lysis of the erythrocytes (Skals et al. 2010). We found that this shrinkage is caused by Ca2+ activation of KCa3.1 and TMEM16A (Skals et al. 2010). Interestingly we observed the same response in python erythrocytes; HlyA induced a significant shrinkage before the erythrocytes swelled and lysed (Skals et al. 2010). It is likely that this response is also caused by KCl efflux in a Ca2+ dependent mechanism, as it was blocked by PPADS and addition of ATP in itself is enough to trigger the volume reduction.
Finally, we addressed whether the resistance to HlyA was caused by a reduced binding of the toxin to the python erythrocyte membrane. We transformed the E. coli ARD6 strain, that express the export system for HlyA, with a HlyA variant containing a cystein in the C-terminal. This allowed us to fluorescently label HlyA with BADAN, and monitor the binding of fluorescent toxin to erythrocyte membranes. The fluorescent toxin bound equally well to human and python erythrocyte ghosts, indicating that the resistance to HlyA in python erythrocytes was not the result of decreased affinity of HlyA to the python erythrocyte membrane.
In conclusion, the large resistance to HlyA displayed by P. regius and P. molurus erythrocytes cannot be explained by lack of purinergic receptors or pannexin channels, increased osmotic resistance, or a decreased affinity of HlyA to the python erythrocyte membrane. This means that very few alternatives are left to explain this remarkable resistance. Some possible explanations could be blockage of the HlyA pore by an as yet unknown mechanism or substance present in the python erythrocyte cytosol. Another possibility is that HlyA is actively removed from the membrane, as has been suggested for renal epithelial cells by Koschinski et al. (2006). The latter mechanism has been observed in keratinocytes, which have been reported to protect themselves against S. aureus α-toxin by internalization and subsequent externalization of vesicles containing α-toxin (Husmann et al. 2009). We did not investigate whether such mechanisms were the cause of HlyA resistance, but vesicle formation or budding of from the plasma membrane was not observed in any of our time lapse studies. Finally, we cannot rule out differences exist in the signal transduction pathway that after HlyA insertion could explain the variation in sensitivity between the species. This could, for instance, be differences in the kinetics of ATP release or pannexin channel opening.
Solving this conundrum requires additional studies; it would be interesting to know why some species are protected against one of the major virulence factors from E. coli strains that are invasive in humans. The HlyA resistance displayed by python erythrocytes could in principle protect pythons against infections with hemolytic E. coli strains. Pythons routinely subject themselves to sources of E. coli infections; they swallow whole prey head first, and they do not avoid contaminated drinking water (in fact, they tend to defecate in their drinking water when kept in captivity). Thus, a protective mechanism against E. coli infections would certainly be beneficial to pythons.
References
Aldrich K, Saunders DK (2001) Comparison of erythrocyte osmotic fragility among ectotherms and endotherms at three temperatures. J Thermal Biol 26:179–182
Beraldo FH, Garcia CR (2007) Divergent calcium signaling in RBCs from Tropidurus torquatus (Squamata, Tropiduridae) strengthen classification in lizard evolution. BMC Physiol 7:7
Beraldo FH, Sartorello R, Gazarini ML, Caldeira W, Garcia CR (2002) Red blood cells of the lizards Ameiva ameiva (Squamata, Teiidae) display multiple mechanisms to control cytosolic calcium. Cell Calcium 31:79–87
Bhakdi S, Mackman N, Nicaud JM, Holland IB (1986) Escherichia coli hemolysin may damage target-cell membranes by generating transmembrane pores. Infect Immun 52:63–69
Bhakdi S, Mackman N, Menestrina G, Gray L, Hugo F, Seeger W, Holland IB (1988) The hemolysin of Escherichia coli. Eur J Epidemiol 4:135–143
Cassidy PS, Harshman S (1973) The binding of staphylococcal 125I-alpha-toxin (B) to erythrocytes. J Biol Chem 248:5545–5546
Cavalieri SJ, Bohach GA, Snyder IS (1984) Escherichia coli α-hemolysin—characteristics and probable role in pathogenicity. Microbiol Rev 48:326–343
Cohen WD (1991) The cytoskeletal system of nucleated erythrocytes. Int J Cytol 130:37–84
Conly JM, Stein K (1992) Quantitative and qualitative measurements of vitamin-K in human intestinal contents. Am J Gastroenterol 87:311–316
Cortajarena AL, Goni FM, Ostolaza H (2001) Glycophorin as a receptor for Escherichia coli alpha-hemolysin in erythrocytes. J Biol Chem 276:12513–12519
Cortajarena AL, Goni FM, Ostolaza H (2003) A receptor-binding region in Escherichia coli α-haemolysin. J Biol Chem 278:19159–19163
Costa-Junior HM, Mendes AN, Davis GHNG, da Cruza CM, Ventura ALM, Serezani CH, Faccioli LH, Nomizo A, Freire-De-Lima CG, Bisaggio RD, Persechini PM (2009) ATP-induced apoptosis involves a Ca2+-independent phospholipase A2 and 5-lipoxygenase in macrophages. Prostaglandins Other Lipid Mediat 88:51–61
Felmlee T, Pellett S, Welch RA (1985) Nucleotide-sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol 163:94–105
Gadeberg OV, Orskov I, Rhodes JM (1983) Cyto-toxic effect of an alpha-hemolytic Escherichia coli strain on human-blood monocytes and granulocytes invitro. Infect Immun 41:358–364
Gasbjerg PK, Brahm J (1991) Kinetics of bicarbonate and chloride transport in human red-cell membranes. J Gen Physiol 97:321–349
Hudault S, Guignot J, Servin AL (2001) Escherichia coli strains colonising the gastrointestinal tract protect germfree mice against Salmonella typhimurium infection. Gut 49:47–55
Husmann M, Beckmann E, Boller K, Kloft N, Tenzer S, Bobkiewicz W, Neukirch C, Bayley H, Bhakdi S (2009) Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett 583:337–344
Hyland C, Vuillard L, Hughes C, Koronakis V (2001) Membrane interaction of Escherichia coli hemolysin: flotation and insertion-dependent labeling by phospholipid vesicles. J Bacteriol 183:5364–5370
Johnson JR, Stell AL (2000) Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis 181:261–272
Jorgensen SE, Hammer RF, Wu GK (1980) Effects of a single hit from the alpha hemolysin produced by Escherichia coli on the morphology of sheep erythrocytes. Infect Immun 27:988–994
Joseph-Silverstein J, Cohen WD (1984) The cytoskeletal system of nucleated erythrocytes. 3. Marginal band function in mature cells. J Cell Biol 98:2118–2125
Koschinski A, Repp H, Unver B, Dreyer F, Brockmeier D, Valeva A, Bhakdi S, Walev I (2006) Why Escherichia coli alpha-hemolysin induces calcium oscillations in mammalian cells—the pore is on its own. FASEB J 20:973–975
Le Stunff H, Auger R, Kanellopoulos J, Raymond MN (2004) The Pro-451 to Leu polymorphism within the C-terminal tail of P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J Biol Chem 279:16918–16926
Light DB, Dahlstrom PK, Gronau RT, Baumann NL (2001) Extracellular ATP activates a P2 receptor in Necturus erythrocytes during hypotonic swelling. J Membr Biol 182:193–202
Locovei S, Scemes E, Qiu F, Spray DC, Dahl G (2007) Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett 581:483–488
Menestrina G, Mackman N, Holland IB, Bhakdi S (1987) Escherichia coli hemolysin forms voltage-dependent ion channels in lipid-membranes. Biochim Biophysi Acta 905:109–117
Peinado VI, Viscor G, Palomeque J (1992) Erythrocyte osmotic fragility in some artiodactylid mammals—relationships with plasma osmolality and red-cell dimensions. Comp Haematol Int 2:44–50
Schindel C, Zitzer A, Schulte B, Gerhards A, Stanley P, Hughes C, Koronakis V, Bhakdi S, Palmer M (2001) Interaction of Escherichia coli hemolysin with biological membranes—a study using cysteine scanning mutagenesis. Eur J Biochem 268:800–808
Skals M, Jorgensen NR, Leipziger J, Praetorius HA (2009) α-Hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc Natl Acad Sci USA 106:4030–4035
Skals M, Jensen UB, Ousingsawat J, Kunzelmann K, Leipziger J, Praetorius HA (2010) Escherichia coli α-hemolysin triggers shrinkage of erythrocytes via K(Ca)3.1 and TMEM16A channels with subsequent phosphatidylserine exposure. J Biol Chem 285:15557–15565
Slifkin M (1965) Lysis of amphibian and reptile erythrocytes by staphylococcal alpha-hemolysin. Comp Biochem Physiol 15:73–76
Sluyter R, Shemon AN, Hughes WE, Stevenson RO, Georgiou JG, Eslick GD, Taylor RM, Wiley JS (2007) Canine erythrocytes express the P2X7 receptor: greatly increased function compared with human erythrocytes. Am J Physiol Regul Integr Comp Physiol 293:R2090–R2098
Soderblom T, Laestadius A, Oxhamre C, Aperia A, Richter-Dahlfors A (2002) Toxin-induced calcium oscillations: a novel strategy to affect gene regulation in target cells. International J Med Microbiol 291:511–515
Valeva A, Walev I, Kemmer H, Weis S, Siegel I, Boukhallouk F, Wassenaar TM, Chavakis T, Bhakdi S (2005) Binding of Escherichia coli hemolysin and activation of the target cells is not receptor-dependent. J Biol Chem 280:36657–36663
Wormser C, Pore SA, Elperin AB, Silverman LN, Light DB (2011) Potentiation of regulatory volume decrease by a P2-like receptor and arachidonic acid in american alligator erythrocytes. J Membr Biol 242:75–87
Acknowledgments
We would like to thank Anne B. Strandsby, Edith Møller, Christian Westberg, and Rasmus Buchanan for their technical assistance. We also thank Jeppe Praetorius for assistance with production of fluorescent HlyA and binding studies of fluorescent HlyA to erythrocyte ghosts.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 3: ATP-induced shrinkage of P. regius erythrocytes stimulated with 3 mM ATP. Pictures were captured every 30 seconds for 90 minutes after the addition of 3 mM ATP. The frame rate of the movie is 10 pictures s−1, i.e. 1 second in the movie corresponds to 5 minutes. The ATP-induced shrinkage persists for the duration of the experiment, no cells were ever observed to lyse when stimulated with ATP (AVI 5288 kb)
Supplementary material 4: HlyA-induced shrinkage and lysis of P. regius erythrocytes. Pictures were captured every 30 seconds for 90 minutes after the addition of HlyA at EC50. The frame rate of the movie is 10 pictures s−1, i.e. 1 second in themovie corresponds to 5 minutes. Lysis can be observed in a few cells on the lower right quadrant of the movie (AVI 4951 kb)
Supplementary material 5: PPADS blocks HlyA-induced shrinkage and lysis of P. regius erythrocytes. Pictures were captured every 30 seconds for 90 minutes after the addition of HlyA at EC50 and PPADS (500 μM). The frame rate of the movieis 10 pictures s−1, i.e. 1 second in the movie corresponds to 5 minutes. Neither shrinkage nor hemolysis was observed in the presence of PPADS (AVI 5284 kb)
Rights and permissions
About this article
Cite this article
Larsen, C.K., Skals, M., Wang, T. et al. Python Erythrocytes Are Resistant to α-Hemolysin from Escherichia coli . J Membrane Biol 244, 131–140 (2011). https://doi.org/10.1007/s00232-011-9406-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-011-9406-2