Abstract
Phosphatidylserine (PS), which is normally localized in the cytoplasmic leaflet of the membrane, undergoes externalization during aging or trauma of red blood cells (RBCs). A fraction of this PS is shed into the extracellular milieu. Both PS externalization and shedding are modulated by the oxidative state of the cells. In the present study we investigated the effect of calcium (Ca) flux on oxidative stress-induced membrane distribution of PS and its shedding and on the membrane composition and functions. Normal human RBCs were treated with the oxidant t-butyl hydroperoxide, and thalassemic RBCs, which are under oxidative stress, were treated with the antioxidant vitamin C or N-acetylcystein. The intracellular Ca content was modulated by the Ca ionophore A23187 and by varying the Ca concentration in the medium. Ca flux was measured by Fluo-3, PS externalization and shedding were measured by quantitative flow cytometry and membrane composition was measured by 1H-NMR analysis of the cholesterol and phospholipids. The results indicated that increasing the inward Ca flux induced PS externalization and shedding, which in turn increased the membrane cholesterol/phospholipid ratio and thereby increased the RBC osmotic resistance. In addition, these processes modulated the susceptibility of RBCs to undergo phagocytosis by macrophages; while PS externalization increased phagocytosis, the shed PS prevented it. These results indicate that PS redistribution and shedding from RBCs, which are mediated by increased calcium, have profound effects on the membrane composition and properties and, thus, may control the fate of RBCs under physiological and pathological conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The phospholipids (PLs) in the plasma membranes of all cells, including the red blood cells (RBCs), are distributed asymmetrically (Zwaal and Schroit 1997). Whereas lipids with the choline head group such as phosphatidylcholine are mainly present in the outer leaflet of the membrane, aminophospholipids such as phosphatidylserine (PS) are mainly present in the cytoplasmic leaflet (Op den Kamp 1979). Changes in this asymmetry characterize apoptosis of nucleated cells; e.g., PS flip-flops from the inner to the external membrane leaflet. Although mature anucleated RBCs do not undergo the classical pattern of apoptosis, upon trauma or aging their PS undergoes externalization (Kuypers 2007; Lang et al. 2006a). PS externalization is thought to be a major signal for phagocytosis by macrophages and removal from the circulation (Föller et al. 2008). This process occurs during physiological RBC aging (senescence), but it is increased in certain pathological conditions such as β-thalassemia (Zwaal and Schroit 1997).
A fraction of the external PS of RBCs undergoes shedding into the extracellular milieu (Pattanapanyasat et al. 2004; Freikman et al. 2008, 2009). Shedding of PS-containing membranous microvesicles was shown in both normal and pathological RBCs (Pattanapanyasat et al. 2004; Greenwalt 2006; Freikman et al. 2008, 2009). It was hypothesized that vesiculation might be a mechanism for the removal of RBC membrane patches containing molecules that mediate rapid removal of RBCs from the circulation (Willekens et al. 2008). PS externalization was reported to be mediated by the intracellular concentration of calcium (Ca) through activation of the PL transporter scramblase (Zhou et al. 1997; Bassé et al. 1996). It was also reported that release of PS-enriched microvesicles from RBCs was stimulated by sustained elevation of intracellular Ca (Smith et al. 2001). PS externalization and shedding from RBCs are also stimulated by oxidative stress (Föller et al. 2008). Thus, thalassemic RBCs, which are under oxidative stress (Fibach and Rachmilewitz 2008; Kruse et al. 2008), and normal RBCs following stimulation by an oxidant externalize and shed PS to a higher degree than normal untreated RBCs. This could be inhibited by antioxidants (Pattanapanyasat et al. 2004; Freikman et al. 2008).
In the current study we investigated the interrelationship between oxidative stress and intracellular Ca concentration and the distribution of PS across the membranes and its shedding from RBCs as well as the effect of PS shedding on the structure and function of the RBC plasma membrane. For this purpose, we used normal and thalassemic RBCs treated or untreated with an oxidant (t-butyl hydroperoxide, BHP) or the antioxidants vitamin C (Vit.C) and N-acetylcystein (NAC). We previously reported that BHP increased PS externalization and shedding, producing a “thalassemia-like” phenotype in normal oxidized RBCs, while Vit.C and NAC had an opposite effect, producing a “normal-like” phenotype in thalassemic RBCs (Freikman et al. 2008). Intracellular Ca was modulated by treating the cells with the Ca ionophore A23187, which facilitates transport of Ca2+ across the plasma membrane (Dedkova et al. 2000), or by varying the Ca concentration in the medium. By measuring intracellular Ca with Fluo-3, we found that oxidative stress stimulated inward Ca flux. Using a novel quantitative flow-cytometric assay (Freikman et al. 2009), we demonstrated that oxidative stress-induced PS externalization and shedding were mediated by the intracellular Ca concentration.
We also demonstrated that oxidative stress-induced shedding affected the susceptibility of RBCs to undergo phagocytosis by macrophages. While PS externalization increased RBC phagocytosis, addition of PS to the growth medium prevented it. These results indicate that phagocytosis is mediated by the PS exposed on the RBCs but also suggest that shedding of PS reduces RBC phagocytosis, probably by competitive binding to PS receptors on macrophages.
Shedding is considered one of the mechanisms of membrane remodeling (Dello Sbarba and Rovida 2002). RBCs that are characterized by increased shedding, such as RBCs derived from patients with thalassemia or sickle cell disease, have an elevated cholesterol/PL ratio in their membranes (Rank et al. 1988; Allan and Thomas 1981; Föller et al. 2008). Using NMR technology, we now show that oxidative stress-induced shedding increases the cholesterol/PL ratio in the membrane of RBCs. An increased cholesterol/PL ratio in the RBC membrane has been reported to decrease membrane fluidity (Allen et al. 2006) and consequently to elevate osmotic resistance (McGrath et al. 1995; Caprari et al. 1999; Brzeszczynska et al. 2008). In the present study, we show that treatment with BHP or A23187 increased the cholesterol/PL ratio as well as the osmotic resistance.
The results indicate that oxidative stress-induced modulation of PS in the membrane of RBCs is mediated by changes in their Ca flux. Increased flux results in externalization of the PS and shedding part of it into the extracellular milieu. These processes have profound effects on the membrane composition (cholesterol/PL ratio) and properties, including osmotic resistance and susceptibility to undergo phagocytosis by macrophages. Thus, the balance between PS externalization and shedding may control the fate of RBCs in the circulation under physiological and pathological conditions.
Methods
RBCs
Peripheral blood samples were obtained from normal donors and β-thalassemic patients. Clinical data of the patients were reported elsewhere (Amer et al. 2008). Informed consent was obtained in all cases according to the Helsinki Committee Regulations (permit 20-30/03/01, Israel).
Treatment of RBCs
Normal or thalassemic RBCs were washed and diluted (1 × 107/ml) in phosphate-buffered saline (PBS) containing, unless otherwise stated, 2 mM CaCl2 (Ca-PBS) and incubated at 37°C with the oxidant BHP or the antioxidants Vit.C and NAC and the Ca ionophore A23187 (all from Sigma-Aldrich, Rehovot, Israel). Following 1-h incubation, cells were centrifuged at 1,700×g and supernatants were kept at −80°C until analysis. RBCs were washed twice with PBS and immediately analyzed by flow cytometry. The effects of BHP, Vit.C and A23187 were found to be concentration-dependent (data not shown); for the experiments described in this study we used 1 mM for BHP and Vit.C and 2 μM for A23187 in order to reach levels of intracellular Ca and external PS comparable to those in untreated thalassemic RBCs.
Measurement of Intracellular Ca
Intracellular Ca2+ was measured as described (Lang et al. 2006b). Briefly, RBCs were first loaded with Fluo-3/AM (Calbiochem, San Diego, CA) by addition of 4 μl of a stock solution (2.0 mM in DMSO) to 1 ml RBC suspension (107 RBCs/ml in PBS supplemented with 10 mM d-glucose) and incubation at 37°C for 30 min under protection from light. RBCs were then centrifuged at 1,000×g for 5 min at 22°C and washed twice with PBS containing 0.5% bovine serum albumin (Sigma-Aldrich) and once with PBS. About 106 Fluo-3-loaded RBCs were then treated with BHP, A23187 or vehicle (DMSO) and analyzed by flow cytometry.
Measurement of Cellular and Shed PS
Measurement of outer PS using fluorescein isothiocyanate-conjugated human recombinant annexin-V is a well-known procedure (Reutelingsperger et al. 2002). Quantitative measurement of PS was performed by the two-step fluorescence inhibition assay as previously described (Freikman et al. 2009). Briefly, the outer PS of intact RBCs or PS in solution (lysed RBCs or RBC supernatants) was bound to fluorescent annexin-V (IQ Products, Groningen, the Netherlands) (step I), and then the residual, nonbound annexin-V was quantified by binding to PS exposed on apoptotic HL-60 cells, which served as an indicator reagent (step II). The indicator cell fluorescence in step II, measured by flow cytometry, was reciprocally proportional to the amount of PS in step I (Freikman et al. 2009).
Flow Cytometry
RBCs were analyzed by a fluorescence-activated cell sorter (FACScalibur; Becton Dickinson Immunofluorometry Systems, Mountain View, CA). Cells were passed at a rate of ~1,000/s with saline serving as the sheath fluid. A 488-nm argon laser beam was used for excitation. RBCs were gated according to their forward light and side light scatterings. The cellular fluorescence emission at 530 nm was measured, and the arithmetic mean fluorescence index was calculated by the FACS-equipped CellQuestR software (Becton Dickinson Immunofluorometry Systems). The height of the fluorescence signal, Fl-1H, depends on the fluorochrome content per cell rather than its concentration; and therefore, it is not affected by possible changes in cell size.
Measurement of Cholesterol and PLs
PLs and cholesterol were measured by proton 1H-NMR as previously described (Yoshioka et al. 2000). Lipid extraction was performed using a modified Folch method (Folch et al. 1957). Briefly, six volumes of a cold methanol:chloroform (1:2) mixture were added to one volume of an ice-cold RBC sample. Then, four volumes of 0.1 M KCl were added, and the mixture was left overnight for phase separation. The lower (hydrophobic) phase was collected and evaporated. The dry residue was dissolved in chloroform/methanol as previously reported (Folch et al. 1957) and analyzed by NMR.
Spectra were obtained with a Varian Inova500 spectrometer equipped with an 11.74 T, 51-mm Bore Oxford Magnet (Varian Medical Systems, Palo Alto, CA), operating at 500 MHz. A 5-mm switchable probe was used. The spectrometer conditions used were 60° flip angle, delay time of 2 s and number of points −5,000. Signals were fully relaxed at these conditions. The data were processed using the VNMR software, including backward linear prediction (Varian Medical Systems). NMR peak assignments were determined by comparison with the spectra of commercial cholesterol and PLs (all from Sigma-Aldrich) as previously described (Sparling et al. 1989; Yoshioka et al. 2000). The absolute concentrations of lipids were determined using the integral of the NMR signal of residual CHCl3 in the deuterated solvent (chloroform-D1, 99.8%) as a reference. Calibration curves for selected signal integrals relative to the CHCl3 integral were prepared using commercial lipids.
Measurement of Osmotic Resistance and Susceptibility to Phagocytosis
Following treatment with BHP or A23187 and washing with PBS, osmotic resistance was determined by exposing RBCs for 5 min to a hypo-osmotic solution (60% PBS in DDW). The nonlysed cells were centrifuged, redispersed in PBS and counted. Susceptibility to phagocytosis was determined by incubating RBCs with human macrophages. Macrophages were obtained from normal human peripheral blood mononuclear cells cultured according to the two-phase liquid culture procedure (Fibach et al. 1989). Adherent macrophages were collected by trypsinization, washed, resuspended in fresh alpha-medium containing 10% fetal calf serum (Biological Industries, Beit-Haemek, Israel) and recultured in multiwell dishes. RBCs were treated as specified in Results, washed and then added to the macrophages. After 24-h incubation, nonphagocytized RBCs were counted microscopically using a hemocytometer as previously described (Amer et al. 2008). The extent of phagocytosis was calculated as the percent of RBC clearance by macrophages. RBC lysis as a possible cause of decreased RBC count was ruled out by benzidine staining of the supernatants (Fibach 1993). Only traces of hemoglobin, which were not statistically different in controls and treated cells, were detected.
Statistical Analysis
The data were expressed as mean ± SD from at least three independent experiments and further subjected to the two-tailed Student’s t-test. P < 0.05 was considered statistically significant.
Results
Effect of Oxidative Stress on Intracellular Ca
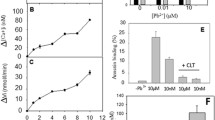
Intracellular Ca was measured in normal and thalassemic RBCs by flow cytometry using Fluo-3 (Lang et al. 2006b). In normal RBCs, 1-h treatment with BHP (1 mM) led to a 1.8-fold increase in Fluo-3 fluorescence (P < 0.01, n = 5) (Fig. 1a, b). Thalassemic RBCs had 20% higher Fluo-3 fluorescence than normal RBCs; treatment with Vit.C (1 mM) decreased it (P < 0.05, n = 5) (Fig. 1c, d). Similar results were obtained with NAC (data not shown). Treatment with A23187 (2 μM), which served as a positive control, increased the fluorescence of normal and thalassemic RBCs by 2.7- and 2.5-fold (P < 0.05, n = 5), respectively, compared with untreated RBCs.
Effect of oxidative stress on intracellular Ca. Fluo-3-loaded normal (a, b) and thalassemic (Thal.) (c, d) RBCs were incubated for 1 h in Ca-PBS with none (Control), A23187 (2 μM), t-butyl hydroperoxide (BHP) (1 mM) or vitamin C (Vit.C, 1 mM) as shown. Cellular Fluo-3 fluorescence was measured by flow cytometry. Fluorescence histograms of representative normal (a) and thalassemic (c) samples are shown. The results of five experiments are summarized in b and d (average ± SD showing the mean fluorescence indices of five different donors). P values of treated RBCs compared to untreated (control) RBCs are indicated
In order to rule out the possibility of hemolysis during the experiment, supernatants were stained with benzidine (Fibach 1993). Only traces of hemoglobin were detected in the supernatants, and there was no significant difference between normal and thalassemic RBCs.
Effect of Oxidative Stress on Cellular PS Distribution and Shedding
To determine the effect of oxidative stress on PS externalization and shedding by RBCs and the involvement of intracellular Ca in this process, we incubated normal RBCs in Ca-PBS with BHP or A23187. The kinetics of changes in the inner, outer and total PS (Fig. 2a–c) as well as in the shed PS (Fig. 2d) were followed for 60 min using a quantitative flow-cytometric assay, as described in “Methods” section. The results showed that untreated (control) RBCs contained 0.18 ± 0.02 and 0.92 ± 0.05 μmol/107 cells outer and inner PS, respectively, which did not significantly change with time (Fig. 2a). Following addition of 1 mM BHP, the outer PS increased by threefold during the first 30 min and plateaued thereafter, while the inner PS continuously decreased during the observation period (Fig. 2b). These changes in PS were reflected by a 28% decrease in the total cellular PS and by continuous shedding of PS into the supernatant, which reached 0.35 μmol/107 cells after 60 min (Fig. 2d). Following addition of 2 μM A23187, the outer PS increased by 2.5-fold during the first 20 min and plateaued thereafter, whereas the inner PS continuously decreased up to 31% (Fig. 2c). The total cellular PS decreased by 30% due to continuous shedding of PS into the supernatant, which reached 0.39 μmol/107 cells (Fig. 2d). The results indicate that although concentration-wise A23187 is much more effective than BHP, oxidative stress and increased Ca are both associated with augmented exposure and shedding of PS.
Effect of BHP and A23187 on the kinetics of PS distribution and shedding. Normal human RBCs were incubated for the indicated durations in Ca-PBS with none (Control) (a) with 1 mM BHP (b) or with 2 μM A23187 (c). Inner, outer and total PS (a–c) as well as shed PS (d) were quantified using indirect fluorescence assay, as described in “Methods” section. Results are expressed as micromoles per 107 RBCs (mean ± SD, n = 5)
To further clarify the involvement of Ca in oxidative stress-induced PS externalization and shedding, normal RBCs were treated with BHP or A23187 in PBS containing different concentrations of Ca (0–2 mM). Only minor changes were observed in the outer, inner or shed PS of untreated RBCs incubated in different Ca concentrations (Fig. 3a, b). Decreasing the Ca concentration during treatment with either 1 mM BHP or 2 μM A23187 resulted in a significant decrease in the outer and shed PS and an increase in the inner and total PS compared to treatment with these compounds in the presence of a physiological concentration of Ca (2 mM). These results indicate a direct correlation between Ca concentration and oxidative stress-induced PS shedding.
Effect of Ca concentration on PS distribution and shedding of normal RBCs. Normal RBCs were incubated in PBS containing the indicated concentrations of CaCl2 with none (control, diamond), BHP (1 mM, triangle) or A23187 (2 μM, square). Outer (a), inner (b) and total cellular PS (c) as well as shed PS (d) were measured after 1 h. Results are expressed as micromoles per 107 RBCs (mean ± SD, n = 5)
We next studied the involvement of Ca in PS externalization and shedding in thalassemic RBCs. Figure 4a, b shows that their outer and shed PS are higher and their inner PS is lower than in normal RBCs. Antioxidants (Vit.C and NAC) reversed these effects, partially correcting their PS phenotype. Figure 4c, d shows the effect of Ca on thalassemic RBCs; lower PS exposure and shedding were observed when Ca was reduced.
Effect of Ca concentration on PS distribution and shedding of thalassemic RBCs. Thalassemic RBCs were incubated for 1 h in Ca-PBS with the antioxidants vitamin C (Vit.C) or N-acetylcystein (NAC) (both at 1 mM) (a, b) or in PBS supplemented with the indicated concentrations of CaCl2 (c, d). Inner, outer and total PS (a, c) as well as shed PS (b, d) were measured. Results are expressed as micromoles per 107 RBCs (mean ± SD, n = 5)
Taken together, these results, which are in agreement with our previous NMR study (Freikman et al. 2008), indicate that PS exposure and shedding are induced by oxidative stress and suggest that they are mediated by the intracellular Ca concentration.
Effects of Oxidative Stress on the RBC Membrane Cholesterol/PL Ratio
Cholesterol and PLs are the major components of the membrane lipids. We used high-resolution 1H-NMR to follow the effect of oxidative stress-induced shedding on the content of these components in RBCs and in their supernatants. NMR signals used for quantification were the C18-CH3 signal at 0.7 ppm for cholesterol (Fig. 5a) and the glycerol backbone –CH2-OP signal at 4.0 ppm for PLs (Fig. 5b). The results showed that in normal RBCs and in their supernatants the cholesterol/PL ratios were 2.9 and 0.7, respectively (Fig. 5c, d). One-hour treatment with BHP (1 mM) or A23187 (2 μM) slightly decreased the cellular cholesterol (by 2.8 ± 1.2 and 3.5 ± 1.4%, respectively) but significantly decreased (by 2.5- and 2.7-fold, respectively) the PL content, thereby increasing the cellular cholesterol/PL ratio by 2.5-fold (Fig. 5c). No change was observed in the cholesterol/PL ratio of the supernatants of untreated RBCs, but treatment with BHP or A23187 decreased the ratio in the supernatants by 1.7- and 2.2-fold (Fig. 5d).
Effect of oxidative stress-induced shedding on the RBC membrane cholesterol/PL ratio. Normal RBCs were treated with none (Control), BHP (1 mM) or A23187 (2 μM), whereas thalassemic RBCs were treated with none (Control), Vit.C or NAC (both at 1 mM). At the indicated time points, RBCs and their supernatants were collected and analyzed by 1H-NMR for cholesterol and PL contents and the cholesterol/PL ratio was calculated. a, b Proton spectrum of RBC lipids—the cholesterol (a) and PL (b) peaks are indicated. Results (mean ± SD, n = 3) show the cholesterol/PL ratio in normal RBCs (c) and supernatants (d) and in thalassemic RBCs (e) and supernatants (f)
In thalassemic RBCs, the cholesterol/PL ratio was 5.8 (2.8-fold higher than normal RBCs), and it was increased to 6.9 during incubation. Treatment with the antioxidants Vit.C and NAC (1 mM) decreased the ratio to 4.2 (Fig. 5e). The ratio in the supernatants of untreated thalassemic RBCs (Fig. 5f) was 0.45 (35% lower than normal RBC supernatants), and it decreased to 0.36 during the experiment. Treatment of thalassemic RBCs with the antioxidants caused a 22% increase in the cholesterol/PL ratio of their supernatants.
Taken together, these results indicate that in response to oxidative stress or an intracellular Ca rise, RBCs shed membrane components selectively, causing relative enrichment of the RBC membrane with cholesterol.
Effects of Shedding on Osmotic Resistance and RBC Phagocytosis
Increased cholesterol in the RBC plasma membrane affects its mechanical properties (Allen et al. 2006), including its fluidity, which in turn was correlated with osmotic resistance (McGrath et al. 1995). To relate PS shedding to osmotic resistance, normal RBCs treated with BHP or A23187 were analyzed for their osmotic resistance. The results showed (Fig. 6a) that BHP (1 mM) or A23187 (2 μM) increased the osmotic resistance by 2.4- and 2.1-fold, respectively.
Effect of oxidative stress on osmotic resistance and susceptibility to phagocytosis. RBCs were diluted to 1 × 106/ml in Ca-PBS and treated for 1 h with none (Control), BHP or A23187. a Osmotic resistance was assayed by exposing RBCs to hypo-osmotic solution (60% PBS in DDW) for 5 min. Percentages of nonhemolyzed RBCs (mean ± SD, n = 5) are shown. b Phagocytosis was assayed by overnight incubation of RBCs with macrophages in the presence or absence of 1 mM PS. Percentages of phagocytosed RBCs (mean ± SD, n = 5) are shown
PS externalization has been suggested to play a role in removing damaged or senescent RBCs from the circulation by phagocytosis (Ayub and Hallett 2004). In order to relate PS externalization and shedding to phagocytosis, normal RBCs treated with BHP or A23187 were analyzed for phagocytosis following coincubation with macrophages. The results showed (Fig. 6b) that only 5.5% of untreated RBCs (control) were removed by phagocytosis, whereas treatment with BHP (1 mM) and A23187 (2 μM) resulted in 64 and 93% phagocytosis, respectively. To probe the role of shed PS in phagocytosis, commercially available PS (Sigma-Aldrich) was suspended in PBS containing 1% BSA and added together with RBCs to macrophage-containing plates. The results show 18.9 and 31.2% inhibition of phagocytosis of RBCs treated with BHP (1 mM) or A23187 (2 μM), respectively (Fig. 6b) compared to phagocytosis of RBCs treated similarly without PS suspension. These results indicate that susceptibility to phagocytosis induced in RBCs by BHP and A23187 is mediated by the outer PS and suggest that shed PS may have an inhibitory effect.
Discussion
In the present study, we investigated the involvement of Ca flux in oxidative stress-induced externalization and shedding of PS from RBCs and its consequences on their membrane composition and properties. For this purpose, we used human normal RBCs as well as thalassemic RBCs, which are known to be under chronic oxidative stress. Oxidative stress was further modulated by treatment with an oxidant (BHP) or antioxidants (Vit.C, NAC) (Amer et al. 2004; Freikman et al. 2008). Using flow cytometry of Fluo-3-labeled RBCs (Fig. 1), we found that thalassemic RBCs had higher Ca flux than normal RBCs. Ca flux was increased in normal RBCs by treatment with BHP and decreased in thalassemic RBCs by treatment with the antioxidants. As a positive control we used RBCs treated with the Ca ionophore A23187. These results are in accordance with the effect of oxidative stress on the regulation of Ca flux as recently reviewed (Hidalgo and Donoso 2008).
We then studied the effects of oxidative stress and intracellular Ca content on the PS distribution across the membrane and its shedding into the extracellular milieu. PS was measured by a novel quantitative indirect flow cytometry (Freikman et al. 2009). We found that treatment of normal RBCs with BHP and A23187 caused the outer PS to increase and the inner PS to decrease, followed by a significant decrease in the total cellular PS due to its shedding (Fig. 2a). These effects were reduced as a function of the intracellular Ca concentration and completely inhibited in Ca-free medium (Fig. 3). Thalassemic RBCs showed increased basal outer PS and shedding compared with normal RBCs (Fig. 4a, b). Treatment of thalassemic RBCs with antioxidants decreased their outer PS and shedding (Fig. 4c, d), acquiring a normal-like phenotype. Moreover, treatment of normal RBCs with antioxidants slightly decreased, while treatment of thalassemic RBCs with oxidants further increased the PS externalization and shedding (data not shown). These results indicate that an increase in Ca flux is involved in the oxidative stress-induced PS externalization and shedding. It should be noted that PS redistribution is the outcome of multiple factors and mechanisms, which may have opposite effects, including changes in cellular Ca concentration and oxidative status, extent of inward PS flow and PS shedding. These dynamic processes are ongoing continuously and simultaneously. PS exposure on thalassemic RBCs, induced by their high intracellular Ca concentration and oxidative status, is blunted by increased shedding. Only when PS externalization overcomes the ability to remove it by shedding are thalassemic RBCs removed by phagocytosis (extravascular hemolysis).
We further studied the effect of oxidative stress-induced shedding on the membrane composition and properties. The PLs and cholesterol, the major lipid membrane components, were measured by 1H-NMR in normal and thalassemic RBC membranes and in their supernatants. We found that treatment of normal RBCs with BHP and A23187 significantly decreased the PLs in the membranes and increased them in the supernatants, while the cholesterol was only slightly decreased in the membrane and was minimal in the RBC supernatants. This resulted in a 2.5-fold increase in the cholesterol/PL ratio in normal RBC membranes. Thalassemic RBCs demonstrated a higher cholesterol/PL ratio than normal RBCs, which was decreased by antioxidants. These findings suggest that shedding is a selective process involving mainly PLs and leading to relative accumulation of cholesterol in the membrane. In this respect, it should be mentioned that while shedding is often described in the context of microparticles, i.e., membrane-bound vesicles (Rank et al. 1988; Allen et al. 2006; Föller et al. 2008), we have previously shown that the majority of the shed PS is not associated with microparticles (Freikman et al. 2008).
Increased cholesterol content in the RBC plasma membrane was reported to affect its mechanical properties (fluidity) (Wilson-Ashworth et al. 2006; Gonzalez et al. 2009). Thus, during physiological aging, senescent RBCs showed an increased cholesterol/PL ratio followed by increased membrane osmotic resistance (Caprari et al. 1999). Increased cholesterol/PL has been observed in various pathological conditions such as diabetes (Allen et al. 2006), chronic alcoholism (Maturu et al. 2010) and multiple sclerosis (Hon et al. 2009). It was also demonstrated that oxidatively stressed RBCs from patients undergoing regular dialysis had decreased membrane fluidity (McGrath et al. 1995). Membrane fluidity is associated with the RBC osmotic resistance (Allen et al. 2006). The results showed (Fig. 6) that RBCs treated with BHP or A23187 had increased osmotic resistance, suggesting that osmotic resistance is related to membrane changes that include PS shedding and accumulation of cholesterol.
A variety of membrane changes in senescent and diseased RBCs have been reported to mediate their removal from the circulation by phagocytosis; external PS is one of these signals (Ayub and Hallett 2004). Shedding might constitute a vital process during development of erythroid precursors (data not shown) in the bone marrow and of the survival of mature RBCs in the circulation. Macrophage phagocytosis of apoptotic bodies was reported to be inhibited by PS liposomes and its structural analogues but not by other anionic PLs (Fadok et al. 1992). We measured phagocytosis of RBCs by cultured macrophages derived from peripheral blood monocytes. The results indicated that while PS externalization (by treatment of normal RBCs with BHP and A23187) increased phagocytosis, addition of PS to the culture medium prevented it. These results are in agreement with a previous report that showed that PS-containing microvesicles inhibited phagocytosis of thalassemic RBCs (Srinivasan and Basu 1996). The results indicate that phagocytosis is mediated, in part, by the PS exposed on the RBCs but also suggest that shedding of PS reduces RBC phagocytosis, probably by competitive binding to PS receptors on macrophages.
The results indicate that oxidatively stressed RBCs undergo modulation of their membrane PS by increasing their Ca flux, leading to membrane shedding which profoundly affects RBC membrane composition and properties, such as their osmotic fragility and susceptibility to undergo phagocytosis (intra- and extravascular hemolysis, respectively) and consequently and consequently regulates the life span of the RBCs in the circulation.
References
Allan D, Thomas P (1981) Ca2+-induced biochemical changes in human erythrocytes and their relation to microvesiculation. Biochem J 198:433–440
Allen HG, Allen JC, Boyd LC, Alston-Mills BP, Fenner GP (2006) Determination of membrane lipid differences in insulin resistant diabetes mellitus type 2 in whites and blacks. Nutrition 22:1096–1102
Amer J, Goldfarb A, Fibach E (2004) Flow cytometric analysis of the oxidative status of normal and thalassemic red blood cells. Cytometry A 60:73–80
Amer J, Atlas D, Fibach E (2008) N-Acetylcysteine amide (AD4) attenuates oxidative stress in beta-thalassemia blood cells. Biochim Biophys Acta 1780:249–255
Ayub K, Hallett MB (2004) Signalling shutdown strategies in aging immune cells. Aging Cell 3:145–149
Bassé F, Stout JG, Sims PJ, Wiedmer T (1996) Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J Biol Chem 271:17205–17210
Brzeszczynska J, Luciak M, Gwozdzinski K (2008) Alterations of erythrocyte structure and cellular susceptibility in patients with chronic renal failure: effect of haemodialysis and oxidative stress. Free Radic Res 42:40–48
Caprari P, Scuteri A, Salvati AM, Bauco C, Cantafora A, Masella R, Modesti D, Tarzia A, Marigliano V (1999) Aging and red blood cell membrane: a study of centenarians. Exp Gerontol 34:47–57
Dedkova EN, Sigova AA, Zinchenko VP (2000) Mechanism of action of calcium ionophores on intact cells: ionophore-resistant cells. Membr Cell Biol 13:357–368
Dello Sbarba P, Rovida E (2002) Transmodulation of cell surface regulatory molecules via ectodomain shedding. Biol Chem 383:69–83
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148:2207–2216
Fibach E (1993) Measurement of total and fetal hemoglobin in cultured human erythroid cells by a novel micromethod. Hemoglobin 17:41–53
Fibach E, Rachmilewitz E (2008) The role of oxidative stress in hemolytic anemia. Curr Mol Med 8:609–619
Fibach E, Manor D, Oppenheim A, Rachmilewitz EA (1989) Proliferation and maturation of human erythroid progenitors in liquid culture. Blood 73:100–103
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Föller M, Huber SM, Lang F (2008) Erythrocyte programmed cell death. IUBMB Life 60:661–668
Freikman I, Amer J, Cohen JS, Ringel I, Fibach E (2008) Oxidative stress causes membrane phospholipid rearrangement and shedding from RBC membranes—an NMR study. Biochim Biophys Acta 1778:2388–2394
Freikman I, Amer J, Ringel I, Fibach E (2009) A flow cytometry approach for quantitative analysis of cellular phosphatidylserine distribution and shedding. Anal Biochem 393:111–116
Gonzalez LJ, Gibbons E, Bailey RW, Fairbourn J, Nguyen T, Smith SK, Best KB, Nelson J, Judd AM, Bell JD (2009) The influence of membrane physical properties on microvesicle release in human erythrocytes. PMC Biophys 2:7
Greenwalt TJ (2006) The how and why of exocytic vesicles. Transfusion 46:143–152
Hidalgo C, Donoso P (2008) Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid Redox Signal 10:1275–1312
Hon GM, Hassan MS, van Rensburg SJ, Abel S, van Jaarsveld P, Erasmus RT, Matsha T (2009) Red blood cell membrane fluidity in the etiology of multiple sclerosis. J Membr Biol 232:25–34
Kruse A, Uehlinger DE, Gotch F, Kotanko P, Levin NW (2008) Red blood cell lifespan, erythropoiesis and hemoglobin control. Contrib Nephrol 161:247–254
Kuypers FA (2007) Membrane lipid alterations in hemoglobinopathies. Hematol Am Soc Hematol Educ Program 2007:68–73
Lang F, Lang KS, Lang PA, Huber SM, Wieder T (2006a) Mechanisms and significance of eryptosis. Antioxid Redox Signal 8:1183–1192
Lang PA, Huober J, Bachmann C, Kempe DS, Sobiesiak M, Akel A, Niemoeller OM, Dreischer P, Eisele K, Klarl BA, Gulbins E, Lang F, Wieder T (2006b) Stimulation of erythrocyte phosphatidylserine exposure by paclitaxel. Cell Physiol Biochem 18:151–164
Maturu P, Vaddi DR, Pannuru P, Nallanchakravarthula V (2010) Alterations in erythrocyte membrane fluidity and Na+/K+-ATPase activity in chronic alcoholics. Mol Cell Biochem 339:35–42
McGrath LT, Douglas AF, McClean E, Brown JH, Doherty CC, Johnston GD, Archbold GP (1995) Oxidative stress and erythrocyte membrane fluidity in patients undergoing regular dialysis. Clin Chim Acta 235:179–188
Op den Kamp JA (1979) Lipid asymmetry in membranes. Annu Rev Biochem 48:47–71
Pattanapanyasat K, Noulsri E, Fucharoen S, Lerdwana S, Lamchiagdhase P, Siritanaratkul N, Webster HK (2004) Flow cytometric quantitation of red blood cell vesicles in thalassemia. Cytometry B Clin Cytom 57:23–31
Rank BH, Moyer NL, Hebbel RP (1988) Vesiculation of sickle erythrocytes during thermal stress. Blood 72:1060–1063
Reutelingsperger CP, Dumont E, Thimister PW, van Genderen H, Kenis H, van de Eijnde S, Heidendal G, Hofstra L (2002) Visualization of cell death in vivo with the annexin A5 imaging protocol. J Immunol Methods 265:123–132
Smith SK, Farnbach AR, Harris FM, Hawes AC, Jackson LR, Judd AM, Vest RS, Sanchez S, Bell JD (2001) Mechanisms by which intracellular calcium induces susceptibility to secretory phospholipase A2 in human erythrocytes. J Biol Chem 276:22732–22741
Sparling ML, Zidovetzki R, Muller L, Chan SI (1989) Analysis of membrane lipids by 500 MHz 1H NMR. Anal Biochem 178:67–76
Srinivasan PT, Basu J (1996) Altered membrane phospholipid organization and erythrophagocytosis in E beta-thalassemia. Biochim Biophys Acta 1285:65–70
Willekens FL, Werre JM, Groenen-Döpp YA, Roerdinkholder-Stoelwinder B, de Pauw B, Bosman GJ (2008) Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol 141:549–556
Wilson-Ashworth HA, Bahm Q, Erickson J, Shinkle A, Vu MP, Woodbury D, Bell JD (2006) Differential detection of phospholipid fluidity, order, and spacing by fluorescence spectroscopy of bis-pyrene, prodan, nystatin, and merocyanine 540. Biophys J 91:4091–4101
Yoshioka Y, Sasaki J, Yamamoto M, Saitoh K, Nakaya S, Kubokawa M (2000) Quantitation by 1H-NMR of dolichol, cholesterol and choline-containing lipids in extracts of normal and pathological thyroid tissue. NMR Biomed 13:377–383
Zhou Q, Zhao J, Stout JG, Luhm RA, Wiedmer T, Sims PJ (1997) Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J Biol Chem 272:18240–18244
Zwaal RF, Schroit AJ (1997) Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 89:1121–1132
Acknowledgments
I. F. performed the research, analyzed data and participated in writing the report. I. R. supervised and analyzed the NMR experiments and data. E. F. designed and supervised the research, analyzed data and wrote the report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freikman, I., Ringel, I. & Fibach, E. Oxidative Stress-Induced Membrane Shedding from RBCs is Ca Flux-Mediated and Affects Membrane Lipid Composition. J Membrane Biol 240, 73–82 (2011). https://doi.org/10.1007/s00232-011-9345-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-011-9345-y