Abstract
Knowledge of the parameters which influence the efficiency of gene electrotransfer has importance for practical implementation of electrotransfection for gene therapy as well as for better understanding of the underlying mechanism. The focus of this study was to analyze the differences in gene electrotransfer and membrane electropermeabilization between plated cells and cells in a suspension in two different cell lines (CHO and B16F1). Furthermore, we determined the viability and critical induced transmembrane voltage (ITVc) for both cell lines. In plated cells we obtained relatively little difference in electropermeabilization and gene electrotransfection between CHO and B16F1 cells. However, significant differences between the two cell lines were observed in a suspension. CHO cells exhibited a much higher gene electrotransfection rate compared to B16F1 cells, whereas B16F1 cells reached maximum electropermeabilization at lower electric fields than CHO cells. Both in a suspension and on plated cells, CHO cells had a slightly better survival rate at higher electric fields than B16F1 cells. Calculation of ITVc in a suspension showed that, for both electropermeabilization and gene electrotransfection, CHO cells have lower ITVc than B16F1 cells. In all cases, ITVc for electropermeabilization was lower than ITVc for gene electrotransfer, which is in agreement with other studies. Our results show that there is a marked difference in the efficiency of gene electrotransfer between suspended and plated cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gene therapy has the potential to treat several diseases for which a conventional cure is unlikely to be found. In gene therapy, DNA or RNA molecules are transferred into living cells in order to change their biological function for therapeutic purposes (Cavazzana-Calvo et al. 2000, 2004; Lehrman 1999; Hacein-Bey-Abina et al. 2003). Several different methods can be used in gene therapy for gene delivery into living cells. To date, viral gene-transfer techniques have been the most effective. Recent trial experiments in the field of HIV treatment using gene therapy show encouraging results (Mitsuyasu et al. 2009). However, viral vectors can lead to a strong undesired response of the immune system (Cavazzana-Calvo et al. 2000). Furthermore, in some cases insertional mutagenesis (Hacein-Bey-Abina et al. 2003) caused the death of the patient years after gene therapy was performed. For this reason, there is a great interest in developing nonviral gene-transfer techniques such as electroporation for gene electrotransfer (Ferber 2001; Cemazar and Sersa 2007; Teissié et al. 2008). Recently, a clinical trial using gene electrotransfer was reported (Daud et al. 2008), which showed great potential for the use of electroporation in gene therapy.
Electroporation (sometimes referred to as “electropermeabilization”) as a method of foreign material delivery into eukaryotic and prokaryotic cells using electric pulses was first described almost three decades ago (Neumann et al. 1982). The exposure of a cell to electric pulses of a sufficient amplitude and duration leads to a transient exchange of matter across the electropermeabilized cell membrane (Neumann et al. 1982; Rols et al. 1998). When the matter transferred into cells by electropermeabilization is DNA, this method is called “gene electrotransfer.” Thus, gene electrotransfer is a nonviral method for delivery of DNA molecules into cells by means of electric pulses. Alongside lipoplex transfection, gene electrotransfer is one of the most promising nonviral methods of gene delivery and is therefore gaining in importance in the field of gene therapy (Ferber 2001; Favard et al. 2007; Li and Huang 2000; Parker et al. 2003).
Various in vitro studies of gene electrotransfer have been performed on different cell lines and showed that one of the prerequisites for successful gene electrotransfection and electropermeabilization is the use of an adequate electric field (Ec) at which the induced transmembrane voltage (ITV) reaches a critical value (ITVc) (Wolf et al. 1994; Rols and Teissié 1998). The ITV is determined by the size and shape of the cell as well as by its orientation in the electric field (Schwan 1957; Weaver and Chizmadzhev 1996; Pucihar et al. 2006). Thus, plated cells may respond differently to specific sets of electric pulses compared to cells in a suspension (Valic et al. 2003). Electric pulse parameters such as pulse amplitude, pulse duration, number of pulses and pulse repetition frequency affect gene electrotransfection and electropermeabilization of cell membranes (Wolf et al. 1994; Pucihar et al. 2002). However, the main difference between gene electrotransfection and electropermeabilization is the fact that plasmid has to be present during exposure of a cell to electric pulses to obtain gene expression, whereas uptake of small molecules can also occur after pulsation (Rols and Teissié 1998; Wolf et al. 1994). Additionally, pulse duration is more important for efficient gene electrotransfection than number of pulses (Rols and Teissié 1998; Teissié et al. 2005).
Various cell lines including Chinese hamster ovary cells (CHO) and mouse melanoma (B16F1) cells in a suspension have already been compared. These comparisons determined that different pulse parameters are needed for optimal transfection of different cell lines (Cegovnik and Novakovic 2004). However, in none of these studies did the authors analyze both gene electrotransfer and electropermeabilization of plated cells and cells in a suspension for different cell lines.
The aim of our study was to analyze and compare gene electrotransfer, electropermeabilization, cell viability and ITVc in parallel for two cell lines: CHO and B16F1 cells. We further analyzed the differences between plated cells and cells in a suspension as well as examined the relation between gene electrotransfer and electropermeabilization on CHO and B16F1 cells on both plated cells and cells in a suspension.
Methods
Cell Culture
Two different cell lines were used in our experiments: CHO-K1 (European Collection of Cell Cultures, Salisbury, UK) cells and mouse melanoma (B16F1) cells. B16F1 cells were grown in Eagle’s minimum essential medium (EMEM) supplemented with 10% fetal bovine serum (Sigma-Aldrich, Deisenhofen, Germany). CHO cells were grown in culture medium F-12 HAM (Dulbecco’s modification of EMEM) supplemented with 10% fetal bovine serum and 0.15 mg/ml l-glutamine (Sigma-Aldrich, St. Louis, MO). A 10 mM isosmolar phosphate buffer (Na2HPO4)/NaH2PO4 with magnesium chloride (MgCl2) and sucrose was used for electroporation.
Plated Cells
Gene Electrotransfer
Both CHO and B16F1 cell lines in the exponential growth phase were plated in two 24-multiwell plates with a cell concentration of 2 × 104/well. The plates were placed in an incubator (37°C, 5% CO2) for 24 h. Before pulsation, the growth medium was removed and 150 μl of an electroporation medium with 10 μg/ml plasmid DNA pEGFP-N1 (Clontech, Palo Alto, CA; 4,649 bp), which expresses green fluorescent protein (GFP; excitation 488 nm, emission 507 nm), was added. After 2–3 min of incubation at room temperature, electric pulses were applied using the Cliniporator™ device (IGEA, Carpi, Italy). Each well was treated with four electric pulses of 200 μs with a pulse repetition frequency of 1 Hz and a specific electric field amplitude, as described previously (Kanduser et al. 2009; Pavlin et al. 2010). The amplitudes of the applied electric fields were 0.3, 0.4, 0.6, 0.8, 1.0, 1.2 and 1.4 kV/cm. The distance between the electrodes was 4 mm. No electric pulses were applied to cells in negative control. Cells were incubated for 5 min to allow cell membrane resealing, then grown in a cell culture medium for 24 h in an incubator.
After 24 h, the cells were observed under an inverted fluorescence microscope (Axiovert 200; Zeiss, Göttingen, Germany) as seen in Fig. 1. Transfected cells (cells expressing GFP) were determined by observing the cells in fluorescence images. The total number of cells in the treated sample was determined by observing the cells in phase-contrast images. Therefore, the ratio of treated cells was determined as the quotient of the number of transfected cells and the total number of cells in the treated sample.
All experiments were repeated six times on different days. Results from different repetitions of experiments were pooled together and are presented as means ± standard deviation.
Cell Electropermeabilization
Electropermeabilization was assayed by propidium iodide (PI, 0.15 mM in pulsing buffer). For electropermeabilization, CHO and B16F1 cells were plated in a concentration of 8 × 104 cells/well. The electric pulse protocol was almost identical to the protocol used for gene electrotransfer. The only difference was that three additional amplitudes (0.1, 0.2 and 1.6 kV/cm) were used for electropermeabilization. Immediately after the growth medium had been removed from each well, 150 μl of PI was added. Then, cells were pulsed. After applying the pulses, cells were incubated for 3 min at room temperature. The extracellular PI was then removed from wells, and 1 ml of phosphate buffer was added. No electric pulses were applied to cells in negative control. The positive control was determined at a 1.6 kV/cm electric field, where all cells were permeabilized.
The uptake of PI for plated cells was evaluated with a spectrofluorometer (excitation 535 nm, emission 617 nm) (Tecan infinite M200; Tecan, Grödig, Austria) using the Magellan program (Tecan). The permeabilization percentage of treated cells was calculated as the ratio of the fluorescence intensity of treated cells and the fluorescence intensity of cells in the positive control.
Cells in a Suspension
Gene Electrotransfer
Gene electrotransfer was performed using CHO and B16F1 cells that were in the exponential growth phase. The cell suspension was prepared by 0.25% trypsin/EDTA solution and centrifuged for 5 min at 1,000 rpm (180g) and 4°C (Sigma-Aldrich, Germany). The cell pellet was resuspended in an electroporative medium at a concentration of 2.5 × 106 cells/ml.
Cuvettes with incorporated aluminum electrodes (interelectrode distance 4 mm; Eppendorf, Hamburg, Germany) were used for electric pulse delivery. The volume of the sample placed in each cuvette was 200 μl. Plasmid DNA (pEGFP-N1) was added to the cell suspension in a concentration of 40 μg/ml. After incubating the DNA with the cells at room temperature for 2–3 min, electric pulses were applied to the samples using the Cliniporator. Each cuvette was treated with four electric pulses of 200 μs with a repetition frequency of 1 Hz and specific electric field amplitude. The amplitudes of the applied electric fields were 0.6, 0.8, 1.0, 1.2, 1.4 and 1.6 kV/cm. No pulses were applied on cells in the negative control.
Treated cells were incubated for 5 min to allow cell membrane resealing and then grown for 24 h in a cell culture medium in the incubator (37°C, 5% CO2). After 24 h, cells were trypsinized, centrifuged for 5 min at 1,000 rpm (180g) at 4°C and resuspended in phosphate-buffered saline (PBS) at a concentration of 1 × 106 cells/ml.
The percentage of GFP-expressing cells was determined using a flow cytometer (Coulter EPICS Altra flow cytometer; Beckman Coulter Electronics, Brea, CA) equipped with a laser emitting at 509 nm, and 10,000 events were recorded.
All experiments were repeated three times on different days. Results from different repetitions of experiments were pooled together and are presented as means ± standard deviation.
Cell Electropermeabilization
To evaluate permeabilization of CHO and B16F1 cells in a suspension, PI was used. The electric pulse protocol was almost identical to the protocol used for gene electrotransfer. The only difference was that two additional amplitudes (0.3 and 0.4 kV/cm) were used for electropermeabilization. In negative control, no electric pulses were applied. After trypsinization, cells were centrifuged for 5 min at 1,000 rpm (180g) at 4°C (Sigma-Aldrich, Germany) and then resuspended in an electroporative medium at a concentration of 2.5 × 106 cells/ml.
The volume of the sample placed in each cuvette was 200 μl. Immediately before electric pulse application, 0.15 mM PI was added to the media (at a ratio of 1:100). Cells were incubated for 3 min at room temperature after pulses were applied and then centrifuged for 5 min at 1,000 rpm (180g) at 4°C to remove extracellular PI that did not enter the cells.
The same method that had been used to measure PI uptake in plated cells was also used here. The only difference was that the positive control here was determined at an electric field of 1.8 kV/cm.
Cell Viability
Plated Cells
Viability was determined 24 h after pulsation by counting cells in phase-contrast images obtained under an inverted fluorescence microscope. Viability was calculated by quantifying the ratio between the number of cells in samples that had been exposed to electric pulses and the number of cells in the negative control. Cells in the negative control were taken as 100% viable.
All experiments were repeated six times on different days. Results from different repetitions of experiments were pooled together and are presented as means ± standard deviation.
Cells in a Suspension
After pulsation, a concentration of 1.0 × 104 for CHO cells and 1.2 × 104 for B16F1 cells was placed into each well on a 96-well microtiter plate (TPP, Trasadingen, Switzerland). Growth medium was added so that the total volume of each well was 100 μl.
After 24 h of incubation, the cell viability test was performed using the MTS-based Cell Titer 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI). Reagent, 20 μl, was added directly to each well. After 2 h of incubation at 37ºC, fluorescence intensity (490 nm emission wavelength) was measured with a spectrofluorometer. The viability of treated cells was calculated as the ratio of fluorescence intensity of the treated cells and fluorescence intensity of cells in the negative control. Cells in the negative control were taken as 100% viable.
The experiments were repeated three times on different days, with at least six parallels made for each parameter each time. Results from different repetitions of experiments were pooled together and are presented as means ± standard deviation.
Cell Size
The average cell size was determined by analyzing phase-contrast images of nonpulsed cells from each experiment. The objective magnification was 20×. ImageJ 1.141o (National Institutes of Health, Bethesda, MD) was used to measure the cells in the phase-contrast images. Plated cells were approximated as a prolate spheroid, and two parameters were measured for every cell: the longest diameter of the cell (D max) and the longest line that runs perpendicular to D max (D min) (Valic et al. 2003). Cells in a suspension were approximated as a sphere, and the diameter (D sus) was measured for each cell.
More than 20 cells per experiment were assayed. The values presented are means ± standard deviations of at least six independent experiments.
Determination of E c and Calculation of ITVc
In order to objectively determine ITVc from graphs, a linear regression line using Sigmaplot v11 (Systat Software, Richmon, CA) was fitted to the first three or four parameters of transfection (Figs. 2a, 3a) and electropermeabilization data (Figs. 2b, 3b). The critical electric field, E c, above which transfection and electropermeabilization were observed was determined as a point where linear fit crossed the electric field axis.
Effect of the electric field on cells in a plated state a on GFP expression for plated CHO (filled circle) and B16F1 (filled triangle) cells, b on PI uptake in plated CHO (filled circle) and B16F1 (filled triangle) cells and c on cell viability for CHO and B16F1 cells. Dotted lines in a and b represent linear fit (see “Methods” section). Cells were treated with four pulses of 200 μs duration and 1 Hz repetition frequency. Values are means of at least six independent experiments ± standard deviation. Please note that the scale in a is different from that in b and c
Effect of the electric field on cells in a suspension a on GFP expression for CHO (filled circle) and B16F1 (filled triangle) cells, b on PI uptake in CHO (filled circle) and B16F1 (filled triangle) cells and c on cell viability for CHO and B16F1 cells. Dotted lines in a and b represent linear fit (see Methods). Cells were treated with four pulses of 200 μs duration and 1 Hz repetition frequency. Values are means of at least three independent experiments ± standard deviation
From experimentally determined E c we calculated ITVc based on the derivation presented below.
The induced transmembrane voltage for cells in a suspension is given by the Schwan equation for an ideal spherical cell:
where E is the applied electric field, R is the cell radius and ϑ is the angle defined between the applied electric field and the point vector of the calculation on the membrane (Valic et al. 2003). ITVc can be obtained by setting \( R = {\frac{{D_{\text{sus}} }}{2}},\quad \vartheta = 0 \) (point vector oriented in parallel to the electric field) and threshold electric field E = E c:
For plated cells, ITVc was calculated for the average size of cells using the Schwan equation for a prolate spheroid (Valic et al. 2003):
where α is the orientation of the electric field, x and z represent the plane where the electric field lies and L x and L z are depolarizing factors. ITVc can be obtained by setting α = 0 (prolate spheroid oriented in parallel to the electric field), z = D max (average long radius of a cell), E = E c and depolarizing factor L z :
where e is
Therefore, the equation used to calculate ITVc for plated cells is
Results
The aim of this study was to analyze CHO and B16F1 cells in parallel for gene electrotransfer, electropermeabilization, viability and ITVc in both plated cells and cells in a suspension.
Plated Cells
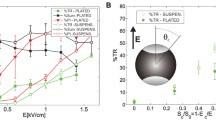
In Fig. 2a, the efficiency of gene electrotransfer on plated cells determined by counting the number of cells that expressed GFP is presented. We obtained E c = 0.20 kV/cm for CHO and E c = 0.165 kV/cm for B16F1 cells. In both cell lines, GFP expression increased to 1.2 kV/cm, where it reached its maximum at 21 and 26% for CHO and B16F1 cells, respectively.
In Fig. 2b, PI uptake in CHO and B16F1 cells as a function of electric field is shown. In CHO cells, permeabilization reached 12% at 0.2 kV/cm, whereas permeabilization of B16F1 cells was already at 30% at the same amplitude. Results show that, although B16F1 cells exhibit higher PI uptake at lower electric fields, both cell lines reached a maximum saturation level of 100% at the same electric field of 1 kV/cm. The threshold values (Ec) were 0.10 kV/cm for CHO cells and 0.08 kV/cm for B16F1 cells.
If we compare GFP expression with PI uptake on plated cells for both cell lines, we can see that at the electric field of 0.3 kV/cm the permeabilization is approximately 30% for CHO cells and 50% for B16F1 cells, while GFP expression at the same electric field is only around 2% in both cell lines.
Furthermore, we compared the cell viability of the two cell lines (Fig. 2c). The viability of CHO cells started to decline at field strengths above 0.8 kV/cm, whereas field strength above 0.3 kV/cm is already damaging for B16F1 cells (Fig. 2c). Altogether plated CHO cells can withstand higher electric fields and have an overall better survival rate than plated B16F1 cells.
Cells in a Suspension
In Fig. 3a, it can be observed that an increase in electric field leads to an increase in electrotransfection efficiency in both cell lines. The thresholds are 0.57 and 0.55 kV/cm for CHO and B16F1 cells, respectively. The transfection efficiency is significantly higher in CHO cells compared to B16F1 cells. The maximum transfection was achieved at the maximum field strength of 1.6 kV/cm, and it was around 70% for CHO cells and around 30% for B16F1 cells.
As can be seen in Fig. 3b, the PI uptake for both cell lines is very similar at lower electric fields. However, at higher pulse amplitudes B16F1 cells have a higher PI uptake compared to CHO cells. At an electric field of 1.2 kV/cm, the PI uptake in CHO cells is around 60%, whereas B16F1 cells are already at the saturation level. The results suggest that the saturation of PI uptake occurs faster in B16F1 cells. The threshold was obtained at 0.44 kV/cm for CHO cells and at 0.41 kV/cm for B16F1 cells.
We also tested the effect of electric field on cell viability in a suspension. An increase in electric field (see Fig. 3c) to 1.0 kV/cm had no effect on the viability of CHO and B16F1 cells. Above this amplitude, we can see a gradual decrease in cell survival for B16F1 cells to about 50% at 1.6 kV/cm. For CHO cells, viability was around 80% at the same field strength.
Determination of Cell Size and ITVc
In Table 1, we present measured diameters for plated cells and cells in a suspension as well as ITVc for PI uptake and GFP expression. ITVc was calculated from E c using Eq. 2 (for cells in a suspension) or Eq. 6 (for plated cells), where we assumed that the cells were oriented in parallel to the electric field (α = 0 and ϑ = 0) in order to obtain ITVc of the cells which permeabilize first.
As can be seen in Table 1, ITVc obtained for GFP expression in plated cells was 0.42 V for CHO and 0.30 V for B16F1 cells. ITVc for PI uptake was 0.23 V for CHO and 0.15 V for B16F1 cells. However, it is important to note that these results are only a rough estimate of the ITVc for plated cells due to large variations of cell sizes within one cell line (D max, D min).
For cells in a suspension, ITVc for transfection was 0.50 V in CHO and 0.65 V in B16F1 cells. ITVc for PI uptake was 0.39 V for CHO and 0.49 V for B16F1 cells.
Discussion and Conclusions
Knowledge of the parameters which influence the efficiency of gene electrotransfer has importance for practical implementation of electrotransfection for gene therapy as well as for better understanding of the underlying mechanism. A number of different studies showed that although successful electropermeabilization is a prerequisite for efficient electric field–mediated gene transfer (Wolf et al. 1994; Rols et al. 1998; Golzio et al. 2001), the process of electrotransfection is more complex and involves several steps: permeabilization, contact of DNA with the cell membrane, translocation and gene expression (Golzio et al. 2002; Favard et al. 2007; Cemazar and Sersa 2007). It was also shown that both biophysical parameters (ITV, medium composition, temperature, fluidity of the membrane, etc.) as well as biological parameters (state of the cell [plated or in a suspension], ability to recover after electric pulses, etc.) determine the efficiency of gene electrotransfer (Neumann et al. 1989; Rols et al. 1998) in vitro. In vivo, additional aspects are important, such as extracellular matrix, which hinders plasmid mobility and tissue inhomogenity that affect local electric field distribution (Gehl et al. 1998; Zaharoff et al. 2002; Corovic et al. 2008; Hojman et al. 2008).
A variety of studies (Wolf et al. 1994; Rols et al. 1998; Rols and Teissié 1998) also analyzed the relationship between electropermeabilization and gene electrotransfer. However, none of these studies compared and analyzed electropermeabilization and gene electrotransfer on plated cells and cells in a suspension in parallel. In this study we therefore focused on comparing plated cells and cells in a suspension. We analyzed the relation between electropermeabilization (PI uptake) and gene electrotransfer (GFP expression) on plated cells and cells in a suspension for two cell lines: mammalian CHO and mouse melanoma (B16F1) cells.
Furthermore, we calculated the ITVc obtained from experimentally determined critical electric fields (Ec) for uptake of PI (permeabilization) and expression of GFP (transfection) (see Table 1) using Eqs. 2 and 6.
Marked differences were observed between plated cells and cells in a suspension. In plated cells, results for electropermeabilization and transfection were similar for both cell lines. However, in a suspension, significant differences between the two cell lines were observed. CHO cells exhibited a much higher transfection rate compared to B16F1, while B16F1 cells reached maximal permeabilization at lower electric fields than CHO cells. For both plated cells and cells in a suspension, CHO cells had a slightly better survival rate at higher electric fields than B16F1 cells.
It is also important to note that, due to a much more homogeneous cell size in suspension, it is much easier to obtain a higher transfection rate in a suspension compared to plated cells. Furthermore, two parameters were determined by measuring cell sizes: ITVc for PI and ITVc for GFP. ITVc was calculated by Eqs. 2 and 6 from experimentally determined E c. The E c values for PI uptake and GFP expression were different, which can be partially explained by the difference in the size of molecules used. Moreover, gene electrotransfer is a much more complex process compared to diffusion of small molecules such as PI.
In plated cells, the E c was found to be similar for both cell lines: around 0.2 kV/cm for GFP expression and around 0.1 kV/cm for uptake of PI. However, due to a large standard deviation of measured radii of plated cells, the ITVc values are only rough estimates. As already demonstrated (Pucihar et al. 2006; Towhidi et al. 2008), exact ITVc for plated cells should always be calculated only for a specific cell. Otherwise, large errors are obtained due to large variations of cell sizes, shapes and orientations.
In a suspension, however, cells have a spherical shape with a much smaller deviation in size; therefore, ITVc can be determined more accurately. Both ITVc for PI and ITVc for GFP were lower for CHO cells compared to B16F1 cells, which agrees with values obtained from measuring electric conductivity during the electric pulses (Pavlin et al. 2005). Together with the observation that viability is comparably better in CHO cells, we can thus explain why, in a suspension, CHO cells are “easier” to transfect compared to B16F1 cells. Although it may be easier for small molecules to penetrate B16F1 cell membranes, CHO cells in a suspension are better at handling other factors of the complex procedure responsible for overall better transfection. In general, higher gene expression can be obtained in cells which are in a better physiological state.
Furthermore, when analyzing the ITVc values presented in Table 1, we found that, in all cases, the E c and ITVc values for electropermeabilization were smaller than those for gene electrotransfer. This confirms that permeabilization is a crucial step for successful gene electrotransfer, though not sufficient.
Our results again demonstrate that, even though electropermeabilization is a prerequisite for successful gene electrotransfer, there is no direct relation between the efficiency of electropermeabilization (uptake of PI) and the efficiency of gene electrotransfer (expression of GFP), as already shown in another study (Rols et al. 1998).
Our results also suggest that cells in a suspension or plated cell respond to electric fields quite differently and that there is no simple relation describing the rate of transfection or permeabilization between plated cells and cells in a suspension. Results in vitro are usually used for optimization and analysis of parameters in vivo; therefore, it is important to use plated cells, which are more similar to cells in tissue (realistic shape), compared to cells in a suspension, which have an ideal spherical shape and homogeneous cell size distribution.
To conclude, electropermeabilization and gene electrotransfer are both threshold phenomena, where critical electric field depends on several parameters. Our results demonstrate that there is no simple rule which would enable us to extrapolate results from electropermeabilization to the efficiency of gene electrotransfer. Furthermore, comparison between plated cells and cells in a suspension indicates that plated cells behave rather differently from cells in a suspension, which can be partially explained by the large distribution of cell sizes and shapes. The observed differences between plated cells and cells in a suspension can be explained using the results of other studies, where it was shown that the cytoskeleton has an important role both in permeabilization (Rols and Teissié 1992), where it affects the resealing of the cell membrane, and in transfection (Vaughan and Dean 2006), where it is involved in intracellular plasmid trafficking. When comparing CHO and B16F1 cell lines, we obtained similar transfection and permeabilization in plated cells. However, in a suspension, CHO cells exhibited higher transfection rate compared to B16F1 cells, while B16F1 cells were “easier” to permeabilize (higher permeabilization rate at lower electric fields). Calculations of ITVc in a suspension showed that CHO cells have lower ITVc than B16F1 cells for both permeabilization and transfection. In plated cells, the distribution of sizes is so large that we can only estimate that ITVc values are similar. However, further studies are necessary in order to understand the relationship between phenomena observed in vitro in a suspension and in plated cells.
References
Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Le Deist F, Fischer A (2000) Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288:669–672
Cavazzana-Calvo M, Thrasher A, Mavilio F (2004) The future of gene therapy. Nature 427:779–781
Cegovnik U, Novakovic S (2004) Setting optimal parameters for in vitro electrotransfection of B16F1, SA1, LPB, SCK, L929 and CHO cells using predefined exponentially decaying electric pulses. Bioelectrochemistry 62:73–82
Cemazar M, Sersa G (2007) Electrotransfer of therapeutic molecules into tissues. Curr Opin Mol Ther 9:554–562
Corovic S, Zupanic A, Miklavcic D (2008) Numerical modeling and optimization of electric field distribution in subcutaneous tumor treated with electrochemotherapy using needle electrodes., 36:1665–1672
Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL, Heller R (2008) Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol 26:5896–5903
Favard C, Dean DS, Rols MP (2007) Electrotransfer as a nonviral method of gene delivery. Curr Gene Ther 7:67–77
Ferber D (2001) Gene therapy: safer and virus-free? Science 294:1638–1642
Gehl J, Skovsgaard T, Mir LM (1998) Enhancement of cytotoxicity by electropermeabilization: an improved method for screening drugs. Anti Cancer Drugs 9:319–325
Golzio M, Teissié J, Rols MP (2001) Control by membrane order of voltage-induced permeabilization, loading and gene transfer in mammalian cells. Bioelectrochemistry 53:25–34
Golzio M, Teissié J, Rols MP (2002) Direct visualization at the single cell level of electrically mediated gene delivery. Proc Natl Acad Sci USA 99:1292–1297
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M (2003) LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302:415–419
Hojman P, Gissel H, Franck MA, Cournil-Henrionnet C, Eriksen J, Gehl J, Mir ML (2008) Physiological effects of high- and low-voltage pulse combinations for gene electrotransfer in muscle. Hum Gene Ther 19:1249–1260
Kanduser M, Miklavcic D, Pavlin M (2009) Mechanisms involved in gene electrotransfer using high- and low-voltage pulses—an in vitro study. Bioelectrochemistry 74:265–271
Lehrman S (1999) Virus treatment questioned after gene therapy death. Nature 401:517–518
Li S, Huang L (2000) Nonviral gene therapy: promises and challenges. Gene Ther 7:31–34
Mitsuyasu RT, Merigan TC, Carr A, Zack JA, Winters MA, Workman C, Bloch M, Lalezari J, Becker S, Thornton L, Akil B, Khanlou H, Finlayson R, McFarlane R, Smith DE, Garsia R, Ma D, Law M, Murray JM, von Kalle C, Ely JA, Patino SM, Knop AE, Wong P, Todd AV, Haughton M, Fuery C, Macpherson JL, Symonds GP, Evans LA, Pond SM, Cooper DA (2009) Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat Med 15:285–292
Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH (1982) Gene transfer into mouse lymoma cells by electroporation in high electric fields. EMBO J 7:841–845
Neumann E, Sowers AE, Jordan CA (1989) Electroporation and electrofusion in cell biology. Plenum Press, New York
Parker AL, Newman C, Briggs S, Seymour L, Sheridan PJ (2003) Nonviral gene delivery: techniques and implications for molecular medicine. Expert Rev Mol Med 5(22):1–15
Pavlin M, Kanduser M, Rebersek M, Pucihar G, Hart FX, Magjarevic R, Miklavcic D (2005) Effect of cell electroporation on the conductivity of a cell suspension. Biophys J 88:4378–4390
Pavlin M, Flisar K, Kanduser M (2010) The role of electrophoresis in gene electrotransfer. J Memb Biol. doi:10.1007/s00232-010-9276-z
Pucihar G, Mir LM, Miklavcic D (2002) The effect of pulse repetition frequency on the uptake into electropermeabilized cells in vitro with possible applications in electrochemotherapy. Bioelectrochemistry 57:167–172
Pucihar G, Kotnik T, Valic B, Miklavcic D (2006) Numerical determination of transmembrane voltage induced on irregularly shaped cells. Ann Biomed Eng 34:642–652
Rols MP, Teissié J (1992) Experimental evidence for the involvement of cytoskeleton in mammalian cell electropermeabilization. Biochim Biophys Acta 1111:45–50
Rols MP, Teissié J (1998) Electropermeabilization of mammalian cells to macromolecules: control by pulse duration. Biophys J 75:1415–1423
Rols MP, Delteil C, Golzio M, Teissié J (1998) Control by ATP and ADP of voltage-induced mammalian-cell-membrane permeabilization, gene transfer and resulting expression. Eur J Biochem 254:382–388
Schwan HP (1957) Electrical properties of tissue and cell suspensions. Adv Biol Med Phys 5:147–209
Teissié J, Golzio M, Rols MP (2005) Mechanisms of cell membrane electropermeabilization: a minireview of our present (lack of?) knowledge. Biochim Biophys Acta 1724:270–280
Teissié J, Escoffre JM, Rols MP, Golzio M (2008) Time dependence of electric field effects on cell membranes. A review for a critical selection of pulse duration for therapeutical applications. Radiol Oncol 42:196–206
Towhidi L, Kotnik T, Pucihar G, Firoozabadi SMP, Mozdarani H, Miklavcic D (2008) Variability of the minimal transmembrane voltage resulting in detectable membrane electroporation. Electromagn Biol Med 27:372–385
Valic B, Golzio M, Pavlin M, Schatz A, Faurie C, Gabriel B, Teissié J, Rols MP, Miklavcic D (2003) Effect of electric field induced transmembrane potential on spheroidal cells: theory and experiment. Biophys J 32:519–528
Vaughan EE, Dean DA (2006) Intracellular trafficking of plasmids during transfection is mediated by microtubules. Mol Ther 3:422–428
Weaver JC, Chizmadzhev YA (1996) Theory of electroporation: a review. Bioelectrochem Bioenerg 41:135–160
Wolf H, Rols MP, Boldt E, Neumann E, Teissié J (1994) Control by pulse parameters of electric field-mediated gene transfer in mammalian cells. Biophys J 66:524–531
Zaharoff DA, Barr RC, Li CY, Yuan Y (2002) Electromobility of plasmid DNA in tumor tissues during electric field–mediated gene delivery. Gene Ther 9:1286–1290
Acknowledgements
This research was supported by the Slovenian Research Agency under grants J2-9770 and P2-0249. We thank also Rosana Hudej (Faculty of Chemistry and Chemical Technology, University of Ljubljana) and Marko Ušaj (Faculty of Electrical Engineering, University of Ljubljana) for help in experimental procedures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marjanovič, I., Haberl, S., Miklavčič, D. et al. Analysis and Comparison of Electrical Pulse Parameters for Gene Electrotransfer of Two Different Cell Lines. J Membrane Biol 236, 97–105 (2010). https://doi.org/10.1007/s00232-010-9282-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-010-9282-1