Abstract
Efficient electroporation of cells in physical contact induces cell fusion, and this process is known as electrofusion. It has been shown that appropriate hypotonic treatment of cells before the application of electric pulses can cause a significant increase in electrofusion efficiency. First, the amplitudes of the electric field were determined spectrofluorometrically, where sufficient permeabilization in hypotonic buffer occurred for B16-F1 and CHO cells. In further electrofusion experiments 14 ± 4% of fused cells for B16-F1 and 6 ± 1% for CHO was achieved. These electrofusion efficiencies, determined by double staining and fluorescence microcopy, are comparable to those of other published studies. It was also confirmed that successful electroporation does not necessarily guarantee high electrofusion efficiency due to biological factors involved in the electrofusion process. Furthermore, not only the extension of electrofusion but also cell survival depends on the cell line used. Further studies are needed to improve overall cell survival after electroporation in hypotonic buffer, which was significantly reduced, especially for B16-F1 cells. Another contribution of this report is the description of a simple modification of the adherence method for formation of spontaneous cell contact, while cells preserve their spherical shape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell–cell electrofusion is a safe, nonviral and nonchemical method used for preparation of hybrid cells for various biomedical applications and can be properly adjusted for different cell types (Scott-Taylor et al. 2000). Electrofusion is a two-condition process: (1) cell membranes have to be brought into a fusogenic state and (2) physical contact between two fusogenic membranes has to be established (Teissie and Rols 1986). The contact needed for electrofusion can be obtained before or immediately after the fusogenic state of membranes is achieved, with better electrofusion efficiency reported in the former case (Wu et al. 1992). A fusogenic state of the cell membrane is achieved by electroporation, which is already widely used in biomedical applications, such as gene electrotransfer, electrochemotherapy and tissue ablation (Escoffre et al. 2009; Marty et al. 2006; Mir, 2009; Rubinsky et al. 2007). Electroporation causes a dramatic increase in the membrane permeability of cells exposed to short and intense electric pulses (Miklavcic and Towhidi 2010). The external electric field induces transmembrane voltage, which provides energy for structural rearrangement of lipid bilayers and cell membrane permeabilization. Induced transmembrane voltage of spherical cell can be calculated as
where r is the radius of the cell, E is the strength of the external electric field and φ is the angle between the direction of the electric field and the selected point on the cell surface (Kotnik et al. 1997; Neumann et al. 1989; Pucihar et al. 2009).
As mentioned above, the second condition required for cell fusion is physical contact between cells, which has to be established while their membranes are in a fusogenic state. Physical contact between cells can be achieved with various methods. Cells can be brought into close contact mechanically with the use of a specific fusion chamber (Jaroszeski et al. 1994), filters (Ramos et al. 2002) or centrifugation (Abidor et al. 1993; Sowers 1986; Teissie and Rols 1986). Another possibility for achieving cell contact is the adherence method, where cell contact is established spontaneously in confluent culture (Finaz et al. 1984; Salomskaite-Davalgiene et al. 2009; Sukharev et al. 1990; Teissie and Blangero 1984; Teissie et al. 1982). Yet another, and the most widely used, method is application of alternating electric fields, causing dielectrophoretic forces in cells that result in cell migration and pearl chain formation (Vienken and Zimmermann 1985; Zimmermann 1982).
Electrofusion is affected by medium proprieties such as ionic composition, electrical conductivity and osmolarity (Abe and Takeda 1988; Matibiri and Mantell 1995; Mizukami et al. 1993; Ohno-Shosaku and Okada 1985; Rols and Teissie 1989; Schnettlera and Zimmermann 1992; Stenger et al. 1988, 1991).
One of the earliest approaches to improving electrofusion efficiency is the use of hypotonic electrofusion buffer, which results in a considerable increase of fusion efficiency (Ahkong and Lucy 1986; Schmitt and Zimmermann 1989; Sukhorukov et al. 2006). In order to improve fusion efficiency in hypotonic buffer, the duration and osmolarity of the hypotonic treatment have to be determined (Usaj et al. 2009).
Even though electrofusion of biological cells is potentially useful, achieving sufficient efficiency still requires further studies (Hayashi et al. 2002; Scott-Taylor et al. 2000; Trontelj et al. 2008). The low number of fused cells remains the main problem of electrofusion (James and Bell 1987; Mally et al. 1992; Neil and Zimmermann 1993; Stevens et al. 1979; Yu et al. 2008).
Electrofusion can be successfully used for different types of cells. Cells differ in their electroporation behavior; therefore, they also differ in electrofusion. While part of the difference in electrofusion behavior can be attributed to differences in cell size (Eq. 1), another part is related to the biological characteristics of treated cells (Cegovnik and Novakovic 2004; Cemazar et al. 1998; Neil and Zimmermann 1993; O’Hare et al. 1989; Yu et al. 2008).
The aim of this study was to optimize the electrofusion protocol through the use of hypotonic buffer. Experiments were conducted on two different cell lines: mouse melanoma cells (B16-F1) and Chinese hamster ovary (CHO) cells. The electroporation efficiency in hypotonic buffer was determined when cells were close to their maximal size. These experiments helped to determine the amplitudes of the electric field needed for successful electroporation. The relation between electroporation, electrofusion efficiency and the effect of applied treatment on cell survival was also studied. A simple modification of the adherence method is also described, where good cell–cell contacts required for electrofusion were achieved spontaneously. Instead of the confluent cell culture described in previous studies (Finaz et al. 1984; Salomskaite-Davalgiene et al. 2009; Sukharev et al. 1990; Teissie and Blangero 1984; Teissie et al. 1982), cell–cell contacts were achieved by plating cells at appropriate concentration for a short time. Electrofusion was performed on slightly adhered cells, which formed spontaneous contacts among themselves, while their spherical shape was preserved.
Materials and Methods
Chemicals, Cell Culture Media
Eagle’s minimal essential medium (EMEM), Ham’s Nutrient Mixtures (F-12 HAM), fetal bovine serum (FBS), l-glutamine, sucrose, dipotassium hydrogen phosphate (K2HPO4), potassium dihydrogen phosphate (KH2PO4), magnesium chloride (MgCl2), crystal violet, trypsin and EDTA were obtained from Sigma-Aldrich (Taufkirchen, Germany). Antibiotics (crystacillin and gentamicin) were obtained from Lek (Ljubljana, Slovenia). Propidium iodide, CMFDA and CMRA cell trackers were obtained from Molecular Probes/Invitrogen (Carlsbad, CA).
Cells
All cell lines were cultured in humidified atmosphere at 37°C and 5% CO2 (Kambič, Semič, Slovenia) in the following culture media: mouse melanoma (B16-F1) in EMEM supplemented with 10% FBS, antibiotics (gentamicin, crystacillin) and l-glutamine; CHO cells in F-12 HAM supplemented with 10% FBS, antibiotics and l-glutamine. Cell lines were grown in a 25-cm2 culture flask (TPP, Trasadingen, Switzerland) until they reached 80–90% confluence.
Isotonic and Hypotonic Buffers
Iso- and hypotonic buffers (phosphate-buffered saline [PBS]) of osmolarities 260 and 93 mOsm (mOsmol/kg), conductivity 1.62 mS/cm and pH 7.2 were used (Table 1) in the experiments. The osmolarity of solutions was determined with a Knauer vapor pressure osmometer (K-7000; Knauer, Wissenschaftliche Gerätebau, Germany).
Electroporation
On the day of the experiment, cell suspension was prepared with 0.25% trypsin/EDTA solution. Trypsin solution was removed, and 5 ml of the culture media was added. Cells were gently rinsed from the bottom with the use of a plastic pipette, and homogenous cell suspension was prepared. Wire electrodes (Pl/Ir = 90/10) with a 5-mm gap were used for the electroporation. The cell suspension prepared in cell culture media was counted and the concentration adjusted to 5 × 105 cells/ml for B16-F1 or 1 × 106 cells/ml for CHO. We plated 40-μl drops in the middle of 24 wells (three wells per parameter) with premarked electrode position, and they were incubated for 20 min. During the incubation period, cells adhered slightly to the plate surface but preserved their round shape. Cells were washed with 0.5 ml of isotonic buffer. Electroporation was performed 2 min after 350 μl of hypotonic buffer containing 10 mM propidium iodide was added. At that time, cells were close to their maximal size induced by hypotonic cell swelling, as described in our previous study, where the kinetics of the process were analyzed in detail (Usaj et al. 2009). Eight rectangular pulses (pulse duration 100 μs, repetition frequency 1 Hz) were used for the electroporation with different pulse amplitudes (from 0 [negative control] to 1,600 [positive control] V/cm, in 200-V/cm steps). No electric pulses were delivered for the negative control. Electroporation efficiency was determined spectrofluorometrically by means of propidium iodide uptake in a microplate reader (Infinite M200; Tecan, Männedorf, Switzerland) at 535 nm excitation and 617 nm emission wavelength 3 min after pulse application. The percentage of permeabilized cells was calculated. The value obtained from negative control was subtracted from the value of treated sample and divided by that from positive control. The average values (±SD) for given pulse amplitudes were calculated on the basis of at least three independent experiments. Differences in electroporation efficiency between cell lines for each electric field amplitude were tested using independent samples t-test (SPSS Statistics; SPSS, Inc., Chicago, IL).
Electrofusion
Florescence microscopy was used for the detection and quantification of fused cells. For this purpose, cells were stained with two different cell tracker dyes. Cells, which were grown in two 25-cm2 culture flasks, were washed with 3 ml bicarbonate-free Krebs-HEPES buffer (Salvi et al. 2002). Cells in one flask were stained with green CMFDA (excitation/emission = 492 nm/517 nm), while cells in the other flask were stained with red CMRA (excitation/emission = 548 nm/576 nm). Loading solutions (7 μM) were prepared by mixing 2.1 μl of each stock solution with 3 ml of bicarbonate-free Krebs-HEPES buffer and incubating at 37°C for 30 min. After 30 min, the loading solutions were replaced with culture media and cells were maintained in the incubator for another 2 h. Cells were then trypsinized and mixed together in a 1:1 ratio in order to obtain an equal mixture of green and red cells.
Close cell–cell contacts were established by means of a modified adherence method (Fig. 1). Thus, a cell concentration was determined which was suitable for establishing a monolayer of spherical cells in close contact. These concentrations were 5 × 105 and 106 cells/ml for B16-F1 and CHO cells, respectively. Cell suspension (1 ml) was plated into a 24-well multiplate (TPP) and incubated for 20 min. During the incubation period, cells adhered slightly to the plate surface and formed spontaneous contacts but still preserved their round shape. In order to be able to confirm that hypotonic treatment improved fusion yield in our experimental conditions, separate experiments of electrofusion in isotonic and hypotonic buffers were performed. Experiments were performed only on B16-F1 cells using different electric field amplitudes for hypotonic (800 and 1,200 V/cm) and isotonic (920 and 1,380 V/cm) buffers in order to induce the same transmembrane voltages (see Eq. 1 and Table 2) in both buffers. Experiments were performed in the same way as described below for electrofusion of B16-F1 and CHO cells in hypotonic buffer. Before electroporation, cells were washed with isotonic buffer and 350 μl of hypotonic (or isotonic) buffer was added. Two minutes later, electric pulses were delivered with an electric pulse generator (Cliniporator; IGEA, Carpi, Italy) using two parallel wire electrodes (Pl/Ir = 90/10) with a 5-mm gap. Electrical field amplitudes for electrofusion in hypotonic buffer were determined in previous electroporation experiments: 800, 1,200 and 1,600 V/cm corresponding to 400, 600 and 800 V, respectively. After delivery of pulses, cells were left undisturbed for 10 min in order for the cell fusion to take place. Thereafter, fusion yield was determined.
Fusion yield was determined with the help of double color fluorescence microscopy (Jaroszeski et al. 1995) and the use of two emission filters, one at 535 nm (HQ535/30m, for CMFDA) and the other at 510 nm (D510/40m, for CMRA), both manufactured by Chroma (Brattleboro, VT), and a monochromator (Polychrome IV; Visitron, Puchheim, Germany). Cells were observed under an inverted microscope (Axiovert 200; Zeiss, Oberkochen, Germany) with 20× objective magnification. Three images (phase contrast, red and green fluorescence) were acquired from five randomly chosen fields in each well, using a cooled CCD video camera (VisiCam 1280, Visitron) and PC software MetaMorph 7.1 (Molecular Devices, Palo Alto, CA).
Image-processing software ImageJ (NIH Image, Bethesda, MD) was used to create three channel images (Fig. 2) from each image triplet (phase contrast, red and green fluorescence). Cells were manually counted for each image, while the fusion efficiency was calculated as a percentage of double labeled cells (Ndouble/Ntotal × 100). Electrofusion efficiency is presented as an average value (±SD) for a given cell line and amplitude obtained from at least four independent experiments.
Three channel microscopic image (red, green, phase contrast) of B16-F1 cells 10 min after being exposed to 1,600 V/cm. Cells were stained with CMRA (red cytoplasm) and CMFDA (green cytoplasm). Overlapping of the two colors and phase-contrast imaging result in easier detection of double labeled fused cells (arrows)
The hypotonic treatment used in our experiments induces swelling of the treated cells. In order to fairly compare fusion efficiency between B16-F1 and CHO cells, the maximum induced transmembrane voltage was calculated with the help of Eq. 1. Since cell size affects the maximum induced transmembrane voltage and putatively the efficiency of electroporation, the cell radii for B16-F1 and CHO was calculated before and 2 min after hypotonic treatment. Thirty-five cells from four independent experiments were measured with phase-contrast microscopy at 40× objective magnification, as described in detail in our previous study (Usaj et al. 2009). Independent samples t-test was used to determine the difference in cell sizes between B16-F1 and CHO.
The fusion efficiency obtained at different electric field amplitudes for a given cell line was statistically tested using one-way ANOVA, with Bonferroni’s post hoc test (SPSS Statistics). Observed differences between cell lines at the same amplitudes were statistically tested using independent samples t-test.
Cell Viability
The viability of hypotonically treated and electroporated cells was analyzed in a separate set of experiments. Viability was determined 24 h after electroporation experiments with the use of a modified crystal violet (CV) viability assay (Gillies et al. 1986). Cell suspension was prepared as described above. Aliquots of cell suspension in culture medium of 5 × 105 cells for B16-F1 and 106 cells for CHO were prepared and centrifuged (290×g, 5 min, 4°C). Supernatant was carefully removed, and cells were resuspended in 1 ml of hypotonic buffer or culture medium for control. Two minutes after hypotonic buffer was added, 800 μl of cell suspension was electroporated. Amplitudes of the electric field were the same as for the electrofusion experiments. No pulses were delivered during the control treatment. After electroporation, cells were kept at room temperature for 10 min. We plated 105 B16-F1 cells or 2 × 105 CHO cells from each parameter in a microplate well (24-well microplates, TPP) in three replicates. Cells were incubated for 24 h.
Then, the culture medium was removed and cells were washed with isotonic buffer. Cells were stained with 0.1% CV solution. After 30 min, dye was carefully removed and cells were lysed by 10% acetic acid. Absorption of lysate was measured with a microplate reader (Infinite M200, Tecan) at 595 nm wavelength, while cell viability was determined as described before (Castro-Garza et al. 2007; Golzio et al. 1998; Usaj et al. 2009). The average values (±SD) for a given cell line were calculated from at least three independent experiments. Differences between electroporated and nonelectroporated (control) cells were tested by the paired samples t-test (SPSS Statistics).
Results
Electroporation
Electroporation of cells was performed 2 min after cells were transferred from isotonic to hypotonic buffer, when they were close to their maximal size induced by hypotonic cell swelling, as described in our previous study (Usaj et al. 2009). Table 2 shows how cell-size changes produced by hypotonic treatment are presented. The difference in cell radii of B16-F1 and CHO cells in suspension in isotonic buffer was statistically significant (P < 0.05), while in hypotonic buffer the difference was reduced and was no longer statistically significant.
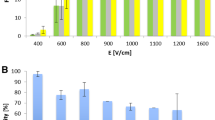
During further experiments, the electroporation of both cell lines in hypotonic buffer was studied. The results are presented in Fig. 3. No statistically significant differences in electroporation efficiency at any of the applied electric field amplitudes were found between B16-F1 and CHO cells. More than 70% of cells were permeabilized at 800 V/cm, while at 1,000 V/cm >92% of cells were permeabilized.
Electroporation efficiency determined with propidium iodide uptake: Cells were electroporated 2 min after hypotonic buffer was added with a train of pulses (8 × 100 μs, 1 Hz) applied at different electric field amplitudes. Each data point represents the average value ± SD of at least four independent experiments
Three different pulse amplitudes for electrofusion experiments in hypotonic buffer were selected from electroporation experiments: 800 V/cm, where 70% permeabilization was observed, 1,200 and 1,600 V/cm, where the permeabilization reached a plateau value.
Electrofusion
Electrofusion was performed on cells in close contact established by the modified adherence method. Electric pulses were delivered 2 min after cells were exposed to isotonic or hypotonic buffer. The data obtained from comparison of electrofusion yield in isotonic and hypotonic buffers for B16-F1 cells confirmed that hypotonic treatment improved fusion yield in our experimental conditions (Table 3). Therefore, all further experiments were performed in hypotonic buffer.
Figure 4 shows cell fusion in hypotonic buffer 10 min after electric pulse application; cytoplasm of fused cells is not completely merged, but fused cells (indicated by arrows) can be clearly identified. Three channel images (both fluorescence and phase contrast) make manual counting of fused cells easier.
Three channel microscopic images of B16-F1 (left) and CHO (right) cells fused at 1,200 and 1,600 V/cm, respectively. Cells were stained with CMRA (red cytoplasm) and CMFDA (green cytoplasm). Images were captured 10 min after electric pulse treatment under 20× objective magnification. In order to keep images clearer, only some fused cells are marked with arrows
Figure 5a presents the percentage of double labeled cells. The results show that electrofusion yield increased with the increased electric field amplitude and reached a maximum of 14% for B16-F1 and 6% for CHO. In comparison to CHO cells, higher fusion yield was obtained in electrofusion of B16-F1 cells, even when they were exposed to lower maximum induced transmembrane voltage. As shown in Table 4, 12% of double labeled cells were detected for B16-F1 at maximum induced transmembrane voltage 1.68 V, while at 1.84 V only 6% of double labeled CHO cells were detected. However, it should be emphasized that only half of the fused cells can be detected with the double color approach.
Electrofusion efficiency (a) and viability (b) of B16-F1 and CHO cell lines for different electric field amplitudes. The increase of electric field amplitude improved electrofusion efficiency of each cell line, whereas cell viability was significantly reduced. Asterisks represent statistically significant differences (* P < 0.05, ** P ≤ 0.001). Columns represent the average value ± SD of at least four independent experiments
In addition to high electrofusion efficiency, cell viability needed to be preserved in order to produce viable fused cells. If the hypotonic treatment was used in combination with electroporation, we had to determine how those two stressful treatments affect cell viability. The results show that the survival rate of B16-F1 cells was significantly lower at 800 V/cm and only 53% of the treated cells survive. However, the treatment did not have such a drastic effect on the survival of CHO cells since the survival rate was 63% (Fig. 5b) even at 1,600 V/cm.
Discussion
The aim of the present study was to optimize the parameters for electrofusion in hypotonic buffer for two different cell lines: mouse melanoma (B16-F1) and CHO. Hypotonic treatment induces an increase in cell size; therefore, the electroporation efficiency of cells exposed to hypotonic buffer was determined, and from these experiments three pulse amplitudes for electrofusion were selected. Contacts between cells required for efficient cell fusion were obtained by a modification of the adherence method.
In the first part of the study electroporation of cells in hypotonic buffer was determined. Significant differences in the size of B16-F1 and CHO cells presented in isotonic buffer disappeared after hypotonic treatment (Table 2). Cell size dynamic in hypotonic buffer was found to be cell line–dependent (Usaj et al. 2009). In comparison to B16-F1 cells (dmax/d0 = 1.18), CHO cells, which are in isotonic buffer smaller than B16-F1 cells, increase their average size to a greater extent (dmax/d0 = 1.29). Even though only cell sizes were measured, we were aware that the growth caused by water influx is not the only consequence of the hypotonic treatment. Hypotonic treatment induces many other responses in the cell accompanied by loss of ions and organic osmolytes, production of reactive oxygen species (ROS), cytoskeletal rearrangement and changes in enzyme activity (Lambert 2007). However, it seems that these changes do not affect electroporation of the cell lines studied. Experiments where cells were electroporated 2 min after hypotonic treatment, when they were close to their maximal size, showed that both cell lines were permeabilized at the same electric field amplitude (Fig. 3). Comparison of electroporation in isotonic buffer (Kanduser et al. 2006) with electroporation in hypotonic buffer suggests that the major factor affecting electroporation of cells is their size since the same level of electroporation was observed at the same induced transmembrane voltage for both buffers. The results confirm those of previous studies, where authors reported that the increase of cell size, induced by hypotonic treatment, resulted in lowering of the electric field strength needed for electroporation (Barrau et al. 2004; Kotnik et al. 1997; Neumann et al. 1989; Zimmermann et al. 2000).
The second part of the study determined the electrofusion efficiency for both cell lines. Electroporation is a prerequisite for effective electrofusion; therefore, it is important to use such electric pulse parameters, where sufficient permeabilization is achieved (Teissie and Ramos 1998; Zimmermann 1982). Three different electric field amplitudes were selected: 800 V/cm, where 70% permeabilization was observed, 1,200 and 1,600 V/cm, where the permeabilization reached a plateau value. It was confirmed that in the experimental conditions higher electrofusion efficiency was achieved when hypotonic buffer was used instead of the isotonic one (Table 3). One of the reasons for the effect of hypotonic buffer on electrofusion could be the increase in cell surface area and unfolding of undulations and invaginations of the cell membrane (Knutton et al. 1976; Sukhorukov et al. 1993), resulting in a decrease of repulsive forces and, consequently, better cell–cell contacts (Evans and Parsegian 1986; Golzio et al. 1998; McIntosh et al. 1995, 1999; Rols and Teissie 1990). Some authors suggest that osmotic pressure caused by hypotonic treatment is the main driving force for efficient cell fusion (Ahkong and Lucy 1986), while others give more emphasis to biological factors. According to them, electrofusion in hypotonic buffer provokes dissolution of the cytoskeleton and increases mobility of membrane components, resulting in improved fusion yield (Neil and Zimmermann 1993; Schmitt and Zimmermann 1989; Sukhorukov et al. 2006; Zimmermann and Neil 1996).
Even though further studies are needed to understand the biological factors involved in electrofusion, these results are important for optimization of the process since the method, which results in good fusion yield, will help in investigating the mechanism underlying cell electrofusion. The fusion yields obtained in this study (Fig. 5a) are comparable to other published results (Gabrijel et al. 2009; Gottfried et al. 2002; Scott-Taylor et al. 2000; Sukhorukov et al. 2006). Even though in some of the studies the authors reported relatively high fusion efficiency, up to 25–35% (Lee et al. 2005; Ramos et al. 2002), these differences can be attributed to the method used for quantification of cell fusion. The high rates were determined by flow cytometry, which gives higher values of fused cells than can be obtained by microscopy. Overestimations of fusion yield obtained by flow cytometry are attributed to aggregated cells and in some cases to inappropriate color compensation (Gabrijel et al. 2004; Gong et al. 2000; Hayashi et al. 2002; Stuhler and Walden 1994). A review of the published literature showed that different authors use different electrofusion protocols, detection methods and quantifications of fused cells (Gabrijel et al. 2008; Jaroszeski et al. 1994; Ramos et al. 2002; Scott-Taylor et al. 2000; Wang and Lu 2006); therefore, the results of different studies cannot be directly compared.
In addition, in these studies different cell lines were used, which is a factor affecting electrofusion efficiency (Mekid and Mir 2000; Salomskaite-Davalgiene et al. 2009; Yu et al. 2008). Fusion yield is related to biological characteristics of the treated cells, such as integrity of the cytoskeleton, mitochondrial activity and membrane composition (Blangero et al. 1989; Neumann et al. 1989; Ohno-Shosaku and Okada 1985; Sowers 1989; Sukharev et al. 1990; Urano et al. 1998; Zheng and Chang 1991). These biological characteristics affect either electroporation, which induces cell fusion, or electrofusion itself. The results from this study show that the differences in electrofusion between B16-F1 and CHO cells are not caused by electroporation since similar electroporation efficiency was obtained with the same electric field amplitude (Fig. 3). Nevertheless, it was observed that B16-F1 cells are more fusogenic than CHO cells (Fig. 5a, Table 4). These results confirm that even though optimal cell electroporation is necessary, it is not the only condition determining fusion efficiency (Salomskaite-Davalgiene et al. 2009).
For the use of electrofusion in biotechnological applications, high fusion efficiency is not the only condition that needs to be fulfilled because only viable fused cells can perform highly specific tasks, such as production of monoclonal antibodies and stimulation of the immune system. Hybridoma colony counting is still the most accurate and reliable method of evaluation for a new fusion strategy (Foung et al. 1990; Schmitt and Zimmermann 1989; Sukhorukov et al. 2005). Nevertheless, first steps for optimization of the electrofusion strategy can be made with other less accurate and reliable but faster tests as well. Therefore, this study used a fast viability test to determine the effect of hypotonic treatment and electroporation parameters on cell survival. We observed that B16-F1 and CHO cells differ in their response to this electrofusion protocol. In B16-F1, cell viability was drastically lower (Fig. 5b) for the amplitudes of the electric field where the maximum fusion efficiency (Fig. 5a) was achieved, while CHO viability was not significantly affected by increasing the electric field amplitude (Fig. 5b). These results indicate that B16-F1 cells were more susceptible to the combination of hypotonic treatment and electric pulse application. Other authors obtained similar results, even though they used a different viability test and/or monitored cell survival at different time intervals after treatment (Gottfried et al. 2002; Jantscheff et al. 2002; Ramos et al. 2002; Scott-Taylor et al. 2000; Wang and Lu 2006). However, cell viability could be improved by using a smaller number of shorter electric pulses and by adjusting buffer osmolarity. Previous studies showed that sufficient electroporation can be obtained with different pulse parameters and that cell survival can be preserved by reducing the number and duration of electric pulses (Macek-Lebar and Miklavcic 2001).
Another contribution of this study is the description of the modified adherence method for establishing spontaneous contacts between cells. Instead of using confluent cell culture, as described in some published studies (Finaz et al. 1984; Salomskaite-Davalgiene et al. 2009; Sukharev et al. 1990; Teissie and Blangero 1984; Teissie et al. 1982), contacts are achieved by plating cells in suitable concentration and allowing them to form contacts while their shape remains spherical (Fig. 1). With plating time of approximately 20 min, experiments can be performed faster and the cell membrane geometry, which might affect cell fusion (Chen et al. 2007; Martens and McMahon 2008), remains the same as when experiments are performed on cell suspension. Therefore, the results from this study can be compared with other methods, where cell suspensions are used. In addition, the number of cells that fuse into one cell is lower (Fig. 2) than in the original adherent method, while fusion efficiency is comparable. Hybrid cells with lower numbers of nuclei have a better chance of surviving and dividing. Thus, fused cells produced with the modified adherence method are also potentially more efficient.
To conclude, hypotonic treatment and electric pulse parameters that result in >70% of permeabilized cells can be successfully used for electrofusion. However, not only successful permeabilization but the biological characteristics of the cell line affect electrofusion efficiency and cell survival after electroporation in hypotonic buffer as well. That is why further studies are needed to improve cell survival and to determine which biological factors are involved in cell electrofusion. The cell contacts required for electrofusion can be obtained by a modification of the adherence method, resulting in spontaneous contact formation while cells preserve their spherical shape. The electrofusion efficiencies obtained are comparable to those from other more sophisticated and time-consuming methods.
References
Abe S, Takeda J (1988) Effects of La3+ on surface charges, dielectrophoresis, and electrofusion of barley protoplasts. Plant Physiol 87:389–394
Abidor IG, Barbul AI, Zhelev DV, Doinov P, Bandrina IN, Osipova EM, Sukharev SI (1993) Electrical properties of cell pellets and cell electrofusion in a centrifuge. Biochim Biophys Acta 1152:207–218
Ahkong QF, Lucy JA (1986) Osmotic forces in artificially induced cell fusion. Biochim Biophys Acta 858:206–216
Barrau C, Teissie J, Gabriel B (2004) Osmotically induced membrane tension facilitates the triggering of living cell electropermeabilization. Bioelectrochemistry 63:327–332
Blangero C, Rols MP, Teissié J (1989) Cytoskeletal reorganization during electric-field-induced fusion of Chinese hamster ovary cells grown in monolayers. Biochim Biophys Acta 981:295–302
Castro-Garza J, Barrios-Garcia HB, Cruz-Vega DE, Said-Fernandez S, Carranza-Rosales P, Molina-Torres CA, Vera-Cabrera L (2007) Use of a colorimetric assay to measure differences in cytotoxicity of Mycobacterium tuberculosis strains. J Med Microbiol 56:733–737
Cegovnik U, Novakovic S (2004) Setting optimal parameters for in vitro electrotransfection of B16F1, SA1, LPB, SCK, L929 and CHO cells using predefined exponentially decaying electric pulses. Bioelectrochemistry 62:73–82
Cemazar M, Jarm T, Miklavcic D, Macek-Lebar A, Ihan A, Kopitar NA, Sersa G (1998) Effect of electric-field intensity on electropermeabilization and electrosensitivity of various tumor-cell lines in vitro. Electromagn Biol Med 17:263–272
Chen EH, Grote E, Mohler W, Vignery A (2007) Cell–cell fusion. FEBS Lett 581:2181–2193
Escoffre J-M, Portet T, Wasungu L, Teissie J, Dean D, Rols M-P (2009) What is (still not) known of the mechanism by which electroporation mediates gene transfer and expression in cells and tissues. Mol Biotechnol 41:286–295
Evans EA, Parsegian VA (1986) Thermal–mechanical fluctuations enhance repulsion between bimolecular layers. Proc Natl Acad Sci USA 83:7132–7136
Finaz C, Lefevre A, Teissie J (1984) Electrofusion. A new, highly efficient technique for generating somatic cell hybrids. Exp Cell Res 150:477–482
Foung S, Perkins S, Kafadar K, Gessner P, Zimmermann U (1990) Development of microfusion techniques to generate human hybridomas. J Immunol Methods 134:35–42
Gabrijel M, Repnik U, Kreft M, Grilc S, Jeras M, Zorec R (2004) Quantification of cell hybridoma yields with confocal microscopy and flow cytometry. Biochem Biophys Res Commun 314:717–723
Gabrijel M, Kreft M, Zorec R (2008) Monitoring lysosomal fusion in electrofused hybridoma cells. Biochim Biophys Acta 1778:483–490
Gabrijel M, Bergant M, Kreft M, Jeras M, Zorec R (2009) Fused late endocytic compartments and immunostimulatory capacity of dendritic–tumor cell hybridomas. J Membr Biol 229:11–18
Gillies RJ, Didier N, Denton M (1986) Determination of cell number in monolayer cultures. Anal Biochem 159:109–113
Golzio M, Mora M-P, Raynaud C, Delteil C, Teissié J, Rols M-P (1998) Control by osmotic pressure of voltage-induced permeabilization and gene transfer in mammalian cells. Biophys J 74:3015–3022
Gong J, Avigan D, Chen D, Wu Z, Koido S, Kashiwaba M, Kufe D (2000) Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells. Proc Natl Acad Sci USA 97:2715–2718
Gottfried E, Krieg R, Eichelberg C, Andreesen R, Mackensen A, Krause SW (2002) Characterization of cells prepared by dendritic cell–tumor cell fusion. Cancer Immun 2:15
Hayashi T, Tanaka H, Tanaka J, Wang R, Averbook BJ, Cohen PA, Shu S (2002) Immunogenicity and therapeutic efficacy of dendritic–tumor hybrid cells generated by electrofusion. Clin Immunol 104:14–20
James K, Bell GT (1987) Human monoclonal antibody production. J Immunol Methods 100:5–40
Jantscheff P, Spagnoli G, Zajac P, Rochlitz C (2002) Cell fusion: an approach to generating constitutively proliferating human tumor antigen-presenting cells. Cancer Immunol Immunother 51:367–375
Jaroszeski MJ, Gilbert R, Fallon PG, Heller R (1994) Mechanically facilitated cell–cell electrofusion. Biophys J 67:1574–1581
Jaroszeski MJ, Gilbert R, Heller R (1995) Cytometric detection and quantitation of cell–cell electrofusion products. In: Nickoloff JA (ed) Animal cell electroporation and electrofusion protocols. Humana Press, Totowa, NJ, pp 355–363
Kanduser M, Sentjurc M, Miklavcic D (2006) Cell membrane fluidity related to electroporation and resealing. Eur Biophys J 35:196–204
Knutton S, Jackson D, Graham JM, Micklem KJ, Pasternak CA (1976) Microvilli and cell swelling. Nature 262:52–54
Kotnik T, Bobanovic F, Miklavcic D (1997) Sensitivity of transmembrane voltage induced by applied electric fields—a theoretical analysis. Bioelectrochem Bioenerg 43:6
Lambert IH (2007) Activation and inactivation of the volume-sensitive taurine leak pathway in NIH3T3 fibroblasts and Ehrlich Lettre ascites cells. Am J Physiol Cell Physiol 293:C390–C400
Lee WT, Shimizu K, Kuriyama H, Tanaka H, Kjaergaard J, Shu S (2005) Tumor–dendritic cell fusion as a basis for cancer immunotherapy. Otolaryngol Head Neck Surg 132:755–764
Macek-Lebar A, Miklavcic D (2001) Cell electropermeabilization to small molecules in vitro: control by pulse parameters. Radiol Oncol 35:10
Mally MI, McKnight ME, Glassy MC (1992) Protocols of electroporation and electrofusion for producing human hybridomas. In: Chang D, Chassy B, Saunders J, Sowers A (eds) Guide to electroporation and electrofusion. Academic Press, San Diego, pp 507–522
Martens S, McMahon HT (2008) Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol 9:543–556
Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M, Billard V, Geertsen PF, Larkin JO, Miklavcic D, Pavlovic I, Paulin-Kosir SM, Cemazar M, Morsli N, Rudolf Z, Robert C, O’Sullivan GC, Mir LM (2006) Electrochemotherapy—an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Suppl 4:3–13
Matibiri EA, Mantell SH (1995) Comparative effects of fusion facilitators on electrofusion attributes of N. tabacum mesophyll protoplasts. Plant Cell Tissue Organ Culture 40:125–131
McIntosh TJ, Advani S, Burton RE, Zhelev DV, Needham D, Simon SA (1995) Experimental tests for protrusion and undulation pressures in phospholipid bilayers. Biochemistry 34:8520–8532
McIntosh TJ, Kulkarni KG, Simon SA (1999) Membrane fusion promoters and inhibitors have contrasting effects on lipid bilayer structure and undulations. Biophys J 76:2090–2098
Mekid H, Mir LM (2000) In vivo cell electrofusion. Biochim Biophys Acta 1524:118–130
Miklavcic D, Towhidi L (2010) Numerical study of the electroporation pulse shape effect on molecular uptake of biological cells. Radiol Oncol 44:8
Mir LM (2009) Nucleic acids electrotransfer-based gene therapy (electrogenetherapy): past, current, and future. Mol Biotechnol 43:167–176
Mizukami Y, Kito H, Okauchi M (1993) Factors affecting the electrofusion efficiency of Porphyra protoplasts. J Appl Psychol 5:29–36
Neil GA, Zimmermann U (1993) Electrofusion. Methods Enzymol 220:174–196
Neumann E, Sowers AE, Jordan CA (1989) Electroporation and electrofusion in cell biology. Springer-Verlag, New York
O’Hare MJ, Ormerod MG, Imrie PR, Peacock JH, Asche W (1989) Electropermeabilization and electrosensitivity of different types of mammalian cells. In: Neumann E, Sowers AE, Jordan CA (eds) Electroporation and electrofusion in cell biology. Plenum Press, New York, pp 319–330
Ohno-Shosaku T, Okada Y (1985) Electric pulse-induced fusion of mouse lymphoma cells: roles of divalent cations and membrane lipid domains. J Membr Biol 85:269–280
Pucihar G, Miklavcic D, Kotnik T (2009) A time-dependent numerical model of transmembrane voltage inducement and electroporation of irregularly shaped cells. IEEE Trans Biomed Eng 56:1491–1501
Ramos C, Bonenfant D, Teissie J (2002) Cell hybridization by electrofusion on filters. Anal Biochem 302:213–219
Rols MP, Teissie J (1989) Ionic-strength modulation of electrically induced permeabilization and associated fusion of mammalian cells. Eur J Biochem 179:109–115
Rols MP, Teissie J (1990) Modulation of electrically induced permeabilization and fusion of Chinese hamster ovary cells by osmotic pressure. Biochemistry 29:4561–4567
Rubinsky B, Onik G, Mikus P (2007) Irreversible electroporation: a new ablation modality–clinical implications. Technol Cancer Res Treat 6:37–48
Salomskaite-Davalgiene S, Cepurniene K, Satkauskas S, Venslauskas MS, Mir LM (2009) Extent of cell electrofusion in vitro and in vivo is cell line dependent. Anticancer Res 29:3125–3130
Salvi A, Quillan J, Sadée W (2002) Monitoring intracellular pH changes in response to osmotic stress and membrane transport activity using 5-chloromethylfluorescein. AAPS J 4:21–28
Schmitt JJ, Zimmermann U (1989) Enhanced hybridoma production by electrofusion in strongly hypo-osmolar solutions. Biochim Biophys Acta 983:42–50
Schnettlera R, Zimmermann U (1992) Zinc ions stimulate electrofusion of Hansenula polymorpha protoplasts. FEMS Microbiol Lett 106:47–51
Scott-Taylor TH, Pettengell R, Clarke I, Stuhler G, La Barthe MC, Walden P, Dalgleish AG (2000) Human tumour and dendritic cell hybrids generated by electrofusion: potential for cancer vaccines. Biochim Biophys Acta 1500:265–279
Sowers AE (1986) A long-lived fusogenic state is induced in erythrocyte ghosts by electric pulses. J Cell Biol 102:1358–1362
Sowers AE (1989) Evidence that electrofusion yield is controlled by biologically relevant membrane factors. Biochim Biophys Acta 985:334–338
Stenger DA, Kubiniec RT, Purucker WJ, Liang H, Hui SW (1988) Optimization of electrofusion parameters for efficient production of murine hybridomas. Hybridoma 7:505–518
Stenger DA, Kaler KV, Hui SW (1991) Dipole interactions in electrofusion. Contributions of membrane potential and effective dipole interaction pressures. Biophys J 59:1074–1084
Stevens RH, Macy E, Morrow C, Saxon A (1979) Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol 122:2498–2504
Stuhler G, Walden P (1994) Recruitment of helper T cells for induction of tumour rejection by cytolytic T lymphocytes. Cancer Immunol Immunother 39:342–345
Sukharev SI, Bandrina IN, Barbul AI, Fedorova LI, Abidor IG, Zelenin AV (1990) Electrofusion of fibroblasts on the porous membrane. Biochim Biophys Acta 1034:125–131
Sukhorukov VL, Arnold WM, Zimmermann U (1993) Hypotonically induced changes in the plasma membrane of cultured mammalian cells. J Membr Biol 132:27–40
Sukhorukov VL, Reuss R, Zimmermann D, Held C, Müller KJ, Kiesel M, Geßner P, Steinbach A, Schenk WA, Bamberg E, Zimmermann U (2005) Surviving high-intensity field pulses: strategies for improving robustness and performance of electrotransfection and electrofusion. J Membr Biol 206:187–201
Sukhorukov VL, Reuss R, Endter JM, Fehrmann S, Katsen-Globa A, Gessner P, Steinbach A, Muller KJ, Karpas A, Zimmermann U, Zimmermann H (2006) A biophysical approach to the optimisation of dendritic–tumour cell electrofusion. Biochem Biophys Res Commun 346:829–839
Teissie J, Blangero C (1984) Direct experimental evidence of the vectorial character of the interaction between electric pulses and cells in cell electrofusion. Biochim Biophys Acta 775:446–448
Teissie J, Ramos C (1998) Correlation between electric field pulse induced long-lived permeabilization and fusogenicity in cell membranes. Biophys J 74:1889–1898
Teissie J, Rols MP (1986) Fusion of mammalian cells in culture is obtained by creating the contact between cells after their electropermeabilization. Biochem Biophys Res Commun 140:258–266
Teissie J, Knutson VP, Tsong TY, Lane MD (1982) Electric pulse-induced fusion of 3T3 cells in monolayer culture. Science 216:537–538
Trontelj K, Rebersek M, Kanduser M, Serbec VC, Sprohar M, Miklavcic D (2008) Optimization of bulk cell electrofusion in vitro for production of human–mouse heterohybridoma cells. Bioelectrochemistry 74:124–129
Urano N, Higashikawa R, Hirai H (1998) Effects of mitochondria on electrofusion of yeast protoplasts. Enzyme Microbial Technol 23:107–112
Usaj M, Trontelj K, Hudej R, Kanducer M, Miklavcic D (2009) Cell size dynamics and viability of cells exposed to hypotonic treatment and electroporation for electrofusion optimization. Radiol Oncol 43:108–119
Vienken J, Zimmermann U (1985) An improved electrofusion technique for production of mouse hybridoma cells. FEBS Lett 182:278–280
Wang J, Lu C (2006) Microfluidic cell fusion under continuous direct current voltage. Appl Physics Lett 89:234102–234103
Wu Y, Montes JG, Sjodin RA (1992) Determination of electric field threshold for electrofusion of erythrocyte ghosts. Comparison of pulse-first and contact-first protocols. Biophys J 61:810–815
Yu X, McGraw PA, House FS, Crowe JE Jr (2008) An optimized electrofusion-based protocol for generating virus-specific human monoclonal antibodies. J Immunol Methods 336:142–151
Zheng Q, Chang DC (1991) Reorganization of cytoplasmic structures during cell–cell fusion. J Cell Sci 100:431–442
Zimmermann U (1982) Electric field–mediated fusion and related electrical phenomena. Biochim Biophys Acta 694:227–277
Zimmermann U, Neil GA (1996) Electromanipulation of cells. CRC, Boca Raton, FL
Zimmermann U, Friedrich U, Mussauer H, Gessner P, Hamel K, Sukhoruhov V (2000) Electromanipulation of mammalian cells: fundamentals and application. IEEE Trans Plasma Sci 28:72–82
Acknowledgements
This research was supported by the Slovenian Research Agency (ARRS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ušaj, M., Trontelj, K., Miklavčič, D. et al. Cell–Cell Electrofusion: Optimization of Electric Field Amplitude and Hypotonic Treatment for Mouse Melanoma (B16-F1) and Chinese Hamster Ovary (CHO) Cells. J Membrane Biol 236, 107–116 (2010). https://doi.org/10.1007/s00232-010-9272-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-010-9272-3