Abstract
This study analyzes changes in the distribution, electrophysiological properties, and proteic composition of voltage-gated sodium channels (NaV) in cultured adult rat skeletal muscle fibers. Patch clamp and molecular biology techniques were carried out in flexor digitorum brevis (FDB) adult rat skeletal muscle fibers maintained in vitro after cell dissociation with collagenase. After 4 days of culture, an increase of the NaV1.5 channel type was observed. This was confirmed by an increase in TTX-resistant channels and by Western blot test. These channels exhibited increased activation time constant (τm) and reduced conductance, similar to what has been observed in denervated muscles in vivo, where the density of NaV1.5 was increasing progressively after denervation. By real-time polymerase chain reaction, we found that the expression of β subunits was also modified, but only after 7 days of culture: increase in β1 without β4 modifications. β1 subunit is known to induce a negative shift of the inactivation curve, thus reducing current amplitude and duration. At day 7, τh was back to normal and τm still increased, in agreement with a decrease in sodium current and conductance at day 4 and normalization at day 7. Our model is a useful tool to study the effects of denervation in adult muscle fibers in vitro and the expression of sodium channels. Our data evidenced an increase in NaV1.5 channels and the involvement of β subunits in the regulation of sodium current and fiber excitability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Voltage-dependent Na channels (NaV) play a key role in cellular excitability. Thus, the balance between activation and inactivation of NaV is crucial for skeletal muscle contraction (Catterall et al. 2005; Goldin 2003). Many data (Dennis and Dow 2007; Desaphy et al. 2001; Fitts et al. 2001; Rossignol et al. 2008) exist concerning modifications in vivo of the functional properties of muscle (force, shortening velocity, and power) and the possible cellular mechanisms corresponding to the observed changes after muscle adaptation (Dennis and Dow 2007). Furthermore, some pathologies (immobilization, sepsis) involving alterations in NaV gating lead to reduce performance and weakness of skeletal muscle (Rossignol et al. 2008).
The NaV channel gene family contains multiple members (NaV1.1–NaV1.9, NaVX), which are grouped according to their sensitivity to tetrodotoxin (TTX). They are separated into TTX-sensitive and TTX-resistant channels (Catterall et al. 2005; Yang et al. 1991). NaV1.4 is classically the major pore-conducting subunit in skeletal muscle. However, it was recently found that phenotype changes in NaV channels during myogenesis. The decrease of the TTX-resistant NaV1.5 isoform reflects a normal process of fiber maturation. This α subunit has been now cloned from a number of species (Kallen et al. 1990; Noda et al. 1984, 1986; Rogart et al. 1989; Zwerling et al. 1991). Although NaVα are sufficient to form functional channels when expressed alone, the presence of regulatory β subunits (NaVβ) fine-tunes the channel activity.
In most of the tissues, four β subunits have been described (NaVβ1–4) (David et al. 2008) for which isoforms have been cloned: β1 (Isom et al. 1992), β2 (Isom et al. 1995a), β3 (Morgan et al. 2000), and β4 (Yu et al. 2003). The mechanisms by which the different auxiliary NaVβ subunits act to modulate channel functions are still under investigation. For example, the properties of cardiac Na channel α subunits (NaV1.5) are modulated by β1 and β3 in heterologous expression systems (Goldin 2001), whereas β2 is thought to have no functional role in the heart. Similarly, in Chinese hamster ovary cells, β subunits modulate NaV1.5 gating with important differences between coexpression of β1 and β3 alone and β1/β3 together (Ko et al. 2005). Most of the performed studies of NaVβ subunits role are realized in transfected cells that coexpressed α and β subunits: mammalian cells (Isom et al. 1995b; Qu et al. 2001), Xenopus oocytes (Morgan et al. 2000), or a myoblast cell line used in order to study β regulation during myogenesis (David et al. 2008). However, this role is still unknown in adult skeletal muscle.
Furthermore, in vivo denervation of muscle induces expression of a juvenile form of sodium channels, namely NaV1.5, in addition to the adult isoform of the sodium channel NaV1.4 (Lupa et al. 1995; Rich et al. 1999). NaV1.5 is relatively resistant to TTX and exhibits a shallower voltage dependence of fast inactivation (Filatov and Rich 2004) and slow inactivation, which could account for fiber hyperexcitability (Kallen et al. 1990; Pappone 1980).
A model of adult cultured fibers is a major way to study α- and β-subunit expression of NaV channels independently of influence of nerve and to find out how denervation-induced alterations in NaV expression could be related to sodium current and muscle excitability. In the literature, in vitro data on striated fibers are extrapolated from myotubes obtained by fusion of satellite cells or by a skeletal muscle cell line (Zebedin et al. 2004), but such experimental models are more appropriate to study the mechanisms of muscle cellular differentiation.
Concerning adult fibers, sodium channels have been extensively studied just after dissociation (Lupa et al. 1995; Ruff 1992), but there is a lack of information for fibers in culture for a longer time (Brown and Schneider 2002; Cifelli et al. 2007). Bekoff and Betz (1977) described for the first time a technique for isolating rat muscle fibers and maintaining fibers viable up to 16 days in culture. Nevertheless, the effect of in vitro maintenance on NaV α- and β-subunit repartition in denervated fibers is unknown. We thus sought to follow the evolution of NaV (α and β subunits) in adult muscle fibers maintained for a week in culture by measuring mRNA and protein levels and, in parallel, sodium channel electrophysiological characterization by patch clamp.
Material and Methods
Dissociation and Culture of Muscle Fibers
All experiments were authorized by a departmental agreement (no. B29-019-4) and were carried out in accordance with the recommendations of our ethical regional committee and of the European Community (no. 86/609). Adult female Wistar rats (body weight 200–220 g, age 2 months; Centre d’élevage Dépré, Saint-Doulchard, France) were killed by cervical dislocation and exsanguination. Fast-twitch flexor digitorum brevis (FDB) were rapidly excised from rats and placed in HEPES-buffered physiological solution containing 3.0 mg ml−1 of a commercial type of collagenase, liberase III (Blendzyme III; Roche). The FDB were placed for 3 h at 37°C for enzymatic dissociation. After this incubation period, dissociated fibers were transferred to 35-mm petri dishes in Dulbecco modified Eagle medium (Sigma) supplemented with 10% fetal calf serum, 10−2 mol l−1 HEPES, 4.5 g l−1 glucose, pH 7.3, 300 mOsm, penicillin 50 IU ml−1 and streptomycin 50 μg ml−1. Every 2 days, fibers were transferred in new dishes to eliminate satellite cells attached to the plastic.
The fluorescein diacetate test (40 μM; Sigma) was used to assess viability of fibers after 1, 4, and 7 days in culture with a fluorescence microscope (excitation filter 470–490 nm, emission filter 510–560 nm).
Patch Clamp
As previously reported (Rannou et al. 2009), fast sodium currents were recorded in cell-attached configuration with the macropatch clamp technique at room temperature (22 ± 2°C). A GeneClamp 500B amplifier equipped with a CV5-1GU head stage (Axon Instruments, Foster City, CA) was used. Pipettes had resistance averaging 2 MΏ when filled with the standard saline solution (150 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 30 mM HEPES; pH 7.4). Voltage-clamp protocols and data acquisition were performed with WinWCP V4.2 (Whole Cell program; University of Strathclyde, Glasgow, Scotland, UK). Currents were low-pass filtered at 5 kHz and digitized at 48 kHz.
Because sodium channel density is highly variable near the end plate (Ruff 1992), sodium currents were recorded at a site located at more than 200 μM from the end plate. This could be visualized with phase contrast under an inverted microscope (Olympus IX 70) and with a progressive-scan digital camera (Donpisha; Sony, Japan). The fibers were placed in a bath recording solution containing Cs+ as the main cation (145 mM CsCl, 5 mM EGTA [ethylene glycol-bis(β-aminoethylether)-N,N,N′,N′-tetra-acetic acid], 1 mM MgCl2, 10 mM HEPES, pH 7.3) to inhibit potassium currents and to depolarize the membrane. To eliminate the residual capacitance transient and the leak current, we used the P/4 subtraction procedure.
Current–Voltage Relationship and Determination of Maximal Conductance
The holding potential was set to −100 mV. The current–voltage relationship was measured by applying to the patch membrane a cycle of 20-ms test pulses from the holding potential to increasing potentials (from −60 to +130 mV in 10-mV increments). The interval between each test pulse was long enough (3 s) to allow the complete recovery of the sodium channel from inactivation. This protocol was repeated three times for each patch to ensure sodium current stability. The patches with no reliable peak current amplitude were discarded. I max corresponds to the peak sodium current in nA. The maximal sodium conductance (g Na max) was given by the slope of the quasi-linear part of the current–voltage relationship (I/V curve). TTX (Alomone Lab, Israel) at the concentration of 300 nM, was used according to a previously described protocol (Filatov and Rich 2004) to evidence NaV1.5 current by blocking NaV1.4 channels.
Activation Determination
The activation curve was obtained by plotting g Na/g Na max as a function of imposed membrane potential. g Na max was calculated as reported here above; g Na was calculated for each imposed potential from −60 to +30 mV, according to the following relation: g Na = I Na/(V m − V Na) where I Na is the sodium current, V m the membrane potential, and V Na the equilibrium potential for sodium, determined from the current–potential curve. Calculated values of g Na/g Na max were fitted with the Boltzmann equation,
where V a1/2 is the potential at which half of the channels are activated and Ka is the slope factor.
Time Constant Determination
The activation and inactivation constants of the sodium currents (τm and τh, respectively) were calculated according to Hodgkin-Huxley relation:
where A indicates the voltage-depending part of the current, p is the exponent of m (around 3), h inf is the equilibrium value reached by h according to the potential, and t is time in milliseconds.
Fast Inactivation Determination
Steady-state fast inactivation was measured by applying 50-ms conditioning prepulses of various holding potentials from −120 to +10 mV, followed by a 20-ms test pulse up to −20 mV to activate Na+ current. To calculate the slope factor K h and half-inactivation voltage V h1/2, the steady-state fast inactivation relationships were fitted with the Boltzmann equation:
where I Na is the sodium current and I Na max the maximal sodium current.
Sodium Channel Immunocytochemistry
Fibers were preincubated in 0.1 M phosphate-buffered saline (PBS), pH 7.4, containing 0.3% Triton X-100 and 10% goat serum (solution A) for 2 h at room temperature. For channel labeling, fibers were exposed to anti-skeletal muscle type 1 sodium channel (SkM1) at a dilution of 1:50 in solution A for overnight at 4°C. The monoclonal antibody recognizes an intracellular epitope of the TTX-S muscle NaCh (SP19, S6936, Sigma). After overnight incubation, fibers were treated with a secondary goat anti-mouse IgG Fc specific FITC conjugated (1:200) for 1 h at room temperature. The samples were rinsed 3 times with 0.1 M PBS, pH 7.4 containing 0.1% Triton X-100 between incubations.
Observations were made under a Olympus fluorescence microscope and a laser scanning confocal instrument (Leica Microsystems).
Reverse Transcription–Real-time Polymerase Chain Reaction
Real-time polymerase chain reaction (PCR) has been already reported (Rossignol et al. 2008). Briefly, RNA was extracted using TriZol reagent. To minimize DNA contamination, eluted RNA solutions were treated with DNAse. Reverse transcription reaction was realized with 1 μg of total RNA. For real-time PCR, all oligonucleotide primers were designed from published sequence (Fig. 5a). GAPDH (d-glyceraldehyde 3-phosphate dehydrogenase) and β2 microglobulin were selected as controls. The specificity of forward and reverse primers was verified on the Infobiogen Web site (http://www.infobiogen.fr/). All real-time PCR assays were conducted with ABI Prism 7000 SDS (Applied Biosystems, Foster City, CA) 7000 System software version 1.2 (Applied Biosystems) with designed primers. For each sample, 2 μl of cDNA was combined with 12.5 μl of Power SYBR Green PCR master mix (Applied Biosystems) and 10 pmol of each primer, then adjusted to 25 μl by adding RNAse-free water. The PCR assay was carried out in duplicate for each sample. The reactions were carried out with standard conditions as follows: 50°C for 2 min and 95°C for 10 min, then 40 cycles consisting of 95°C for 15 s and 60°C for 1 min.
In real-time PCR, a fluorescent dye (SYBR Green) is added to the medium. SYBR Green, a DNA-binding dye, emits fluorescence when incorporated into double-strand DNA. At the end of each PCR cycle, fluorescence is measured. Ct corresponds to the fluorescence threshold appearance and is proportional to mRNA content. Each Ct of the studied mRNA is normalized by reference to housekeeping genes to obtain ΔCt (ΔCt = Ct target gene − Ct housekeeping gene). For each increase of ΔCt of 1, the amount of mRNA is divided by 2. Determinations of the relative gene expression levels were made using the ΔΔCt method as described by Gahr et al. (2004). ΔΔCt allows comparison between two conditions for the studied mRNA, ΔΔCt = ΔCt condition 1 − ΔCt condition 2. The difference of mRNA content R between two conditions is given by the formula R = 2−ΔΔCt.
Protein Content and Immunoblotting of NaV Channel
Isolated fibers were homogenized in buffer with 100 mM choline chloride, 50 mM phosphate potassium, 0.5 mM calcium chloride, 0.5 mM magnesium chloride, 1 mg/ml pepstatin, 1 mg/ml aprotinin, 1 mg/ml leupeptin, 1 mg/ml benzamidine, 8 μg/ml calpaïn I and II inhibitors, and 0.2 mM PMSF, pH 7.4. After a first centrifugation at 500×g for 10 min, the clear supernatant was removed and centrifuged for 1 h at 100,000×g to pellet the membranes (with a Ti502 rotor; Beckman Instruments, Fullerton, CA), which was subsequently resuspended in the same homogenization buffer by gentle agitation. The protein content was measured by the Bradford method. Proteins were diluted 1:1 in Laemmli sample buffer (Sigma) and subjected to separation by one-dimensional sodium dodecyl sulfate–polyacrylamide 10% gel electrophoresis. The separated proteins were transferred to nitrocellulose membrane (0.45 μm; Millipore), and the blot was blocked for 1 h at room temperature with 5% milk and Tween 20 in PBS and incubated with mouse or rabbit primary antibodies against NaV1.4 (3 μg/ml anti-SkM1, monoclonal mouse IgG clone L/D3; Sigma), and NaV1.5 (4 μg/ml polyclonal rabbit IgG; Sigma), respectively. Antibody against GAPDH (Santa Cruz Biotech, Santa Cruz, CA) was used as control.
Revelation was performed by goat anti-mouse (1/1250, A2429, Sigma) or goat anti-rabbit (1/1250, A3687, Sigma) secondary antibodies conjugated to alkaline phosphatase followed by detection with NBT (Sigma)/BCIP (Sigma) substrate. Membranes were photographed and densitometric tracing was obtained with densitometry software (Mesurim Pro, http://pedagogie.acamiens.fr/).
Data Analysis and Statistics
Excel was used to analyze experimental data and to perform curve fittings. All values are given as mean ± standard error of the mean (SEM). Statistical differences were determined by performing the Student t-test after checking the normality of distribution. Paired t-test was used to compare TTX value to control at a given day; unpaired t-test was used to compare the value between the different days.
For each gene, ΔCts were compared between days of culture by the Student t-test for nonpaired data. Differences were considered significant at P < 0.05.
Results
Morphological Observations
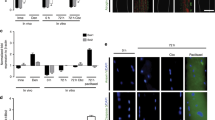
Cross-striation was clearly visible microscopically in about 80% of cultured fibers during 7 days of culture (Fig. 1a, b, d). This percentage of viability was systematically confirmed by the fluorescein diacetate test (Fig. 1c). Clusters of NaV, revealed by immunocytochemistry (Fig. 1e), normally located in the postsynaptic membrane of striated fibers, gradually disappeared from the synaptic region. At day 7, fluorescence was observed throughout fibers (Fig. 1f).
Cultured muscle fibers enzymatically dissociated from adult rat flexor digitorum brevis (FDB) muscle at day 1 (D1) (a, d) and at day 7 (D7) (b) observed with Hoffman phase-contrast microscope. (c) Fluorescein diacetate fluorescence at D7 (original magnification, 1.5×). (e, f) Fluorescent NaV after anti-PAN-sodium channel labeling, concentration of the NaV at the end plate at D1 (e) and (f) the dispersion throughout the fiber at D7
Current–Voltage Relationship
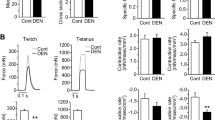
Sodium currents in single FDB fibers were elicited by test pulses between −60 to 130 mV from the holding potential of −100 mV (Rannou et al. 2009; Talon et al. 2005) after different times of culture. Examples of recorded current are shown in Fig. 2a; the current potential relationships are shown in Fig. 2b. A decrease in sodium current amplitude was observed after TTX administration. Moreover, a negative shift of the maximum current toward hyperpolarization was noticed. The characteristics of these currents are reported in Table 1. It could be noticed that peak current amplitude (I max) was decreased at day 4, followed by a reincrease after 7 days of culture, reaching a value not significantly different from day 1 value (Fig. 2a). The same profile was also evidenced for g Na max conductance: a diminution after 4 days and a reincrease at 7 days, compared to day 1. In addition, we showed that a TTX induced a partial inhibition of I max depending on the day of culture, thus showing a 50% ratio of TTX-sensitive current carried by NaV1.4 type vs. TTX-resistant current carried by NaV1.5 type. Moreover, compared to control fibers, a progressive decrease of TTX inhibition was shown: it was about 50% at day 1, 37% at day 2, and 24% at day 7 (Fig. 2a).
Patch clamp records (a) of sodium currents in flexor digitorum brevis (FDB) fibers at different culture times, before (left traces) and after application of 300 nM tetrodotoxin (right traces), at day 1 (D1) (top), day 4 (D4) (middle), and day 7 (D7) (bottom) of culture. (b) Sodium current potential relationship with or without tetrodotoxin (TTX) at D1, D4, and D7
Time constants corresponding to activation (τm) and fast inactivation (τh) were calculated for a depolarizing pulse of 0 mV. A progressive increase of τm was observed according to culture duration. Those values remained unchanged even after TTX exposure. The τh constant significantly decreased after 4 days, whereas the value obtained for day 7 was not significantly different than the day 1 value. However, TTX significantly reduced, τh value corresponding to I Na diminution, whereas τm was not modified.
Activation and Fast Inactivation
To allow comparison in activation curves of sodium current, conductance values were normalized to g Na max (Fig. 3a). Similarly, current values were normalized to maximal current to allow for comparison in fast inactivation curves (Fig. 3b). Values calculated by fitting experimental points according to the Boltzmann equation are shown in Table 1. V a1/2, the voltage dependence of activation, was characterized by a significant shift toward more positive potential from day 1 to day 4 and a partial recovery at day 7. All the curves were shifted toward a more negative potential by TTX exposure. However, the same pattern of increase at day 4 and partial recovery at day 7 was still observed (Fig. 3a). Concerning the voltage dependence of sodium current fast inactivation (V h1/2), no significant shift was noticed, either in control condition or in the presence of TTX, indicating that culture duration did not influence Na channel fast inactivation (Fig. 3b).
Activation (a) and fast inactivation (b) curves of voltage-gated sodium channels in single fibers from flexor digitorum brevis (FDB), identified according to culture time (day 1 [D1], day 4 [D4], day 7 [D7]). Curves were obtained by fitting the data with Boltzmann equation. b g Na/g Na max for each tested potential between −60 and +30 mV for the holding potential of −100 mV. c Steady-state fast inactivation was determined for the holding potential of −100 mV. The 50-ms inactivation prepulses to potentials ranging between −120 to +10 mV were applied; then peak sodium current amplitudes were measured during the 20-ms test pulse at which amplitude was selected to give a response near the maximum
Protein Expression
The evaluation of NaV1.4 and NaV1.5 protein expression was measured after 1, 4, and 7 days of culture to check a possible modification in channel type repartition. Specificity of anti-NaV1.5 and anti-NaV1.4 antibodies was checked by Western blot test on skeletal muscle and heart membrane protein extract because the NaV1.5 isoform is known to be predominant in heart. As expected, only one band at 260 kDa was detected with anti-NaV1.5 antibody in the heart, whereas NaV1.4 was predominant in the skeletal muscle. A decrease in NaV1.4 protein expression depending on culture duration was observed, corresponding to a 50% decrease between day 1 and day 7. On the other hand, NaV1.5 protein expression was dramatically increased between 1 and 7 days of culture (Fig. 4a, b). However, it should be noted that at day 1, a NaV1.5 protein expression was already detected.
α and β Subunits mRNA Expression
Concerning the transcript level, real-time PCR was used to quantify the expression of NaV1.4 and NaV1.5 mRNAs at different days of culture. Moreover, regulatory subunits β1 to β4 were also evaluated because they are involved in channel regulation. No significant variation can be observed in NaV1.4 mRNA levels at the different days of culture. Rather, we showed a strong increase of NaV1.5 expression from day 4 to day 7. Variations of β expression levels were more complex: β1 mRNA was not modified at day 4 but reached a peak of expression at day 7. No modification of β4 was observed (Fig. 5b). β2 and β3 subunit were expressed at a very low level.
a Sequences of forward and reverse primers used to amplify sodium voltage gated channel α and β subunits and the size expected for each polymerase chain reaction (PCR)-amplified product. b Relative abundance of mRNA level coding for NaV1.4, NaV1.5, β1, and β4, determined by real-time PCR. It should be noted that the results are expressed in ΔCt and that a one-point increase in ΔCt corresponds to a decrease of 50% in mRNA expression. *Significant difference from day 1 (P < 0.05)
Discussion
In this study, adult skeletal muscle fibers were cultured during 7 days. Methods of dissociation and culture of FDB muscle fibers have already been described (Bekoff and Betz 1977; Ravenscroft et al. 2007). Our technique, which uses liberase instead of collagenase, allowed a shorter time of dissociation and provided an increased number of isolated viable fibers. By both staining assays and an electrophysiological approach, we demonstrated that these fibers remained viable and functional in vitro during 7 days of culture. However, we described morphological, biochemical, and physiological modifications of sodium channel in these fibers. In the present work, simultaneous localization (immunostaining), protein isoform detection (Western blot test), mRNA expression (real-time PCR), and electrophysiological properties (patch clamp) of sodium channels were carried out in cultured adult muscle fibers. Conversely, earlier studies focused either on sodium channel mRNA (Kallen et al. 1990) or proteins (Casadei et al. 1984; Haimovich et al. 1984; Kraner et al. 1989; Zwerling et al. 1991).
By using PAN-antibody labeling, it was observed that the end plate disappeared after 7 days in culture, whereas the staining corresponding to extrajunctional channels was dramatically increased. This result was in agreement with previous studies using BTX staining (Grohovaz et al. 1993).
According to culture duration, modifications of electrophysiological properties were observed: I Na max and g Na max decreased at day 4 and reincreased at day 7. Maximal current and conductance can be related to the number of activable sodium channels and/or to the difference in type or regulation of the sodium channels. Time constants are mainly related to sodium channels α and/or β subunits types and not to channel density. Our study shows that activation time constant τm exhibited a progressive enhancement from day 1 to day 7. However, the inactivation time constant τh decreased from day 1 to day 7. These results indicated a modification in the type and/or the regulation of the sodium channels.
After the differential TTX inhibition and the formula proposed by Lupa et al. (1995), we have calculated the respective participation of NaV1.4 and NaV1.5 to maximal sodium current at the different culture times (Table 2). Our results indicate that NaV1.5 contribution to total current increased throughout culture. A possible interference due to satellite cells expressing NaV1.5 channels was avoided by elimination of most of these cells in the early stages of culture.
This result is in keeping with data obtained in denervation models showing that TTX induced a different inhibition of sodium channel. In one example, Wang et al. (2005) showed an increase of NaV1.5 sodium channel in a model of aging mice corresponding to a denervation model. On the other hand, Desaphy et al. (1998) reported an increase of NaV1.4 in aging rats but with no correlation to denervation.
Filatov and Rich (2004) showed that the inactivation process depends on the channel type. A negative shift of inactivation curve was observed with NaV1.5 compared to NaV1.4. At day 7, such a negative shift is observed in our study in agreement with an increase of NaV1.5 isoform expression. With culture duration, TTX did not induce a shift of inactivation curve between days and 7, suggesting that other mechanisms than NaV1.4/NaV1.5 ratio were present in sodium current regulation. In reference to David et al. (2008), β subunits can change properties of sodium channel. The activation curve at day 4 showed a shift toward more positive potential, in agreement with an increase of TTX resistant channel density as reported by Pappone (1980). It should be noted that a trend to recovery was observed at day 7. Because the density of NaV1.5 did not decrease at day 7, it could be hypothesized that this shift is due to a regulatory effect of β subunits.
In normal conditions, a quite low level of expression of the NaV1.5 was observed in adult skeletal muscle, and similarly, an extremely low level of mRNA was detected in FDB adult muscle with real-time PCR. This is in agreement with Kallen et al. (1990), who have obtained the same result in Northern blot analysis. Our molecular biology results go along with the electrophysiological data. First, NaV.1.4 mRNA and corresponding proteins were present in fibers throughout the culture. Second, for NaV1.5, there is an mRNA peak at day 4, as reported previously (Lupa et al. 1995; Yang et al. 1991), and maximal protein expression occurred at day 7. This can be related to the time needed for translation of mRNA into proteins and for their insertion into the membrane as functional channels (Lupa et al. 1995). The channel lifetime is still unknown in adult muscle and can be related to the contractile activity of the fibers: denervation and stress conditions can modify the turnover of ionic channels (Qu et al. 2001). The discrepancy between mRNA level and protein expression can be related to trafficking modifications in relation to β subunits variations, as reported by Isom et al. (1992) in another model. In spite of these studies, to our knowledge, the cellular signals involved in the regulation of sodium channel expression and distribution have not been fully described until now. Another possibility is an alteration of NaV proteins by endogenous protease leading to unfunctionnal channels (Kraner et al. 1989). Moreover, it should be noticed that expression of NaV protein was carried out from membrane fraction, not on the total cellular amount.
It is well known that sodium channel α subunit determines the basic properties of the channel, while β subunits modulate the channel properties. Functional studies in a heterologous system have demonstrated that depending on the type of coexpressed α subunit, β subunits are able to modulate almost all aspects of the channel properties, including voltage-dependent gating, activation, and inactivation, as well as strong increase in the number of functional channels present in the plasmic membrane (Qin et al. 2003).
Some papers have reported an effect of β subunits, but most of them concern channels expressed in Xenopus oocytes (Lenkowski et al. 2003). Information is still lacking about the evolution of β-subunits and their potential role in adult cultured fibers, or even in denervated muscle. In a model of hind limb unloaded rat, Desaphy et al. (2001) showed an increase in sodium current parallel to an increase in β1 subunit and a decrease in NaV1.4 alpha subunit after 3 weeks. This would suggest that an increase in β1 is correlated with an increase in sodium current carried by NaV1.5. This is in agreement with the present findings. We demonstrated that β1 and β4 were expressed more than β2 and β3 at day 1. The low level of β2 and β3 reduces the significance of further variations; however, β1 showed a noticeable twofold increase at day 7. Sodium channel β subunits are multifunctional. They modulate channel gating and regulate the level of channel expression at the plasmic membrane level. Studies showed that β subunits also function as cell adhesion molecules in terms of interaction with extracellular matrix molecules, regulation of cell migration, cellular aggregation, and interaction with the cytoskeleton (Isom 2001, 2002).
Concerning β1, Patino et al. (2009) showed that this subunit induced a negative shift in another type of sodium channel (NaV1.2). It can be hypothetized that a similar effect can be induced in the muscle channels and correspond to a reduction in sodium currents. Then the increase in the sodium channel density, mainly related to NaV1.5 expression, would induce an increase in the sodium current at day 4 and an increase in fiber excitability, possibly leading to spontaneous contractions, as in denervated muscle (Caldwell et al. 1986). The modifications observed in the β subunit ratio increase in β1 with β4 stability could constitute a regulation process and lead to a decrease of the sodium current. This regulatory role of β subunits in muscle has to be confirmed because results are frequently contradictory. These differences could be attributed to the availability to glycosylation and/or sialylation of mammalian cells in comparison with Xenopus oocytes. It is known that in Xenopus oocytes, some of the posttranslational events observed in mammalian cells are altered or absent (Isom et al. 1995b).
In a model of C2C12 skeletal muscle, Zebedin et al. (2004) demonstrated that NaV1.5 channel expression was enhanced, corresponding to cell differentiation in relation with myosin composition. During the early embryonic development, NaV1.5 is present in fiber membrane. After the maturation time, a switch toward adult sodium channel isoform (NaV1.4) is observed (Lupa et al. 1993), which is presumably the result of the effects of an increased muscle activity. Conversely, our model of adult cultured muscle fibers showed an increase in a NaV1.5 and β1 expression and can be useful for the study of postdenervation changes. Cultured fibers “denervated” by enzymatic dissociation can be compared with what was previously described in immature (Lupa et al. 1993) and denervated muscle (Rich et al. 1999) from in vivo models. The different published models of denervation have also shown a reexpression of NaV1.5 isoform. Often in these studies, the sodium current sensitivity to TTX was used to investigate the status of muscle denervation.
In conclusion, we suggest three main mechanisms to account for these changes: (1) the disappearance of nervous trophic factors involved in the control of the phenotypic expression, as reported for the AChRs (McArdle 1983); (2) the inactivity of the muscle fibers after removal of the nerve input (Rich et al. 1999); and (3) nerve injury products, important for the initiation and control of the denervation phenomena (Cangiano 1985). This last mechanism cannot be taken into account concerning cultured fibers. Because of the denervation mechanism complexity, it would be of great importance to have adult cultured muscle fibers model in which postdenervation phenomena could be monitored to control dedifferentiation mechanisms. That is why further studies are now carried out to establish a coculture model with rodent neurons and glial cells: to get a better understanding in nerve–muscle interactions.
References
Bekoff A, Betz W (1977) Properties of isolated adult rat muscle fibres maintained in tissue culture. J Physiol 271:537–547
Brown LD, Schneider MF (2002) Delayed dedifferentiation and retention of properties in dissociated adult skeletal muscle fibers in vitro. In Vitro Cell Dev Biol Anim 38:411–422
Caldwell JH, Campbell DT, Beam KG (1986) Na channel distribution in vertebrate skeletal muscle. J Gen Physiol 87:907–932
Cangiano A (1985) Denervation supersensitivity as a model for the neural control of muscle. Neuroscience 14:963–971
Casadei JM, Gordon RD, Lampson LA, Schotland DL, Barchi RL (1984) Monoclonal antibodies against the voltage-sensitive Na+ channel from mammalian skeletal muscle. Proc Natl Acad Sci USA 81:6227–6231
Catterall WA, Goldin AL, Waxman SG (2005) International Union of Pharmacology. XLVII. Nomenclature and structure–function relationships of voltage-gated sodium channels. Pharmacol Rev 57:397–409
Cifelli C, Bourassa F, Gariepy L, Banas K, Benkhalti M, Renaud JM (2007) KATP channel deficiency in mouse flexor digitorum brevis causes fibre damage and impairs Ca2+ release and forces development during fatigue in vitro. J Physiol 582:843–857
David M, Martinez-Marmol R, Gonzalez T, Felipe A, Valenzuela C (2008) Differential regulation of Na(v)beta subunits during myogenesis. Biochem Biophys Res Commun 368:761–766
Dennis RG, Dow DE (2007) Excitability of skeletal muscle during development, denervation, and tissue culture. Tissue Eng 13:2395–2404
Desaphy JF, De Luca A, Imbrici P, Conte Camerino D (1998) Modification by ageing of the tetrodotoxin-sensitive sodium channels in rat skeletal muscle fibres. Biochim Biophys Acta 1373:37–46
Desaphy JF, Pierno S, Leoty C, George AL Jr, De Luca A, Camerino DC (2001) Skeletal muscle disuse induces fibre type-dependent enhancement of Na(+) channel expression. Brain 124:1100–1113
Filatov GN, Rich MM (2004) Hyperpolarized shifts in the voltage dependence of fast inactivation of Nav1.4 and Nav1.5 in a rat model of critical illness myopathy. J Physiol 559:813–820
Fitts RH, Riley DR, Widrick JJ (2001) Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol 204:3201–3208
Gahr SA, Palti Y, Rexroad CE III (2004) Genomic characterization of a novel pair of ID genes in the rainbow trout (Oncorhynchus mykiss). Anim Genet 35:317–320
Goldin AL (2001) Resurgence of sodium channel research. Annu Rev Physiol 63:871–894
Goldin AL (2003) Mechanisms of sodium channel inactivation. Curr Opin Neurobiol 13:284–290
Grohovaz F, Lorenzon P, Ruzzier F, Zorec R (1993) Properties of acetylcholine receptors in adult rat skeletal muscle fibers in culture. J Membr Biol 136:31–42
Haimovich B, Bonilla E, Casadei J, Barchi R (1984) Immunocytochemical localization of the mammalian voltage-dependent sodium channel using polyclonal antibodies against the purified protein. J Neurosci 4:2259–2268
Isom LL (2001) Sodium channel beta subunits: anything but auxiliary. Neuroscientist 7:42–54
Isom LL (2002) The role of sodium channels in cell adhesion. Front Biosci 7:12–23
Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA (1992) Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science 256:839–842
Isom LL, Ragsdale DS, De Jongh KS, Westenbroek RE, Reber BF, Scheuer T, Catterall WA (1995a) Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell 83:433–442
Isom LL, Scheuer T, Brownstein AB, Ragsdale DS, Murphy BJ, Catterall WA (1995b) Functional co-expression of the beta 1 and type IIA alpha subunits of sodium channels in a mammalian cell line. J Biol Chem 270:3306–3312
Kallen RG, Sheng ZH, Yang J, Chen LQ, Rogart RB, Barchi RL (1990) Primary structure and expression of a sodium channel characteristic of denervated and immature rat skeletal muscle. Neuron 4:233–242
Ko SH, Lenkowski PW, Lee HC, Mounsey JP, Patel MK (2005) Modulation of Na(v)1.5 by beta1- and beta3-subunit co-expression in mammalian cells. Pflugers Arch 449:403–412
Kraner S, Yang J, Barchi R (1989) Structural inferences for the native skeletal muscle sodium channel as derived from patterns of endogenous proteolysis. J Biol Chem 264:13273–13280
Lenkowski PW, Shah BS, Dinn AE, Lee K, Patel MK (2003) Lidocaine block of neonatal Nav1.3 is differentially modulated by co-expression of beta1 and beta3 subunits. Eur J Pharmacol 467:23–30
Lupa MT, Krzemien DM, Schaller KL, Caldwell JH (1993) Aggregation of sodium channels during development and maturation of the neuromuscular junction. J Neurosci 13:1326–1336
Lupa MT, Krzemien DM, Schaller KL, Caldwell JH (1995) Expression and distribution of sodium channels in short- and long-term denervated rodent skeletal muscles. J Physiol 483(pt 1):109–118
McArdle JJ (1983) Molecular aspects of the trophic influence of nerve on muscle. Prog Neurobiol 21:135–198
Morgan K, Stevens EB, Shah B, Cox PJ, Dixon AK, Lee K, Pinnock RD, Hughes J, Richardson PJ, Mizuguchi K, Jackson AP (2000) Beta 3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc Natl Acad Sci USA 97:2308–2313
Noda M, Shimizu S, Tanabe T, Takai T, Kayano T, Ikeda T, Takahashi H, Nakayama H, Kanaoka Y, Minamino N et al (1984) Primary structure of electrophorus electricus sodium channel deduced from cDNA sequence. Nature 312:121–127
Noda M, Ikeda T, Suzuki H, Takeshima H, Takahashi T, Kuno M, Numa S (1986) Expression of functional sodium channels from cloned cDNA. Nature 322:826–828
Pappone PA (1980) Voltage-clamp experiments in normal and denervated mammalian skeletal muscle fibres. J Physiol 306:377–410
Patino GA, Claes LR, Lopez-Santiago LF, Slat EA, Dondeti RS, Chen C, O’Malley HA, Gray CB, Miyazaki H, Nukina N, Oyama F, De Jonghe P, Isom LL (2009) A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci 29:10764–10778
Qin N, D’Andrea MR, Lubin ML, Shafaee N, Codd EE, Correa AM (2003) Molecular cloning and functional expression of the human sodium channel beta1B subunit, a novel splicing variant of the beta1 subunit. Eur J Biochem 270:4762–4770
Qu Y, Curtis R, Lawson D, Gilbride K, Ge P, DiStefano PS, Silos-Santiago I, Catterall WA, Scheuer T (2001) Differential modulation of sodium channel gating and persistent sodium currents by the beta1, beta2, and beta3 subunits. Mol Cell Neurosci 18:570–580
Rannou F, Droguet M, Giroux-Metges MA, Pennec Y, Gioux M, Pennec JP (2009) Differences in sodium voltage-gated channel properties according to myosin heavy chain isoform expression in single muscle fibres. J Physiol 587:5249–5258
Ravenscroft G, Nowak KJ, Jackaman C, Clement S, Lyons MA, Gallagher S, Bakker AJ, Laing NG (2007) Dissociated flexor digitorum brevis myofiber culture system—a more mature muscle culture system. Cell Motil Cytoskeleton 64:727–738
Rich MM, Kraner SD, Barchi RL (1999) Altered gene expression in steroid-treated denervated muscle. Neurobiol Dis 6:515–522
Rogart RB, Cribbs LL, Muglia LK, Kephart DD, Kaiser MW (1989) Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci USA 86:8170–8174
Rossignol B, Gueret G, Pennec JP, Morel J, Rannou F, Giroux-Metges MA, Talarmin H, Gioux M, Arvieux CC (2008) Effects of chronic sepsis on contractile properties of fast twitch muscle in an experimental model of critical illness neuromyopathy in the rat. Crit Care Med 36:1855–1863
Ruff RL (1992) Na current density at and away from end plates on rat fast- and slow-twitch skeletal muscle fibers. Am J Physiol 262:C229–C234
Talon S, Giroux-Metges MA, Pennec JP, Guillet C, Gascan H, Gioux M (2005) Rapid protein kinase C-dependent reduction of rat skeletal muscle voltage-gated sodium channels by ciliary neurotrophic factor. J Physiol 565:827–841
Wang ZM, Zheng Z, Messi ML, Delbono O (2005) Extension and magnitude of denervation in skeletal muscle from ageing mice. J Physiol 565:757–764
Yang JS, Sladky JT, Kallen RG, Barchi RL (1991) TTX-sensitive and TTX-insensitive sodium channel mRNA transcripts are independently regulated in adult skeletal muscle after denervation. Neuron 7:421–427
Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R (2003) Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci 23:7577–7585
Zebedin E, Sandtner W, Galler S, Szendroedi J, Just H, Todt H, Hilber K (2004) Fiber type conversion alters inactivation of voltage-dependent sodium currents in murine C2C12 skeletal muscle cells. Am J Physiol Cell Physiol 287:C270–C280
Zwerling SJ, Cohen SA, Barchi RL (1991) Analysis of protease-sensitive regions in the skeletal muscle sodium channel in vitro and implications for channel tertiary structure. J Biol Chem 266:4574–4580
Acknowledgments
All authors contributed to the different steps of analysis and interpretation of data, drafting, and discussion of the manuscript. All experiments were performed at the Laboratory of Physiology, EA 4326, School of Medicine, 22 Avenue Camille Desmoulins, 29238 Brest, France. The authors thank Laurent Corcos and Jean-François Clément for helpful assistance concerning real-time PCR and confocal observations. Public funding from the French Ministry of Research supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morel, J., Rannou, F., Talarmin, H. et al. Sodium Channel NaV1.5 Expression is Enhanced in Cultured Adult Rat Skeletal Muscle Fibers. J Membrane Biol 235, 109–119 (2010). https://doi.org/10.1007/s00232-010-9262-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-010-9262-5