Abstract
COPI-coated vesicles are protein and liquid carriers that mediate transport within the early secretory pathway. In this Topical Review, we present their main protein components and discuss current models for cargo sorting. Finally, we describe the striking similarities that exist between the COPI system and the two other characterized types of vesicular carriers: COPII- and clathrin-coated vesicles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
EUKARYOTIC CELLS AND COMPARTMENTALIZATION
Eukaryotic cells are separated from their environment by the plasma membrane, allowing the existence of complex internal structures like cytosol-embedded organelles. The membrane-encased organelles define distinct chemical environments which in turn allow the spatial separation of a variety of reactions. The identity of an organelle is defined by its protein- and lipid composition, and therefore demands individual transport of membrane-associated proteins, their bound cargo molecules and lipids (Munro, 2004). This transport is mainly mediated via spherical vesicles.
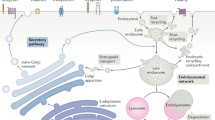
OVERVIEW: SECRETORY PATHWAY
The existence of an intracellular pathway of newly synthesized proteins was first shown in exocrine pancreatic cells (Caro & Palade, 1964). The endoplasmic reticulum (ER) and the Golgi-apparatus (Jamieson & Palade, 1966) are involved in this transport. Proteins that are transported along the secretory pathway are membrane proteins, soluble lysosomal/vacuolar proteins and secreted proteins – which will be termed as cargo throughout the review – that have common signal sequences which direct them to the ER and make them distinct from cytosolic proteins or proteins targeted to organelles like mitochondria or the nucleus (Blobel & Dobberstein, 1975a; Blobel & Dobberstein, 1975b). Besides allowing post-translational modifications such as protein-folding, glycosylation, disulfide bond formation, proline-hydroxylation, and tyrosine sulfation, the secretory machinery has to fulfill another important task, namely the distribution of correctly folded proteins to their final destination. In terms of the machinery involved, secretory transport can be dissected into at least four distinct steps: ER import/quality control, ER to Golgi transport, intra-Golgi transport/ER retrieval and post-Golgi transport.
The first step for protein secretion is the translation of a protein’s signal sequence, causing an immediate arrest of ribosome-mediated polypeptide elongation and the association of the ribosomal machinery with the ER membrane (Blobel & Potter, 1967; Walter & Blobel, 1981). This targeting includes binding of the signal recognition particle (SRP) to the signal sequence followed by binding to the ER-associated SRP receptor (reviewed in Wild et al., 2004). After transfer of the polypeptide to the protein – conducting channel called the ER translocon, membrane proteins are inserted into the ER membrane, while soluble secreted proteins and lysosomal/vacuolar proteins are translocated inside the ER lumen. There are two interdependent ER-associated systems to prevent accumulation of proteins that are misfolded in the ER, the unfolded protein response (UPR) and the ER-associated degradation (ERAD) (Friedlander et al., 2000; Travers et al., 2000). Protein misfolding can be identified by aggregation, free thiol groups or incomplete N-glycosylation. Both systems lead to re-translocation of the protein into the cytosol followed by proteasome–dependent proteolysis.
Once secretory proteins have passed the ER quality control, they are taken up by COPII (coat protein complex II) vesicles, the first class of coated vesicles involved in secretory transport (Barlowe et al., 1994). The vesicle coat contains the small GTPase Sar1, the heterodimeric Sec23/24 complex and the heterotetrameric Sec13/31 complex, which are sequentially recruited from the cytosol to the donor ER membrane (Matsuoka et al., 1998). The next step of transport towards the Golgi-apparatus involves the ERGIC (ER-Golgi intermediate compartment), a compartment defined by the presence of the lectin ERGIC-53 (Hauri et al., 2000). There is still an ongoing debate as to whether the ERGIC forms de novo by homotypic fusion of COPII vesicles or if it is a pre-existing compartment which takes up newly made COPII vesicles via heterotypic fusion (Bannykh & Balch, 1998). Both COPII- and COPI-markers (COPI see below) are associated with the ERGIC, therefore it functions as an interface between COPII- and COPI-mediated transport. Since ER/Golgi recycling membrane proteins are concentrated in COPI-positive structures at the ERGIC, whereas secretory cargo is predominantly found in COPI-negative regions of it, it is believed that the ERGIC represents the first entity that discriminates between anterograde and retrograde transport (Martinez-Menarguez et al., 1999). This hypothesis is strengthened by a recent live-cell imaging study in which a dissociative process between GFP-tagged ERGIC-53 and fluorescent-labeled anterograde cargo could be observed (Ben-Tekaya et al., 2005). Retrograde cargo retrieval to the ER is most likely mediated by COPI vesicles (see below) whereas anterograde ERGIC to Golgi transport possibly occurs in a COPI-independent fashion since structures that contain anterograde cargo (pre-Golgi carriers) are COPI-negative, and are much larger than the expected vesicle size (Presley et al., 1997; Martinez-Menarguez et al., 1999).
After entering the cis-Golgi, cargo is transported along the Golgi-apparatus, which consists of four to six distinct cisternae with different protein compositions, finally exiting at the trans-Golgi network (TGN, anterograde direction). COPI vesicles seem to mediate further anterograde transport within the Golgi-apparatus (Orci et al., 1986; Ostermann et al., 1993), and to recycle material back to the ER (Cosson & Letourneur, 1994; Letourneur et al., 1994; Majoul et al., 2001) (retrograde direction). The exact role of COPI vesicles within intra-Golgi transport is still under debate and will be discussed below.
Finally, post-Golgi cargo has to be sorted at the TGN. The TGN combines the secretory and the endocytic transport routes, and targets lysosomes, endosomes and the plasma membrane (reviewed in Gu et al., 2001; Rodriguez-Boulan & Musch, 2005). Here, the secretory pathway can be subdivided into constitutive and regulated: the regulated pathway is only present within endocrine and neuroendocrine cells (reviewed in Tooze et al., 2001), whereas the constitutive pathway exists in all cell types and involves clathrin-coated vesicles (CCVs) and tubular carriers. A comprehensive description is beyond the scope of this review and is discussed elsewhere (Gleeson et al., 2004; Rodriguez-Boulan & Musch, 2005; Traub, 2005). Like in the COPII system, CCV biogenesis mainly involves sequential binding of a small GTPase, Arf1 (for clathrin adaptor proteins AP-1, 3, 4), Arf6 (for AP-2) (Paleotti et al., 2005), the clathrin adaptor protein complexes (reviewed in Owen et al., 2004), and the heterohexameric coat protein clathrin.
COPI-mediated Transport
ROLE OF COPI VESICLES IN INTRA-GOLGI TRANSPORT
Two alternative models for intra-Golgi transport were initially proposed: cisternal progression/maturation and vesicular transport. The cisternal progression/maturation model postulates the transport of anterograde cargo en bloc with cisternae (Glick & Malhotra, 1998). Transport would be achieved by assembly of new cisternae at the cis-Golgi (potentially from fusion of pre-Golgi carriers), the cisternae would then mature and progress along the Golgi-apparatus, and finally disassemble at the trans-Golgi. Therefore, anterograde cargo would not leave the lumen of cisternae. Maintenance of cisternae with distinct compositions would be mediated by exclusively retrograde directed COPI vesicles removing resident cargo (e.g., glycosyltransferases) from the more trans- to the more cis-located cisternae, leading to the maturation of a given cisternae. In contrast, the vesicular transport model postulates the transport of anterograde cargo between static cisternae via coordinated budding and fusion reactions of anterograde-directed COPI vesicles (Rothman & Wieland, 1996). Since the continuous loss of material at the trans-Golgi would be antagonized by retrograde-directed COPI vesicles, the existence of at least two distinct populations of COPI vesicles, one mediating anterograde, and one mediating retrograde transport, would be required. However, both models have caveats since the cisternal progression/maturation model does not explain the presence of anterograde cargo within COPI vesicles (Nickel et al., 1998; Pepperkok et al., 2000), or different transport rates of anterograde cargo (Karrenbauer et al., 1990; Bonfanti et al., 1998). Likewise, the vesicular transport model is unable to explain transport of cargo much bigger than COPI vesicles (e.g., procollagen) (Bonfanti et al., 1998). When the presence of two different COPI vesicle populations was shown, one containing anterograde cargoes and the Golgi-restricted SNARE GS28 (SNARE proteins see below), and the other one carrying the retrograde, ER-directed KDEL receptor (Orci et al., 1997; Orci et al., 2000b), a combination of both models was suggested: the percolating vesicle model (Orci et al., 2000b; Pelham & Rothman, 2000). Here, one COPI vesicle population would mediate the fast transport of anterograde cargo between all cisternae in a bi-directional random walk, as suggested by the even distribution of GS28 over the Golgi apparatus (Orci et al., 2000b). Directional transport of anterograde cargo would still be achieved due to the steady-state entry of biosynthetic cargo at the cis-Golgi and its exit at the trans-Golgi. The second COPI vesicle population would allow recycling material back to the ER. The percolating vesicle model also integrates a slow anterograde transport pathway mediated by cisternae progression/maturation that accounts for transport of large cargo that would not fit in COPI vesicles, and explains the different transport rates observed.
All models provided so far exhibit one common feature: the presence of Golgi resident enzymes in COPI vesicles. Whereas some studies showed the presence of Golgi residents in a subpopulation of COPI vesicles (Lanoix et al., 2001; Malsam et al., 2005), others stated the depletion or even the exclusion of Golgi enzymes in COPI vesicles (Sonnichsen et al., 1996; Orci et al., 2000a; Cosson et al., 2002; Kweon et al., 2004). A reduction of Golgi residents in COPI vesicles as compared to donor Golgi would be inconsistent with a pure cisternal progression/maturation model, but would still fit the other two Golgi transport models described above. In contrast, a complete absence of Golgi enzymes in COPI vesicles would be incompatible with a COPI-mediated vesicular transport model. In electron tomography-based studies, a cargo-induced effect on the formation of tubular inter-cisternal connections was reported (Marsh et al., 2004; Trucco et al., 2004). This observation led to the formulation of continuity-based models (reviewed in Mironov et al., 2005). Here, transport of cargo would be mediated through polymorphic carriers having the ability to bridge adjacent cisternae in a process resembling ERGIC to cis-Golgi cargo transport. Cisternal maintenance including Golgi enzyme transport would be achieved by tubules, which would only connect subsequent cisternae. The suggested roles of COPI vesicles would solely be the control of inter-cisternal fusion events via active uptake of SNARE proteins into COPI vesicles, and establishment of Golgi morphology. In conclusion, it is still under debate which model(s) is/are describing best how intra-Golgi transport is achieved. More experiments will be needed to address this crucial issue.
IDENTIFICATION OF THE MAIN PLAYERS INVOLVED IN COPI-VESICLE FORMATION
Cytosolic Components
COPI vesicles were first generated in vitro by combining Golgi membranes and the cytosol in the presence of GTPγS, and purified by subsequent density gradient centrifugation, yielding “Golgi-derived coated vesicles”. These vesicles have a polypeptide composition that is distinct from their donor Golgi membranes (Malhotra et al., 1989). The identification of Arf1, a small GTPase of the ras superfamily (Kahn & Gilman, 1984), as a component of the coat suggested that GTP hydrolysis has a regulatory role in COPI vesicle budding (Serafini et al., 1991a). It was also shown that a cytosolic protein complex named coatomer (derived from coat protomer) contains at least four proteins that are found in COPI vesicles (α-, β-, γ- and δ-COP). These can be purified from the cytosol, indicating its role as an unassembled precursor of COPI vesicles (Duden et al., 1991; Serafini et al., 1991b; Waters et al., 1991). Subsequently, other coatomer subunits β′- (Stenbeck et al., 1993), ε- (Hara-Kuge et al., 1994) und ζ-COP (Kuge et al., 1993) were identified. Recently, an additional isoform of γ-COP and of ζ-COP (Blagitko et al., 1999; Futatsumori et al., 2000) was identified. The new isoforms are termed γ2- and ζ2-COP, and the original isoforms are referred to as γ1- and ζ1-COP. Each isoform is, like the other coatomer subunits, present in just one copy, resulting in three different main heptameric protein complexes with only minor amounts of γ2ζ2-Coatomer (Wegmann et al., 2004).
Membrane Proteins
After the discovery of retention signals within the cytoplasmic tail of the adenoviral type I transmembrane protein E3/19K (Nilsson et al., 1989; Jackson et al., 1990), it was shown that coatomer is essential for transport of dilysine-tagged proteins back from the Golgi-apparatus to the ER (Letourneur et al., 1994).
Further proteomic research revealed the stoichiometric presence in COPI vesicles of p24 proteins, which are type I transmembrane proteins that contain a C-terminal dibasic motif (Stamnes et al., 1995; Sohn et al., 1996). In a completely defined system, using synthetic liposomes with physiological lipid compositions, the cytoplasmic components Arf1, Coatomer and GTP alone do not induce vesicle formation. However, in the presence of the cytoplasmic domains of p24 family proteins in liposomes with Golgi-like lipid composition, vesicles are formed efficiently (Bremser et al., 1999), although the p24 protein requirement for this process seems to depend on the liposomes’ lipid composition (Spang et al., 1998).
BASIC MODEL FOR THE FORMATION OF COPI-VESICLES
The first step of COPI biogenesis is the activation of cytosolic Arf1 initially complexed with GDP. Guanine nucleotide exchange factors (GEFs) defined by a central Sec7 domain (Chardin et al., 1996) stimulate the binding of GTP to Arf1, a process which can be inhibited by the fungal metabolite Brefeldin A (BFA) (Donaldson et al., 1992b). This nucleotide exchange causes a conformational change of the GTPase that leads to exposure of an N-terminal myristoyl-anchor and an amphiphatic helix allowing stable membrane association (Franco et al., 1996; Antonny et al., 1997). The interaction of Sec7 domain proteins with Arf1 can strongly be facilitated and modulated by additional lipid-protein interactions, which bring proteins close to the membrane surface (Terui et al., 1994; Itoh & De Camilli, 2004). For the lower molecular weight GEFs like ARNO and cytohesins, a PH domain mediates binding to specific phospho- inositides present in the Golgi membrane (Klarlund et al., 2000; De Matteis et al., 2005). Most recently, it was shown that Gbf1, a large Sec7 domain GEF, is probably the relevant GEF involved in COPI biogenesis since it localizes to the cis-Golgi and its activity is BFA-sensitive (Zhao et al., 2002; Niu et al., 2005). Taken together, the energetically unfavorable exposure of the amphipatic helix/myristoyl moiety of Arf1·GTP in the absence of membranes ensures that no misactivation occurs in the cytosol. In addition, the GEF activity, recruited to membrane by a specific lipid-protein interaction, might contribute to the organelle specificity of the exchange reaction (Lee et al., 2004).
Further work revealed a first recruitment to the Golgi membrane of Arf1·GDP by p23 and probably p24, supporting the idea that such cytoplasmic domains act as primary Arf1·GDP receptors (Gommel et al., 2001; Majoul et al., 2001; Contreras et al., 2004). Recently, another Arf1·GDP receptor was suggested: overexpression of membrin, an early Golgi SNARE, partially protected BFA-mediated Arf1 release from Golgi membranes. Therefore, it was deduced that this interaction occurs preferentially with Arf1·GDP (Honda et al., 2005). Since the binding of Arf1·GDP to Golgi membranes could be efficiently competed with dimeric p23 peptide (Gommel et al., 2001), it is possible that membrin acts after p23 recruitment.
Upon exchange of GDP for GTP the Arf1/p23 complex dissociates, providing a “priming complex” that consists of Arf1·GTP and p24 proteins for subsequent coatomer binding. In contrast to COPII- or CCV-coat proteins, coatomer is recruited en bloc (Hara-Kuge et al., 1994) to the priming complex. Coatomer binds Arf1·GTP through the β- und γ-COP subunits (Zhao et al., 1997; Zhao et al., 1999) and p23 through the γ-COP subunit (Harter et al., 1996). The complex can also bind to cytoplasmic tails of transmembrane proteins bearing a KKXX sequence at their C-termini (Cosson & Letourneur, 1994). This suggests multivalent interactions involved in coatomer recruitment to membranes, and indeed Arf1·GTP alone poorly recruits coatomer from cytosol to membranes in the absence of such cytoplasmic tails (Bremser et al., 1999). Binding to p24 proteins of coatomer finally leads to a conformational change (Reinhard et al., 1999) that is likely to provide the energy to bend the membrane resulting in a coated COPI vesicle.
In order to fuse with its target membrane, a COPI vesicle must be uncoated. The coat remains stably associated with the vesicles until GTP hydrolysis by Arf1 is catalyzed (Tanigawa et al., 1993); this is activated through an interaction with an ArfGAP (Cukierman et al., 1995). Addition of the purified catalytic domain of ArfGAP1 (GTPase activating protein) induced uncoating of in vitro generated COPI vesicles, demonstrating that GTP hydrolysis is sufficient for this process (Reinhard et al., 2003).
The final step in COPI-mediated transport is delivery of cargo to the target membrane. Basically, two classes of proteins contribute specificity to this process: SNAREs (soluble NSF attachment protein receptors) (Sollner et al., 1993) and tethering proteins. SNAREs mediate the final docking stage of the uncoated vesicle with its target membrane and catalyze the membrane fusion reaction (reviewed in Hong, 2005). Fusion is ensured by pairing of complementary SNAREs, which are defined by the presence of a SNARE motif (Weber et al., 1998). These domains are able to form a tetrahelical bundle in which one helix is contributed by the v(vesicle)-SNARE and three helices are provided by the t(target)-SNARE (Parlati et al., 2000). The SNAREs GS15, GS28, Ykt6 and syntaxin 5 are likely to be involved in intra-Golgi COPI-mediated transport (Parlati et al., 2002; Shorter et al., 2002; Xu et al., 2002) while Sec22, Sec20, Use1 and Syn18 might be the SNAREs involved in the fusion of COPI vesicles with the ER membrane (Burri et al., 2003; Dilcher et al., 2003). While it is possible to co-immunoprecipitate coatomer subunits with an anti-GS15 antibody (Xu et al., 2002), no direct coatomer/SNARE interaction could be convincingly shown so far (Xu et al., 2002; our own observations). In fact, ArfGAPs (Rein et al., 2002) or Arf1 (Lee et al., 2005b) could mediate the interaction between SNAREs and the COPI coat. SNAREs were first thought to ensure the specificity of the fusion reaction by allowing only certain v-SNARES to interact with defined t-SNARE complexes (Parlati et al., 2002). However, evidence exists that indicates that the specificity provided by these interactions is not tight enough to explain the fidelity of membrane targeting (Antonin et al., 2000; Wang et al., 2004; Brandhorst et al., 2006).
Tethering factors provide another level of specificity by targeting vesicles to the right membrane before the vesicles interact with t-SNAREs. Coiled-coil tethers are usually dimeric extended α-helical proteins that are localized on defined membranes along the secretory pathway, often in a Rab GTPase- dependent manner (Short et al., 2005), and were proposed to serve as molecular bridges between membranes. Tethering factors involved in the COPI system are giantin, p115 and GM130 (Sonnichsen et al., 1998), and golgin 84 and CASP (Malsam et al., 2005). Giantin is present on COPI vesicles and can interact with GM130 and p115, both localized at the cis-Golgi. In addition, p115 catalyze the formation of the GS15, GS28, Ykt6, syntaxin 5 v/t-SNARE complex, stimulating the final docking and fusion of the vesicles (Shorter et al., 2002). Potential additional roles of tethering factors, including regulation of cargo sorting and coat recruitment, are beyond the scope of this review and are described elsewhere (Sztul & Lupashin, 2006). In order to allow heterotypic fusion, vesicular golgins and v-SNAREs have to be selectively taken up into COPI vesicles (Orci et al., 2000b; Malsam et al., 2005).
As mentioned above, interactions between coatomer, Arf1 and transmembrane receptors are weak. This implies that in vivo, Arf1 and coatomer should be in fast equilibrium between the cytosol and Golgi membranes, and stable association can only be achieved when multiple weak interactions between coatomer, Arf1 and the cytoplasmic tails of transmembrane proteins occur. This was indeed observed in vivo using fluorescently tagged proteins: fast binding and release of coatomer and Arf1 in an apparently stochastic manner could be observed at 4°C, a temperature where COPI coated vesicles can not be formed (Presley et al., 2002). This indicates continuous recruitment of the COPI machinery to membranes, regardless whether this recruitment is productive or not, allowing the cytosolic machinery to probe or remodel the membrane until all requirements for vesicle formation are met. This might include the presence of the right cargo, machinery and lipids. Similarly, a recent study of the related AP-2/clathrin-mediated endocytosis has proposed that clathrin pits are initiated continuously and randomly at the plasma membrane, but collapse unless stabilized, perhaps by cargo capture (Ehrlich et al., 2004).
UPTAKE OF CARGO
Sorting Motifs
The sorting signals that specify the intracellular localization of a protein may bind directly to a coat subunit or to an adaptor protein that links the cargo to the coat. As mentioned above, the KKXX motif has long been described as a canonical signature for ER retrieval of transmembrane proteins (Cosson & Letourneur, 1994). A first study based on yeast temperature-sensitive mutants identified coatomer subunits α- and β′-COP as the binding partners for the KKXX signature (Letourneur et al., 1994), and more recent work identified point mutations in the WD40 repeats present at the N-termini of α- and β′-COP that could abolish binding to different KKXX or KXKXX sequences, defining two distinct but overlapping binding sites for the ER retrieval motif (Eugster et al., 2004). Conflicting data come from cross-linking studies that used a radioactive photoactivatable KKXX-bearing peptide and identified the γ-COP subunit as the sole binding partner of the retrieval motif (Harter et al., 1996; Harter & Wieland, 1998). This discrepancy could be explained by the presence of several binding sites for di-lysine motifs on coatomer. Accordingly, the aminoglycoside antibiotic neomycin (structurally related to a “double KKXX” motif) can precipitate coatomer in a concentration-dependent manner following a bell curve. This suggests that the antibiotic probably cross-links the complex into larger aggregates (Hudson & Draper, 1997). As the simple dipeptide lysyl-lysine could interfere with the precipitation, it is likely that at least two binding sites for di-lysine motifs exist in coatomer. As mentioned above, another class of transmembrane proteins that can bind to coatomer is the p24 family (Fiedler et al., 1996; Sohn et al., 1996). Although they display some similarity to the KKXX- proteins, the p24 proteins have distinct features: (i) a conserved di-phenylalanine motif in their cytoplasmic tails, (ii) most members do not have a canonical KKXX sequence at their C-termini but a variation of it such as KK(X)n or KR(X)n (n≥2). Despite these differences, no functional difference has widely been attributed to p24-proteins as opposed to KKXX-proteins. However, as any change in the KKXX sequence disrupts binding of ER resident proteins to coatomer (Cosson & Letourneur, 1994), it is likely that the p24 proteins use a different mechanism to bind to the COPI coat. Indeed, binding to coatomer relies more on the di-phenylalanine motif than on the two basic residues in p24 proteins that do not have a true KKXX sequence (Fiedler et al., 1996; Sohn et al., 1996; Goldberg, 2000). The coatomer subunit involved in the binding to p24 family members was identified as γ-COP (Harter & Wieland, 1998). Efforts to find other COPI binding motifs involved in ER retrieval led to the identification of the δL motif, where the sequence WXXW/Y/F within a cytoplasmic tail of a transmembrane protein confers the ability to bind to COPI through δ-COP and leads to an ER localization of reporter proteins. The yeast protein Sec71p seems to rely on such a motif to gain ER localization (Cosson et al., 1998). Another ER retrieval motif is the RXR sequence found in subunits of the ATP-sensitive K+ channel (K(ATP)), and located within cytoplasmic loops or C-terminal tails (Zerangue et al., 1999). Coatomer can bind to the RXR motif and ensures trapping of individual subunits in the ER. However, upon assembly of the subunits into a functional receptor, multiple RXR motifs can also bind to the 14-3-3 proteins that will efficiently compete for retrieval via the COPI interaction, and thus allow trafficking of the fully assembled receptor to the plasma membrane (Yuan et al., 2003). The COPI subunit involved in RXR motif recognition is not known to date.
Luminal permanent ER resident proteins must be distinguished from newly synthesized secretory proteins which leave this compartment to reach their final destination. The sequence KDEL present at the C-terminal end of luminal ER resident proteins was identified as their retention signal (Munro & Pelham, 1987). The KDEL sequence does not actually confer ER permanent residency but allows recycling of a KDEL-protein from the early Golgi back to the ER (Pelham, 1988). Identification of the receptor involved in this recycling was first achieved in yeast (Lewis et al., 1990; Semenza et al., 1990), and then in mammalian cells (Lewis & Pelham, 1990). The KDEL receptor is an integral membrane protein with 7 transmembrane spans (Scheel & Pelham, 1998). Binding of a KDEL protein to its receptor is pH dependent (Wilson et al., 1993) and induces redistribution of the receptor from the Golgi apparatus to the ER (Lewis & Pelham, 1992). In vivo FRET experiments showed that the KDEL receptor uses the COPI pathway (Majoul et al., 1998). In addition, ligand binding induces oligomerization of the receptor, a process that might be important for recruitment of the COPI machinery (Majoul et al., 2001). The C-terminal cytoplasmic tail of the KDEL receptor, like the p24 family members, has a double lysine with a longer extension. In the mammalian protein, this di-lysine motif in combination with a phosphorylated serine residue also present in the cytoplasmic tail mediates binding to coatomer and ArfGAP1, whereas one of the motifs alone is not sufficient to support binding (Cabrera et al., 2003). Altogether the KDEL pathway is reminiscent of endocytic processes, possibly with ligand-induced oligomerization of receptors leading to stimulation of the budding machinery, and ligand release in the target compartment upon a change in luminal pH.
Models for Uptake of Cargo
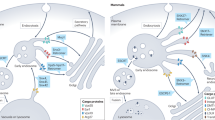
What is the mechanism of cargo uptake? An in vitro assay developed to study packaging of retrograde and anterograde transmembrane cargo proteins shows that both types of cargo can enter COPI-coated vesicles, however, the efficiency of their uptake is strikingly reduced when the assay is performed in the presence of the poorly hydrolyzable analog GTPγS as opposed to GTP (Nickel et al., 1998). The target GTPase involved in cargo uptake was identified as Arf1 both in vitro (Malsam et al., 1999) and in vivo (Pepperkok et al., 2000). Since GTP hydrolysis by Arf seems to be required for efficient sorting, an ArfGAP is likely to play a central role in this process, however, with two seemingly contradictory effects: GTP hydrolysis is needed for cargo sorting at the beginning of vesicle formation, and GTP hydrolysis is needed for the uncoating of vesicles (see above). Kinetic studies of GAP-stimulated GTP hydrolysis by Arf1 revealed a role for coatomer, as the protein complex could promote an efficient stimulation of the reaction (Goldberg, 1999). This study was performed in solution using a truncated mutant of Arf1 lacking its N-terminal myristoylated α-helix (NΔ17Arf1). On Golgi membranes, ArfGAP1-dependent GTP hydrolysis by full length myristoylated Arf1 is faster than in solution, and thus coatomer-induced stimulation is not as dramatic as in solution (Szafer et al., 2001). This might indicate a poor affinity between Arf1 and its GAP that is compensated by their mutual binding to membranes, and points to a role for coatomer in bridging both molecules, hence modulating GTPase activity. Direct binding of ArfGAP2, or its yeast ortholog Glo3p, to γ-COP has been shown, supporting this view (Eugster et al., 2000; Watson et al., 2004). Modulation of GTPase activity could also be shown from cytoplasmic tails exposed by the Golgi. Again the p24 family seems to play a key role. The cytoplasmic tail of p24 inhibited ArfGAP1-mediated GTP hydrolysis (Goldberg, 2000) in an in vitro assay using soluble components (NΔ17Arf1 and ArfGAP1 catalytical domain). Similarly p23 and p24 tails showed inhibition ability in an assay that used the full-length Arf1 and ArfGAP1 on liposomes or Golgi membranes (Lanoix et al., 2001). On the other hand, in the same study, KKXX tagged proteins and Golgi resident enzymes did not induce such inhibition. A sorting mechanism based on kinetic control of Arf1-mediated GTP hydrolysis has been proposed where transmembrane proteins with the ability to slow this process will be selectively enriched into COPI coated vesicles (Fig. 1). In this model, coatomer bound to Arf1 in the absence of these tails will rapidly dissociate from the membrane due to the fast GTPase activity stimulated by non-inhibited ArfGAP. In contrast, coatomer bound to both Arf1 and, for example, p24 will be more stably associated to the membrane due to reduced ArfGAP stimulation. The initiation complex would then have more time to diffuse on the membrane, capture other transmembrane proteins with coat affinity, and build a lattice that would form a vesicle before uncoating occurs. This model can account for sorting mechanisms mediated by p24 proteins. However, it does not explain sorting into COPI vesicles that do not contain any p24 proteins (Malsam et al. 2005), unless other transmembrane proteins with GAP-modulating activities are found in these vesicles.
Kinetic proof-reading model. The p24-protein-dependent uptake of cargo into COPI-vesicles is shown in A, whereas B displays an abortive situation in the absence of p24 proteins. In A, the recruitment of coatomer is initiated by Arf – GTP dissociates from p24 proteins (1). After Arf-GEF catalyzed nucleotide exchange, Arf – GTP dissociates from p24 proteins leading to its stable anchorage at the membrane (2). Coatomer then binds both the p24 oligomer and Arf – GTP via β- and γ-COP interactions (3). Recruitment of AftGAP is likely to be mediated via γ-COP. Cargo can be captured by α- and β′-COP (4a) and after coat polymerization (5), a COPI vesicle can pinch off the donor membrane (6). Finally, uncoating is achieved by ArfGAP-stimulated Arf – GTP-hydrolysis leading to a “nude” vesicle (7), which can deliver cargo to the target membrane. All steps described in B are the same as in A, except that coatomer without the presence of p24 protein tails will rapidly dissociate from the membrane due to the fast, non-inhibited ArfGAP activity (4b). Active components are shown in green and inactive components are shown in red.
Recent data using liposomes with defined sizes revealed that ArfGAP1 is a membrane curvature-sensitive protein, since, in an Arf GTP hydrolysis assay, stimulation of Arf1-mediated GTP hydrolysis increased as the size of the liposomes decreased (Bigay et al., 2003; Bigay et al., 2005). Maximal stimulation was reached using liposomes with a size comparable to COPI vesicles, while activity was minimal with larger liposomes. This effect is due to a lipid-packing sensor motif present in the non-catalytic domain of ArfGAP1 (Bigay et al., 2005). On a highly curved membrane, conserved hydrophobic residues within this domain can insert into the loosely packed lipid leaflet and induce folding into an amphipatic α-helix that allows stable binding to the membrane of the ArfGAP1. On a flat membrane, where lipid packing is tighter, insertion of the conserved hydrophobic residues is less favored, hampering stable binding of ArfGAP1. This spatial regulation of ArfGAP1 on membranes and in vivo data with GFP-tagged proteins made some authors suggest a dynamic model for the mechanism of cargo concentration (Liu et al., 2005). According to this model, (Fig. 2) a continuous flow of coat components occurs from the rims of the nascent bud to its tip. This is due to the fast GTP hydrolysis rate allowed by the highly curved membrane surface of the bud tip where uncoating occurs. Coat components released from the tip would be replaced by the trimeric coatomer/Arf1/ArfGAP1 complex from the rim, where membrane curvature is lower and GTP hydrolysis slower. After budding, uncoating of the newly formed vesicle should occur rapidly, again due to the fast GTP hydrolysis rate allowed on the highly curved vesicle surface. GTP hydrolysis-dependent cargo sorting into COPI vesicles fits into this model. Indeed, any transmembrane cargo that relies on direct binding to the coat in order to be sorted into COPI vesicles would be directed from the rim to the tip of the nascent bud until budding occurs, leading to the presence of the cargo inside the vesicle. If GTP hydrolysis is inhibited, the trimeric complex coatomer/Arf1/ArfGAP1 would be stabilized on membranes whatever their curvature and would eventually lead to vesicle formation without a flow of components. In this case, cargo that relies on direct binding to coat components will be packaged at best in a stoichiometric amount with the coat and at worst at the same concentration as in the donor membrane. However, this model does not explain how cargo molecules that do not interact with the coat are also efficiently packaged into COPI-coated vesicles in an Arf1-mediated GTP hydrolysis manner.
Dynamic curvature sensitivity model. All steps described are the same as in Fig. 1, except that vesicle budding (6) occurs concomitantly with ArfGAP-stimulated uncoating (7). Since ArfGAP activity increases as membrane curvature becomes higher, there is a net coat dissociation form at the tip of a nascent vesicle (−) and a net coat association at the rims (+). This would drag coatomer-bound cargo into the nascent bud until budding occurs, and after detachment of the vesicle from the membrane, lead to a rapid dissociation of the coat. Active components are shown in green and inactive components are shown in red.
It is of note that both models for cargo uptake are not mutually exclusive, but as shown on Fig. 2, can coexist.
Different Populations of COPI Vesicles
Analysis of the protein content of COPI-coated vesicles showed evidence of heterogeneity. Indeed, different populations of vesicles containing either anterograde or retrograde cargos could be observed (Orci et al., 1997, 2000b; Lanoix et al., 2001; Malsam et al., 2005). This implies that there must be a mechanism to distinguish between cargo with respect to destination. Additionally, the uptake of cargo and its corresponding “address molecules” like v-SNAREs and vesicular golgins, and not t-SNAREs or Golgi -associated golgins, is likely to be coupled. Indeed, after homotypic fusion of yeast vacuoles it was shown that a GTPase is able to sequester t-SNAREs, suggesting a v/t-SNARE filtering role (Antonny, 2004; Peters et al., 2004).
A recent study showed that distinct populations of COPI vesicles can be purified according to the tethering factors they contain and pointed to the possibility of a coupled packaging of the address tethering factors and their corresponding cargo (Malsam et al., 2005). In line with different functions for COPI vesicles, in higher eukaryotes these carriers do not represent a homogeneous population with regard to their coat. Rather, two isotypic coatomer subunits, γ2 and ζ2 (Blagitko et al., 1999; Futatsumori et al., 2000), were identified. Three different coatomer complexes could be detected according to their isotype combinations. The ζ2/γ1-COP combination seems to coat a distinct population of COPI vesicles (Wegmann et al., 2004). The different coatomer isotypes might then provide a way to modulate the cargo repertoire of the COPI system through binding to distinct types of transmembrane protein cytoplasmic tails and coating distinct vesicles. Likewise, ArfGAP1 is not the only GAP involved in the COPI machinery: two other GAPs, ArfGAP2 (Randazzo 1997) and ArfGAP3 (Liu et al. 2001) can interact with γ-COP (Watson et al., 2004) and could potentially also modulate the kind of cargo that will be packaged. Finally, different COPI vesicle populations might also be obtained by recruiting coat components to distinct areas on the Golgi membrane. But such a mechanism remains to be addressed.
Comparison with the COPII- and the Clathrin-Coated Vesicle Systems
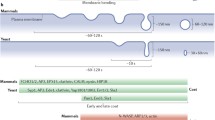
The COPI, COPII and clathrin systems share mechanistical similarity and, in the case of COPI and clathrin systems, also structural analogy (see Table 1). The following general principles apply for the formation of all vesicle types. Each type of coat requires a small GTPase in its GTP-bound form to be recruited to membranes. Sar1 recruits the Sec23/24 complex (Matsuoka et al., 1998), Arf1 recruits COPI (Donaldson et al., 1992a; Palmer et al., 1993), AP-1 (Traub et al., 1993), AP-3 (Ooi et al., 1998) and AP-4 (Boehm et al., 2001), and Arf6 recruits AP-2 (Paleotti et al., 2005). Moreover, the protein coat is made of two layers, one inner layer that directly binds to the small GTPase and one outer layer. In the case of COPII, the inner layer is made of the Sec23/24 complex which is recruited to the membrane first, and the outer layer, the Sec13/31 complex, is subsequently recruited (Matsuoka et al., 1998). Similarly, clathrin adaptors form an inner layer that, once on membranes, recruits the clathrin triskelion (reviewed in Traub, 2005). In contrast, coatomer has both layers in one complex that does not dissociate under physiological conditions (Hara-Kuge et al., 1994). It is, however, possible to dissociate coatomer into subcomplexes in vitro (Lowe & Kreis, 1995; Pavel et al., 1998). A tetrameric β′/δ/γ/ζ-COP subcomplex most likely corresponds to the inner layer, while a trimeric α/β′/ε-COP subcomplex resembles the outer layer. Indeed, protein sequence analysis reveals analogy between the β/δ/γ/ζ-COP subunits and the β/μ2/α2/σ2-adaptins of the AP-2 complex (Schledzewski et al., 1999), and the same is true for the other AP complexes. Two hybrid analysis showed similar architecture of the COPI β/δ/γ/ζ-COP subcomplex and the AP complexes with respect to inter-subunit organization (Faulstich et al., 1996; Eugster et al., 2000). The parentage between COPI and adaptins was strengthened when the structure of a C-terminal fragment of γ-COP was resolved, revealing a striking resemblance with the clathrin adaptor appendage domains (Hoffman et al., 2003; Watson et al., 2004). Therefore, it is likely that, as inferred from sequence comparison, the overall structure of the β/δ/γ/ζ-COP subcomplex is quite similar to that of the adaptin complexes (Collins et al., 2002; Heldwein et al., 2004).
For the COPII system, the inner layer complex Sec23/Sec24 possesses an intrinsic GAP activity. Indeed, Sec23 is the GAP for Sar1 (Yoshihisa et al., 1993), while Sec24 interacts with cargo proteins (Miller et al., 2002). The GAP activity of the Sec23/Sec24 complex is poor and permits a rather long lifetime (30 s) of the Sar1/Sec23/Sec24 ternary complex on liposomes. Strikingly, it is accelerated by one order of magnitude after binding of the Sec13/Sec31 complex (Antonny et al., 2001). It has been suggested that initial recruitment of Sec23/Sec24 by activated Sar1 allows the formation of prebudding complexes that can interact with cargo. These cargo-loaded precomplexes are then crosslinked through binding with Sec13/Sec31 to form the lattice that eventually leads to vesicle formation (Antonny & Schekman, 2001). As mentioned above, ArfGAPs are not sub- units of coatomer but separate cytosolic factors. Coatomer, like the COPII-system, has an influence on the GTP hydrolysis rate as it stimulates ArfGAP1 activity (Szafer et al., 2001). It has been suggested that ArfGAPs are a component of the COPI coat necessary for vesicle formation (Yang et al., 2002; Lewis et al., 2004). While this would mirror the presence in the COPII coat of the SarGAP, it was shown that COPI vesicles do not need an ArfGAP for their formation (Spang et al., 1998; Bremser et al., 1999; Reinhard et al., 2003). A possible significance of ArfGAP activity modulation for cargo capture has been described above. For the clathrin system, a recent study showed that the adaptor complex AP-1 stimulates ArfGAP1 activity (Meyer et al., 2005). The influence of other clathrin adaptors on Arf-mediated GTP hydrolysis remains to be addressed.
All vesicular systems need to generate membrane curvature to allow vesicle formation. COPI, COPII and clathrin systems seem, however, to have evolved different means to achieve membrane deformation. For the clathrin system, the accessory proteins epsin (Ford et al., 2002), amphiphysin (Takei et al., 1999), and endophilin (Farsad et al., 2001) are thought to be responsible for membrane curvature formation. Epsin relies on binding to PIP2 to trigger the folding of an amphipathic α-helix that inserts into a membrane to induce curvature. Amphiphysin and endophilin have BAR domains (reviewed in Gallop & McMahon, 2005) which dimerize to form a structure shaped like a banana. These BAR domains are lipid binding domains that can induce curvature by forcing the membrane to adopt their concave shape. For the COPII system, structure resolution of the Sec24/Sec23 complex revealed that both subunits have a similar fold that forms a dimer with a concave shape on its putative membrane interacting side that would fit the curvature of a COPII vesicle (Bi et al., 2002). The Sec24/Sec23 complex does not however have a BAR domain. Recent work revealed that Sar1 has membrane deformation activity through insertion of its N-terminal amphipathic α-helix in a lipid leaflet (Lee et al., 2005a). It is suggested that Sar1 is responsible for the initial membrane curvature necessary for COPII vesicle formation while the Sec23/Sec24 complex will determine the shape of the vesicle. For COPI, Arf1 shares strong similarity with Sar1, however, no membrane deformation activity has ever been described for this protein. In contrast, only when coatomer is added to liposomes or Golgi membranes, budding profiles can be observed, suggesting that it is coatomer that induces membrane curvature (Orci et al., 1993; Ostermann et al., 1993; Spang et al., 1998; Bremser et al., 1999). The cytoplasmic tail of p24 proteins is able to induce a conformational change within coatomer that leads to the polymerization of the complex (Reinhard et al., 1999). This polymerization might be the driving force that produces membrane deformation and leads to vesicle formation.
Concluding Remarks
With the discovery of three major isotypes of coatomer, and a similarity emerging between β, γ, δ, and ζ-COP and the adaptor complexes of the clathrin system, experiments will be possible in the near future to assign individual transport steps to individual coatomer isotypes. As a result, the general mechanism of protein transport through the Golgi should be better understood at a molecular level.
References
Antonin W., Holroyd C., Tikkanen R., Honing S., Jahn R. 2000. The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes. Mol. Biol. Cell. 11:3289–3298
Antonny B., Beraud-Dufour S., Chardin P., Chabre M. 1997. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry 36:4675–4684
Antonny B., Madden D., Hamamoto S., Orci L., Schekman R. 2001. Dynamics of the COPII coat with GTP and stable analogues. Nat. Cell. Biol. 3:531–537
Antonny B., Schekman R. 2001. ER export: public transportation by the COPII coach. Curr. Opin. Cell. Biol. 13:438–443
Antonny B. 2004. SNARE filtering by dynamin. Cell 119:581–582
Bannykh S.I., Balch W.E. 1998. Selective transport of cargo between the endoplasmic reticulum and Golgi compartments. Histochem. Cell. Biol. 109:463–475
Barlowe C., Orci L., Yeung T., Hosobuchi M., Hamamoto S., Salama N., Rexach M.F., Ravazzola M., Amherdt M., Schekman R. 1994. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77:895–907
Ben-Tekaya H., Miura K., Pepperkok R., Hauri H.P. 2005. Live imaging of bidirectional traffic from the ERGIC. J. Cell. Sci. 118:357–367
Bi X., Corpina R.A., Goldberg J. 2002. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature 419:271–277
Bigay J., Gounon P., Robineau S., Antonny B. 2003. Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature 426:563–566
Bigay J., Casella J.F., Drin G., Mesmin B., Antonny B. 2005. ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. Embo. J. 24:2244–2253
Blagitko N., Schulz U., Schinzel A.A., Ropers H.H., Kalscheuer V.M. 1999. gamma2-COP, a novel imprinted gene on chromosome 7q32, defines a new imprinting cluster in the human genome. Hum. Mol. Genet. 8:2387–2396
Blobel G., Potter V.R. 1967. Studies on free and membrane-bound ribosomes in rat liver. II. Interaction of ribosomes and membranes. J. Mol. Biol. 26:293–301
Blobel G., Dobberstein B. 1975a. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell. Biol. 67:835–851
Blobel G., Dobberstein B. 1975b. Transfer to proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J. Cell. Biol. 67:852–862
Boehm M., Aguilar R.C., Bonifacino J.S. 2001. Functional and physical interactions of the adaptor protein complex AP-4 with ADP-ribosylation factors (ARFs). Embo. J. 20:6265–6276
Bonfanti L., Mironov A.A., Jr., Martinez-Menarguez J.A., Martella O., Fusella A., Baldassarre M., Buccione R., Geuze H.J., Mironov A.A., Luini A. 1998. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell 95:993–1003
Brandhorst D., Zwilling D., Rizzoli S.O., Lippert U., Lang T., Jahn R. 2006. Homotypic fusion of early endosomes: SNAREs do not determine fusion specificity. Proc. Natl. Acad. Sci. USA 103:2701–2706
Bremser M., Nickel W., Schweikert M., Ravazzola M., Amherdt M., Hughes C.A., Sollner T.H., Rothman J.E., Wieland F.T. 1999. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell 96:495–506
Burri L., Varlamov O., Doege C.A., Hofmann K., Beilharz T., Rothman J.E., Sollner T.H., Lithgow T. 2003. A SNARE required for retrograde transport to the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 100:9873–9877
Cabrera M., Muniz M., Hidalgo J., Vega L., Martin M.E., Velasco A. 2003. The retrieval function of the KDEL receptor requires PKA phosphorylation of its C-terminus. Mol. Biol. Cell. 14:4114–4125
Caro L.G., Palade G.E. 1964. Protein Synthesis, Storage, and Discharge in the Pancreatic Exocrine Cell. An Autoradiographic Study. J. Cell. Biol. 20:473–495
Chardin P., Paris S., Antonny B., Robineau S., Beraud-Dufour S., Jackson C.L., Chabre M. 1996. A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature 384:481–484
Collins B.M., McCoy A.J., Kent H.M., Evans P.R., Owen D.J. 2002. Molecular architecture and functional model of the endocytic AP2 complex. Cell 109:523–535
Contreras I., Ortiz-Zapater E., Aniento F. 2004. Sorting signals in the cytosolic tail of membrane proteins involved in the interaction with plant ARF1 and coatomer. Plant. J. 38:685–698
Cosson P., Letourneur F. 1994. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science 263:1629–1631
Cosson P., Lefkir Y., Demolliere C., Letourneur F. 1998. New COP1-binding motifs involved in ER retrieval. Embo. J. 17:6863–6870
Cosson P., Amherdt M., Rothman J.E., Orci L. 2002. A resident Golgi protein is excluded from peri-Golgi vesicles in NRK cells. Proc. Natl. Acad. Sci. USA 99:12831–12834
Cukierman E., Huber I., Rotman M., Cassel D. 1995. The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science 270:1999–2002
De Matteis M.A., Di Campli A., Godi A. 2005. The role of the phosphoinositides at the Golgi complex. Biochim. Biophys. Acta. 1744:396–405
Dilcher M., Veith B., Chidambaram S., Hartmann E., Schmitt H.D., Fischer von Mollard G. 2003. Use1p is a yeast SNARE protein required for retrograde traffic to the ER. Embo. J. 22:3664–3674
Donaldson J.G., Cassel D., Kahn R.A., Klausner R.D. 1992a. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc. Natl. Acad. Sci. USA 89:6408–6412
Donaldson J.G., Finazzi D., Klausner R.D. 1992b. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature 360:350–352
Duden R., Griffiths G., Frank R., Argos P., Kreis T.E. 1991. Beta-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to beta-adaptin. Cell 64:649–665
Ehrlich M., Boll W., Van Oijen A., Hariharan R., Chandran K., Nibert M.L., Kirchhausen T. 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118:591–605
Eugster A., Frigerio G., Dale M., Duden R. 2000. COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. Embo. J. 19:3905–3917
Eugster A., Frigerio G., Dale M., Duden R. 2004. The alpha- and beta’-COP WD40 domains mediate cargo-selective interactions with distinct di-lysine motifs. Mol. Biol. Cell. 15:1011–1023
Farsad K., Ringstad N., Takei K., Floyd S.R., Rose K., De Camilli P. 2001. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J. Cell. Biol. 155:193–200
Faulstich D., Auerbach S., Orci L., Ravazzola M., Wegchingel S., Lottspeich F., Stenbeck G., Harter C., Wieland F.T., Tschochner H. 1996. Architecture of coatomer: molecular characterization of delta-COP and protein interactions within the complex. J. Cell. Biol. 135:53–61
Fiedler K., Veit M., Stamnes M.A., Rothman J.E. 1996. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science 273:1396–1399
Ford M.G., Mills I.G., Peter B.J., Vallis Y., Praefcke G.J., Evans P.R., McMahon H.T. 2002. Curvature of clathrin-coated pits driven by epsin. Nature 419:361–366
Franco M., Chardin P., Chabre M., Paris S. 1996. Myristoylation-facilitated binding of the G protein ARF1GDP to membrane phospholipids is required for its activation by a soluble nucleotide exchange factor. J. Biol. Chem. 271:1573–1578
Friedlander R., Jarosch E., Urban J., Volkwein C., Sommer T. 2000. A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nat. Cell. Biol. 2:379–384
Futatsumori M., Kasai K., Takatsu H., Shin H.W., Nakayama K. 2000. Identification and characterization of novel isoforms of COP I subunits. J. Biochem. (Tokyo) 128:793–801
Gallop, J.L., McMahon, H.T. 2005. BAR domains and membrane curvature: bringing your curves to the BAR. Biochem. Soc. Symp.:223–231
Gleeson P.A., Lock J.G., Luke M.R., Stow J.L. 2004. Domains of the TGN: coats, tethers and G proteins. Traffic 5:315–326
Glick B.S., Malhotra V. 1998. The curious status of the Golgi apparatus. Cell 95:883–889
Goldberg J. 1999. Structural and functional analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell 96:893–902
Goldberg J. 2000. Decoding of sorting signals by coatomer through a GTPase switch in the COPI coat complex. Cell 100:671–679
Gommel D.U., Memon A.R., Heiss A., Lottspeich F., Pfannstiel J., Lechner J., Reinhard C., Helms J.B., Nickel W., Wieland F.T. 2001. Recruitment to Golgi membranes of ADP-ribosylation factor 1 is mediated by the cytoplasmic domain of p23. Embo. J. 20:6751–6760
Gu F., Crump C.M., Thomas G. 2001. Trans-Golgi network sorting. Cell. Mol. Life. Sci. 58:1067–1084
Hara-Kuge S., Kuge O., Orci L., Amherdt M., Ravazzola M., Wieland F.T., Rothman J.E. 1994. En bloc incorporation of coatomer subunits during the assembly of COP-coated vesicles. J. Cell. Biol. 124:883–892
Harter C., Pavel J., Coccia F., Draken E., Wegehingel S., Tschochner H., Wieland F. 1996. Nonclathrin coat protein gamma, a subunit of coatomer, binds to the cytoplasmic dilysine motif of membrane proteins of the early secretory pathway. Proc. Natl. Acad. Sci. USA 93:1902–1906
Harter C., Wieland F.T. 1998. A single binding site for dilysine retrieval motifs and p23 within the gamma subunit of coatomer. Proc. Natl. Acad. Sci. USA 95:11649–11654
Hauri H.P., Kappeler F., Andersson H., Appenzeller C. 2000. ERGIC-53 and traffic in the secretory pathway. J. Cell. Sci. 113 (Pt 4):587–596
Heldwein E.E., Macia E., Wang J., Yin H.L., Kirchhausen T., Harrison S.C. 2004. Crystal structure of the clathrin adaptor protein 1 core. Proc. Natl. Acad. Sci. USA 101:14108–14113
Hoffman G.R., Rahl P.B., Collins R.N., Cerione R.A. 2003. Conserved structural motifs in intracellular trafficking pathways: structure of the gammaCOP appendage domain. Mol. Cell. 12:615–625
Honda A., Al-Awar O.S., Hay J.C., Donaldson J.G. 2005. Targeting of Arf-1 to the early Golgi by membrin, an ER-Golgi SNARE. J. Cell. Biol. 168:1039–1051
Hong W. 2005. SNAREs and traffic. Biochim. Biophys. Acta. 1744:493–517
Hudson R.T., Draper R.K. 1997. Interaction of coatomer with aminoglycoside antibiotics: evidence that coatomer has at least two dilysine binding sites. Mol. Biol. Cell. 8:1901–1910
Itoh T., De Camilli P. 2004. Membrane trafficking: dual-key strategy. Nature 429:141–143
Jackson M.R., Nilsson T., Peterson P.A. 1990. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. Embo. J. 9:3153–3162
Jamieson J.D., Palade G.E. 1966. Role of the Golgi complex in the intracellular transport of secretory proteins. Proc. Natl. Acad. Sci. USA 55:424–431
Kahn R.A., Gilman A.G. 1984. Purification of a protein cofactor required for ADP-ribosylation of the stimulatory regulatory component of adenylate cyclase by cholera toxin. J. Biol. Chem. 259:6228–6234
Karrenbauer A., Jeckel D., Just W., Birk R., Schmidt R.R., Rothman J.E., Wieland F.T. 1990. The rate of bulk flow from the Golgi to the plasma membrane. Cell 63:259–267
Klarlund J.K., Tsiaras W., Holik J.J., Chawla A., Czech M.P. 2000. Distinct polyphosphoinositide binding selectivities for pleckstrin homology domains of GRP1-like proteins based on diglycine versus triglycine motifs. J. Biol. Chem. 275:32816–32821
Kuge O., Hara-Kuge S., Orci L., Ravazzola M., Amherdt M., Tanigawa G., Wieland F.T., Rothman J.E. 1993. zeta-COP, a subunit of coatomer, is required for COP-coated vesicle assembly. J. Cell. Biol. 123:1727–1734
Kweon H.S., Beznoussenko G.V., Micaroni M., Polishchuk R.S., Trucco A., Martella O., Di Giandomenico D., Marra P., Fusella A., Di Pentima A., Berger E.G., Geerts W.J., Koster A.J., Burger K.N., Luini A., Mironov A.A. 2004. Golgi enzymes are enriched in perforated zones of golgi cisternae but are depleted in COPI vesicles. Mol. Biol. Cell. 15:4710–4724
Lanoix J., Ouwendijk J., Stark A., Szafer E., Cassel D., Dejgaard K., Weiss M., Nilsson T. 2001. Sorting of Golgi resident proteins into different subpopulations of COPI vesicles: a role for ArfGAP1. J. Cell. Biol. 155:1199–1212
Lee M.C., Miller E.A., Goldberg J., Orci L., Schekman R. 2004. Bi-Directional Protein Transport Between the ER and Golgi. Annu. Rev. Cell. Dev. Biol. 20:87–123
Lee M.C., Orci L., Hamamoto S., Futai E., Ravazzola M., Schekman R. 2005a. Sar1p N-Terminal Helix Initiates Membrane Curvature and Completes the Fission of a COPII Vesicle. Cell 122:605–617
Lee S.Y., Yang J.S., Hong W., Premont R.T., Hsu V.W. 2005b. ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J. Cell. Biol. 168:281–290
Letourneur F., Gaynor E.C., Hennecke S., Demolliere C., Duden R., Emr S.D., Riezman H., Cosson P. 1994. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell 79:1199–1207
Lewis M.J., Pelham H.R. 1990. A human homologue of the yeast HDEL receptor. Nature 348:162–163
Lewis M.J., Sweet D.J., Pelham H.R. 1990. The ERD2 gene determines the specificity of the luminal ER protein retention system. Cell 61:1359–1363
Lewis M.J., Pelham H.R. 1992. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell 68:353–364
Lewis S.M., Poon P.P., Singer R.A., Johnston G.C., Spang A. 2004. The ArfGAP Glo3 is required for the generation of COPI vesicles. Mol. Biol. Cell. 15:4064–4072
Liu W., Duden R., Phair R.D., Lippincott-Schwartz J. 2005. ArfGAP1 dynamics and its role in COPI coat assembly on Golgi membranes of living cells. J. Cell. Biol. 168:1053–1063
Lowe M., Kreis T.E. 1995. In vitro assembly and disassembly of coatomer. J. Biol. Chem. 270:31364–31371
Majoul I., Sohn K., Wieland F.T., Pepperkok R., Pizza M., Hillemann J., Soling H.D. 1998. KDEL receptor (Erd2p)-mediated retrograde transport of the cholera toxin A subunit from the Golgi involves COPI, p23, and the COOH terminus of Erd2p. J. Cell. Biol. 143:601–612
Majoul I., Straub M., Hell S.W., Duden R., Soling H.D. 2001. KDEL-cargo regulates interactions between proteins involved in COPI vesicle traffic: measurements in living cells using FRET. Dev. Cell. 1:139–153
Malhotra V., Serafini T., Orci L., Shepherd J.C., Rothman J.E. 1989. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell 58:329–336
Malsam J., Gommel D., Wieland F.T., Nickel W. 1999. A role for ADP ribosylation factor in the control of cargo uptake during COPI-coated vesicle biogenesis. FEBS Lett. 462:267–272
Malsam J., Satoh A., Pelletier L., Warren G. 2005. Golgin tethers define subpopulations of COPI vesicles. Science 307:1095–1098
Marsh B.J., Volkmann N., McIntosh J.R., Howell K.E. 2004. Direct continuities between cisternae at different levels of the Golgi complex in glucose-stimulated mouse islet beta cells. Proc. Natl. Acad. Sci. USA 101:5565–5570
Martinez-Menarguez J.A., Geuze H.J., Slot J.W., Klumperman J. 1999. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell 98:81–90
Matsuoka K., Orci L., Amherdt M., Bednarek S.Y., Hamamoto S., Schekman R., Yeung T. 1998. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93:263–275
Meyer D.M., Crottet P., Maco B., Degtyar E., Cassel D., Spiess M. 2005. Oligomerization and dissociation of AP-1 adaptors are regulated by cargo signals and by ArfGAP1-induced GTP hydrolysis. Mol. Biol. Cell. 16:4745–4754
Miller E., Antonny B., Hamamoto S., Schekman R. 2002. Cargo selection into COPII vesicles is driven by the Sec24p subunit. Embo. J. 21:6105–6113
Mironov A.A., Beznoussenko G.V., Polishchuk R.S., Trucco A. 2005. Intra-Golgi transport: a way to a new paradigm? Biochim. Biophys. Acta. 1744:340–350
Munro S., Pelham H.R. 1987. A C-terminal signal prevents secretion of luminal ER proteins. Cell 48:899–907
Munro S. 2004. Organelle identity and the organization of membrane traffic. Nat. Cell. Biol. 6:469–472
Nickel W., Malsam J., Gorgas K., Ravazzola M., Jenne N., Helms J.B., Wieland F.T. 1998. Uptake by COPI-coated vesicles of both anterograde and retrograde cargo is inhibited by GTPgammaS in vitro. J. Cell. Sci. 111 (Pt 20):3081–3090
Nilsson T., Jackson M., Peterson P.A. 1989. Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell 58:707–718
Niu T.K., Pfeifer A.C., Lippincott-Schwartz J., Jackson C.L. 2005. Dynamics of GBF1, a Brefeldin A-sensitive Arf1 exchange factor at the Golgi. Mol. Biol. Cell. 16:1213–1222
Ooi C.E., Dell’Angelica E.C., Bonifacino J.S. 1998. ADP-Ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J. Cell. Biol. 142:391–402
Orci L., Glick B.S., Rothman J.E. 1986. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport within the Golgi stack. Cell 46:171–184
Orci L., Palmer D.J., Ravazzola M., Perrelet A., Amherdt M., Rothman J.E. 1993. Budding from Golgi membranes requires the coatomer complex of non-clathrin coat proteins. Nature 362:648–652
Orci L., Stamnes M., Ravazzola M., Amherdt M., Perrelet A., Sollner T.H., Rothman J.E. 1997. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell 90:335–349
Orci L., Amherdt M., Ravazzola M., Perrelet A., Rothman J.E. 2000a. Exclusion of golgi residents from transport vesicles budding from Golgi cisternae in intact cells. J. Cell. Biol. 150:1263–1270
Orci L., Ravazzola M., Volchuk A., Engel T., Gmachl M., Amherdt M., Perrelet A., Sollner T.H., Rothman J.E. 2000b. Anterograde flow of cargo across the golgi stack potentially mediated via bidirectional "percolating" COPI vesicles. Proc. Natl. Acad. Sci. USA 97:10400–10405
Ostermann J., Orci L., Tani K., Amherdt M., Ravazzola M., Elazar Z., Rothman J.E. 1993. Stepwise assembly of functionally active transport vesicles. Cell 75:1015–1025
Owen D.J., Collins B.M., Evans P.R. 2004. Adaptors for clathrin coats: structure and function. Annu. Rev. Cell. Dev. Biol. 20:153–191
Paleotti O., Macia E., Luton F., Klein S., Partisani M., Chardin P., Kirchhausen T., Franco M. 2005. The small G-protein Arf6GTP recruits the AP-2 adaptor complex to membranes. J. Biol. Chem. 280:21661–21666
Palmer D.J., Helms J.B., Beckers C.J., Orci L., Rothman J.E. 1993. Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J. Biol. Chem. 268:12083–12089
Parlati F., McNew J.A., Fukuda R., Miller R., Sollner T.H., Rothman J.E. 2000. Topological restriction of SNARE-dependent membrane fusion. Nature 407:194–198
Parlati F., Varlamov O., Paz K., McNew J.A., Hurtado D., Sollner T.H., Rothman J.E. 2002. Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc. Natl. Acad. Sci. USA 99:5424–5429
Pavel J., Harter C., Wieland F.T. 1998. Reversible dissociation of coatomer: functional characterization of a beta/delta-coat protein subcomplex. Proc. Natl. Acad. Sci. USA 95:2140–2145
Pelham H.R. 1988. Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. Embo. J. 7:913–918
Pelham H.R., Rothman J.E. 2000. The debate about transport in the Golgi--two sides of the same coin? Cell 102:713–719
Pepperkok R., Whitney J.A., Gomez M., Kreis T.E. 2000. COPI vesicles accumulating in the presence of a GTP restricted arf1 mutant are depleted of anterograde and retrograde cargo. J. Cell. Sci. 113 (Pt 1):135–144
Peters C., Baars T.L., Buhler S., Mayer A. 2004. Mutual control of membrane fission and fusion proteins. Cell 119:667–678
Presley J.F., Cole N.B., Schroer T.A., Hirschberg K., Zaal K.J., Lippincott-Schwartz J. 1997. ER-to-Golgi transport visualized in living cells. Nature 389:81–85
Presley J.F., Ward T.H., Pfeifer A.C., Siggia E.D., Phair R.D., Lippincott-Schwartz J. 2002. Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport. Nature 417:187–193
Rein U., Andag U., Duden R., Schmitt H.D., Spang A. 2002. ARF-GAP-mediated interaction between the ER-Golgi v-SNAREs and the COPI coat. J. Cell. Biol. 157:395–404
Reinhard C., Harter C., Bremser M., Brugger B., Sohn K., Helms J.B., Wieland F. 1999. Receptor-induced polymerization of coatomer. Proc. Natl. Acad. Sci. USA 96:1224–1228
Reinhard C., Schweikert M., Wieland F.T., Nickel W. 2003. Functional reconstitution of COPI coat assembly and disassembly using chemically defined components. Proc. Natl. Acad. Sci. USA 100:8253–8257
Rodriguez-Boulan E., Musch A. 2005. Protein sorting in the Golgi complex: shifting paradigms. Biochim. Biophys. Acta. 1744:455–464
Rothman J.E., Wieland F.T. 1996. Protein sorting by transport vesicles. Science 272:227–234
Scheel A.A., Pelham H.R. 1998. Identification of amino acids in the binding pocket of the human KDEL receptor. J. Biol. Chem. 273:2467–2472
Schledzewski K., Brinkmann H., Mendel R.R. 1999. Phylogenetic analysis of components of the eukaryotic vesicle transport system reveals a common origin of adaptor protein complexes 1, 2, and 3 and the F subcomplex of the coatomer COPI. J. Mol. Evol. 48:770–778
Semenza J.C., Hardwick K.G., Dean N., Pelham H.R. 1990. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell 61:1349–1357
Serafini T., Orci L., Amherdt M., Brunner M., Kahn R.A., Rothman J.E. 1991a. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell 67:239–253
Serafini T., Stenbeck G., Brecht A., Lottspeich F., Orci L., Rothman J.E., Wieland F.T. 1991b. A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein beta-adaptin. Nature 349:215–220
Short B., Haas A., Barr F.A. 2005. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim. Biophys. Acta. 1744:383–395
Shorter J., Beard M.B., Seemann J., Dirac-Svejstrup A.B., Warren G. 2002. Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J. Cell. Biol. 157:45–62
Sohn K., Orci L., Ravazzola M., Amherdt M., Bremser M., Lottspeich F., Fiedler K., Helms J.B., Wieland F.T. 1996. A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J Cell Biol 135:1239–1248
Sollner T., Whiteheart S.W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J.E. 1993. SNAP receptors implicated in vesicle targeting and fusion. Nature 362:318–324
Sonnichsen B., Watson R., Clausen H., Misteli T., Warren G. 1996. Sorting by COP I-coated vesicles under interphase and mitotic conditions. J. Cell. Biol. 134:1411–1425
Sonnichsen B., Lowe M., Levine T., Jamsa E., Dirac-Svejstrup B., Warren G. 1998. A role for giantin in docking COPI vesicles to Golgi membranes. J. Cell. Biol. 140:1013–1021
Spang A., Matsuoka K., Hamamoto S., Schekman R., Orci L. 1998. Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc. Natl. Acad. Sci. USA 95:11199–11204
Stamnes M.A., Craighead M.W., Hoe M.H., Lampen N., Geromanos S., Tempst P., Rothman J.E. 1995. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc. Natl. Acad. Sci. USA 92:8011–8015
Stenbeck G., Harter C., Brecht A., Herrmann D., Lottspeich F., Orci L., Wieland F.T. 1993. beta’-COP, a novel subunit of coatomer. Embo. J. 12:2841–2845
Szafer E., Rotman M., Cassel D. 2001. Regulation of GTP hydrolysis on ADP-ribosylation factor-1 at the Golgi membrane. J. Biol. Chem. 276:47834–47839
Sztul E., Lupashin V. 2006. Role of tethering factors in secretory membrane traffic. Am. J. Physiol. Cell. Physiol. 290:C11–26
Takei K., Slepnev V.I., Haucke V., De Camilli P. 1999. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell. Biol. 1:33–39
Tanigawa G., Orci L., Amherdt M., Ravazzola M., Helms J.B., Rothman J.E. 1993. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J. Cell. Biol. 123:1365–1371
Terui T., Kahn R.A., Randazzo P.A. 1994. Effects of acid phospholipids on nucleotide exchange properties of ADP-ribosylation factor 1. Evidence for specific interaction with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 269:28130–28135
Tooze S.A., Martens G.J., Huttner W.B. 2001. Secretory granule biogenesis: rafting to the SNARE. Trends. Cell. Biol. 11:116–122
Traub L.M., Ostrom J.A., Kornfeld S. 1993. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J. Cell. Biol. 123:561–573
Traub L.M. 2005. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim. Biophys. Acta. 1744:415–437
Travers K.J., Patil C.K., Wodicka L., Lockhart D.J., Weissman J.S., Walter P. 2000. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101:249–258
Trucco A., Polishchuk R.S., Martella O., Di Pentima A., Fusella A., Di Giandomenico D., San Pietro E., Beznoussenko G.V., Polishchuk E.V., Baldassarre M., Buccione R., Geerts W.J., Koster A.J., Burger K.N., Mironov A.A., Luini A. 2004. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat. Cell. Biol. 6:1071–1081
Walter P., Blobel G. 1981. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J. Cell. Biol. 91:557–561
Wang C.C., Ng C.P., Lu L., Atlashkin V., Zhang W., Seet L.F., Hong W. 2004. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev. Cell. 7:359–371
Waters M.G., Serafini T., Rothman J.E. 1991. ‘Coatomer’: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature 349:248–251
Watson P.J., Frigerio G., Collins B.M., Duden R., Owen D.J. 2004. Gamma-COP appendage domain - structure and function. Traffic 5:79–88
Weber T., Zemelman B.V., McNew J.A., Westermann B., Gmachl M., Parlati F., Sollner T.H., Rothman J.E. 1998. SNAREpins: minimal machinery for membrane fusion. Cell 92:759–772
Wegmann D., Hess P., Baier C., Wieland F.T., Reinhard C. 2004. Novel isotypic gamma/zeta subunits reveal three coatomer complexes in mammals. Mol. Cell. Biol. 24:1070–1080
Wild K., Halic M., Sinning I., Beckmann R. 2004. SRP meets the ribosome. Nat. Struct. Mol. Biol. 11:1049–1053
Wilson D.W., Lewis M.J., Pelham H.R. 1993. pH-dependent binding of KDEL to its receptor in vitro. J. Biol. Chem. 268:7465–7468
Xu Y., Martin S., James D.E., Hong W. 2002. GS15 forms a SNARE complex with syntaxin 5, GS28, and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus. Mol. Biol. Cell. 13:3493–3507
Yang J.S., Lee S.Y., Gao M., Bourgoin S., Randazzo P.A., Premont R.T., Hsu V.W. 2002. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J. Cell. Biol. 159:69–78
Yoshihisa T., Barlowe C., Schekman R. 1993. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science 259:1466–1468
Yuan H., Michelsen K., Schwappach B. 2003. 14-3-3 dimers probe the assembly status of multimeric membrane proteins. Curr. Biol. 13:638–646
Zerangue N., Schwappach B., Jan Y.N., Jan L.Y. 1999. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22:537–548
Zhao L., Helms J.B., Brugger B., Harter C., Martoglio B., Graf R., Brunner J., Wieland F.T. 1997. Direct and GTP-dependent interaction of ADP ribosylation factor 1 with coatomer subunit beta. Proc. Natl. Acad. Sci. USA 94:4418–4423
Zhao L., Helms J.B., Brunner J., Wieland F.T. 1999. GTP-dependent binding of ADP-ribosylation factor to coatomer in close proximity to the binding site for dilysine retrieval motifs and p23. J. Biol. Chem. 274:14198–14203
Zhao X., Lasell T.K., Melancon P. 2002. Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein traffic. Mol. Biol. Cell. 13:119–133
Acknowledgments
The authors acknowledge Patricia McCabe for carefully proofreading the manuscript. We apologize to those authors who have publications relevant to the COPI-dependent transport that were not cited in this review due to space limitations. Moreover, in many cases, review articles were cited instead of the original literature, owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Béthune, J., Wieland, F. & Moelleken, J. COPI-mediated Transport. J Membrane Biol 211, 65–79 (2006). https://doi.org/10.1007/s00232-006-0859-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-006-0859-7