Abstract
Gas–liquid mass transfer is an extremely common process in the chemical industry and enhancing this process can help achieve high efficiency and low energy consumption. The addition of nanoparticles in the liquid phase is an important method for enhancing such transfers. In this paper, the preparation methods of nanofluids are briefly described and the parameters associated with nanofluid transport, such as mass-transfer coefficient, liquid volumetric mass-transfer coefficient, mass transfer interface area, and gas holdup, are introduced. Then, the latest experiments and mechanisms for the effect of nanofluids on the gas–liquid mass transfer process are reviewed from the viewpoint of transport parameters. The reasons for the enhancement of gas–liquid mass transfer by nanofluids are given: shuttle effect, mixing of the gas–liquid boundary layer, and inhibition of bubble coalescence. Finally, the problems existing in current research are assessed and, toward enhancing gas–liquid mass transfer using nanoparticles, future research directions are proffered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mass transfer underlies many processes in science, technology, and industry, including traditional industrial activities related to power, metallurgy, petroleum, chemicals, and materials as well as hi-tech fields such as aerospace, electronics, and nuclear energy. By enhancing mass transfer, not only does the mass transfer rate of a device or system increase but energy consumption may be lowered and efficiency may rise [1,2,3]. It is for these reasons that mass transfer technology has received much attention and in recent years has developed rapidly. The main means to enhance mass transfer is to improve device structures, plus the energy transfer field or the addition of dispersed phase particles. Experiments demonstrate that the addition of dispersed phase particles can provide a strong enhancement in mass transfer.
Nanofluids involves adding particles, either metal or nonmetal, and usually as an oxide, to a liquid, following a certain preparation and concentration, to form a new mass transfer medium [4]. Indeed, as the name suggests, a nanofluid is essentially a two-phase suspension of liquid and nanometer-sized particles. In general, liquids that carry solid nanoparticles are called “base fluids”. In terms of their composition, these nanoparticle suspensions are not a completely new concept. The magnetic fluids that emerged in the 1960s are typical nanofluids that improved the energy transfer process of the fluid by exploiting the presence of the magnetic nanoparticles.
Based on research on heat transfer enhancement inside fluids, nanoparticles have been developed to enhance the mass transfer process and their potential applications. For example, Krishnamurthy et al. [5] observed the diffusion of rhodamine dye in deionized water and Al2O3 / H2O nanofluids using photomicrography techniques. They showed that the mass transfer of the rhodamine dye in Al2O3 / H2O nanofluids was faster. Ma et al. [6] found a significantly faster ammonia absorption in aqueous ammonia solution by adding nanoparticles. Compared with micrometer-sized silica particles (1.4 and 7 um), Zhu et al. [7] found that MCM41 nanoparticles (250 nm) can significantly increase the volumetric mass-transfer coefficient; the thiol groups exhibit the largest mass transfer enhancement, equivalent to 1.9 times the base fluid. They suggest that the organic groups adhered to MCM41 nanoparticles giving them a spherical shape that changed the mass transfer enhancement. In addition, CO mass transfer enhancement depends on the interaction between nanoparticle and CO molecule, which is affected by the hydrophobicity of the nanoparticle and the functional groups on it. By adding nano-size oil, nano-emulsion absorbents used to enhance CO2 absorption were prepared for experiments performed by Jeong et al. [8]. An oil and surfactant ratio of 2:1 was best for dispersion stability, and the nano-emulsion with volume fraction of 0.01% gave the best absorption performance. They attributed the enhancements of CO2 absorption to the shuttle effect and the hydrodynamic effect of nanoscale oil droplets. Pang et al. [9] prepared binary nanofluids adding single silver nanoparticles and performed experiments of ammonia absorption in a bubble absorber. They found that the mass transfer of binary nanofluids with coolant increased compared with that in the absence of coolant; the highest absorption rate of the former increased by 55% at a nanoparticles mass fraction of 0.02%. Wang et al. [10] investigated CO2 absorption in ethanolamine-based nanofluids containing three different nanoparticles (silica, titania, and alumina). The overall mass transfer enhancement was observed to be governed by the bubble crushing effect. The experiment results showed that nanofluids with 0.1 wt% TiO2 nanoparticles expended 42% less desorption time under the same condition, compared with that without nanoparticles.
The mass transfer performance in nanofluids is not a common realization although many studies have been published on heat transfer enhancement. In this paper, the recent studies on the enhancement in mass transfer using nanofluids are summarized by comparing the experimental and mechanical results of various studies to present a guide to future studies on this topic. The methods of preparation and transport parameters of nanofluids are described and the latest experimental research results concerning their impact on gas–liquid mass transfer and the underlying mechanisms are reviewed in detail from the perspective of transport parameters. Moreover, the problems confronting in current research are assessed and the direction of future research is outlined based on the summary of relevant studies.
2 Nano-fluid transport parameters
2.1 Preparation of nanofluids
The preparation of nanofluids is a key step in the application of nanoparticles to enhance the mass transfer performance of the base fluid. At present, the preparation of a nanofluid falls under either a one-step or two-step method [11,12,13,14].
-
(1).
One-step method
The nanoparticles are dispersed directly into the base fluid; this saves having to collect and store nanoparticles as well as effectively prevents the metal nanoparticles oxidizing in air, so that agglomeration is minimized and, hence, nanoparticle dispersion and suspension stability in the base fluid is improved [15]. Therefore, a one-step method is particularly suitable for nanofluids suspended with metal nanoparticles of high thermal conductivity. However, this method has drawbacks with high costs, small amounts from preparations, and being ill-suited for batch preparation required for practical applications. One fast and efficient one-step chemical method was developed by Zhu et al. [16] in which non-agglomerated and stably suspended Cu nanofluids was prepared by reducing CuSO4·5H2O with NaH2PO2·H2O in ethylene glycol under microwave irradiation.
-
(2).
Two-step method
The nanoparticles are prepared in the first step and then added to the base fluid at a specified concentration using a prescribed method to form a stable nanoparticle suspension. This method is suitable for preparing all kinds of nanofluids and is simple and easy to conduct. However, the extremely high surface energy arising from the large specific surface area of the nanoparticles makes them agglomerate easily. Therefore, dispersing the nanoparticles uniformly is crucial for the two-step method. Ultrasonic vibration helps to overcome the mutual attraction between the small particles in the agglomerate, freeing the particles and dispersing them [17]. By adsorbing onto the surface of the particle and forming a thin layer, a surfactant changes the original properties of the particle surface so that the particles have a good affinity with the surrounding base liquid molecules and a strong repulsive force between the particles [18]. Hoog et al. [19] synthesized nanofluids containing Fe nanoparticles by dispersing Fe nanocrystalline powder in ethylene glycol. Fe nanoparticles were prepared by chemical vapor condensation, and ultrasonic vibration was used to mitigate nanoparticle aggregation.
2.2 Transport parameters
2.2.1 Mass transfer coefficient
The strength of mass transfer in a process (the amount of material transferred from one phase to another within a unit of time, per unit area, unit concentration, or pressure differential) is quantified by the mass-transfer coefficient. From that perspective, the enhancement factor, i.e., the ratio of the mass-transfer coefficients of the nanofluid and the base fluid, is used to indicate the degree of enhancement in mass transfer. With the different systems and mechanisms, a variety of mass-transfer coefficients arise. Let the mass transfer component be a gas phase denoted by A, its partial pressure by PA, its mass transfer interface area by a, and its mass transfer rate by ΦA; similarly for the liquid phase denoted by A*. Then the mass transferred per unit time is expressed as:
where kG and kL are the gas and liquid mass-transfer coefficients, pA∗ is the partial pressure of component A* in the gas phase at the phase interface, cA∗ and cA are the molar concentrations of components A* and A in the liquid phase body at any instant in time. As measurements of the parameters at the gas–liquid interface are difficult to obtain, it is impractical to calculate the mass transfer rate using eqs. (1) and (2). In accordance with Henry’s law, at the interface,
where H denotes the Henry constant. Substituting (1) and (2) to eliminate the interface parameters cA∗ and pA∗, According to the law of conservation of mass, it is known that the mass transfer rate of the same substance in the gas phase at the phase interface is equal to that in the liquid phase at the phase interface, i.e., eq. (1) is equal to eq. (2). the mass transfer rate obtains,
where KG and KL are the total gas-phase and liquid-phase mass-transfer coefficients, p∗ the gas phase partial pressure of the component A in equilibrium with the liquid phase cA, and c∗ is the molar concentration of component A in equilibrium with the gas phase pA. The coefficients KG and KL are expressible as:
The denominator of these two equations indicates the total mass transfer resistance between the gas and liquid phases and shows that increasing the gas mass-transfer coefficient kG and the liquid mass-transfer coefficient kL are two ways to reduce the mass transfer resistance. As for the gas phase, because the diffusion rate is higher, the gas mass-transfer coefficient kG can be easily increased by increasing the partial pressure and the turbulence of the gas phase. However, the physical properties of the liquid phase undergoing chemical reactions are quite different from those of the gas phase and there may be complex hydrodynamics occurring at the gas–liquid interface. Therefore, a number of aspects need considering to improve the mass transfer performance by kL.
2.2.2 Liquid volumetric mass-transfer coefficient
The influence of the nanoparticles on the liquid volumetric mass-transfer coefficient is the focus of gas–liquid mass transfer. The change of the liquid volumetric mass-transfer coefficient is the result of both kL and a acting together. Nanoparticles change the liquid volumetric mass-transfer coefficient by changing the apparent viscosity of the liquid. If the suspension is regarded as quasi-homogeneous, the liquid volumetric mass-transfer coefficient decreases with increasing apparent viscosity of the suspension. Zhu et al. [7] showed that MCM41 nanoparticles (250 nm) can significantly increase the liquid volumetric mass-transfer coefficient compared with micrometer-sized silica particles (1.4 and 7 μm).
The wettability of the particles has some influence on the liquid volumetric mass-transfer coefficient. The non-wetting particles are more likely to adhere to the gas–liquid interface to reduce the gas–liquid contact area than the wettability particles. Lee et al. [20] found that the volumetric mass-transfer coefficient of CH4-H2O with hydrophilic methyl-modified MSN is higher than that of nanofluids containing methyl-modified MSN with hydrophobic surface. Zhu et al. [7] also found that the wettability of nanoparticles has an impact on the mass transfer in CO absorption experiments with MCM41 nanoparticles.
2.2.3 Gas–liquid mass transfer interface area and gas holdup
The gas–liquid mass transfer interface area and the gas holdup are important parameters in gas–liquid mass transfer. The effect of nanoparticles on both of these is more complicated being influenced by many factors such as the physical-chemical properties of the liquid phase, the size and nature of the nanoparticles, the operating conditions, and the equipment parameters. In general, for bubbles smaller than 1.0 mm in size, nanoparticles would adhere to the surface of the bubbles to inhibit their aggregation and prevent bubbles from expanding, which increases the mass transfer interface area and enhances mass transfer. In the experimental study conducted by Olle et al. [21], the gas–liquid mass transfer interface area was enhanced in the presence of nanoparticles. However, the opposite may also occur.
3 Experimental research enhanced by nanoparticles
Given the different gas–liquid reaction laws, the gas–liquid reaction equipment used in industry is various, including several general categories such as stirring tanks, bubbling, and falling films. From the macroscopic perspective, the gas–liquid mass transfer process varies greatly in the different types of gas–liquid reaction equipment. However, the mass transfer processes have similarities from a microscopic perspective.
3.1 Macroscopic experimental study
3.1.1 Stirring absorption reaction system
Lu et al. [22,23,24] performed a study concerning the effect of carbon nanotube (CNT) particles on CO2 absorption in a stirred tank reactor compared with that in micro-activated carbon (AC). Both of these particles significantly enhanced the absorption of carbon dioxide, but the enhancement exhibited different trends under stirring. With increasing stirring speed, the enhancement factor of the AC suspension decreases whereas that of CNT increases. Differences in the enhancement mechanisms for the particles from their sizes were found by Lu et al. [22,23,24]. They concluded that in addition to grazing effects, the Brownian motion of the nanoparticles and micro-convection arising from the Brownian motion inside the mass transfer boundary layer should be considered, and the agglomeration of nanoparticles is also an important factor in mass transfer enhancement.
In another study, Lu et al. [25] investigated CO2 absorption enhanced by Al2O3 nanofluids with deionized water as base fluid in a thermostatic stirred reactor and analyzed the effects of the particle concentration, the surfactant stirring speed, ultrasonic treatment time, and other factors on the absorption. Sodium dodecyl benzene sulfonate was used to improve the stability of nanofluids. The mass fraction of nanoparticles and ultrasonic treatment time were effective in enhancing CO2 absorption; the combination of surfactant and nanoparticle enhances the absorption of CO2 more effectively than the nanoparticle without surfactant. The effective absorption rate of the Al2O3 suspension in the stable dispersion state decreased with increasing stirring speed, whereas that in a low dispersion state first increased and then decreased. They concluded that the absorption enhancement of Al2O3 nanofluids is attributable to the micro-convective movement from Brownian motion.
The enhancement effect of magnetic nanoparticles (Fe3O4) used in nanofluids for oxygen absorption enhancement was examined by Olle et al. [21]. The experimental results showed that oxygen mass transfer increased six-fold at volume fraction below 1%. The liquid mass-transfer coefficient and the gas–liquid interface area were enhanced in the presence of nanoparticles; the mass-transfer coefficient remained unchanged at volume fraction of 1%, and the enhancement of the volumetric mass-transfer coefficient showed a strong temperature dependence.
Park et al. [26] conducted experiments in respect to CO2 in aqueous nanometer-sized colloidal silica solution in a flat-stirred vessel. They concluded that the volumetric mass-transfer coefficient is related to the viscosity of the aqueous colloidal silica solution. In addition, based on the membrane theory with chemical reaction, the theoretical CO2 absorption rate was estimated and compared with the measured values. Zhang et al. [27] prepared nanofluids adding TiO2 nanoparticles and performed a study of the influence of the solid content and size of nanoparticles on the rate of CO2 absorption. TiO2 nanoparticles improve significantly the CO2 absorption rate. The enhancement factor first increases and then decreases as solid content increases, indicating that there is an optimum solid content, which gradually increases with increasing particle size. They concluded that the shuttle and micro-convection mechanisms contributed to the CO2 absorption enhancement.
3.1.2 Bubble absorption reaction system
Faraj et al. [28] performed a study concerning the absorption of hydrogen sulfide in silica and graphene nanofluids using a bubble column. The experimental results showed that the rate of hydrogen sulfide absorption decreases in the presence of silica nanoparticles whereas that of graphene nanofluids increased by 40% with a mass fraction of 0.02% and decreased with mass fractions above 0.02%. They speculated that the grazing effect was the main reason for the mass transfer enhancement.
Kim et al. [29] investigated the effect of Al2O3 nanoparticles on the absorption and diffusion of CO2 bubbles. The mass transfer enhancement performance of nanoparticles was analyzed by measuring the surface tension and viscosity of the nanofluids. The experimental results showed that the mass-transfer coefficient increases by 26% and the viscosity increased up to 11% at volume fraction of 0.01% compared with pure methanol, but the surface tension remained essentially unchanged.
The enhancement performance of carbon dioxide absorption in methanol-based Al2O3 nanofluids was investigated by Pineda et al. [30]. Experiments were performed under turbulent flow conditions and the range for the rotational Reynolds number was 1.9 × 104–19.2 × 104. For both pure methanol and nanofluids, the volumetric mass-transfer coefficient reached a maximum at 4 × 104 rotational Reynolds number, after which the absorption rate decreases almost linearly with increasing rotational speed. At this Reynolds number, the maximum increase in volumetric mass-transfer coefficient was estimated to be 20% and up to 27% for nanofluids with 0.1 vol% Al2O3 nanoparticles.
Wu et al. [31] conducted a study of the effect of Fe3O4 nanoparticles in combination with the application of an external magnetic field on the absorption of ammonia. The combined effect of both significantly increases the absorption of ammonia bubbles, and the comprehensive factor is higher than any single factor under the same conditions. When the initial concentration of ammonia is 20%, the mass concentration of Fe3O4 nanoparticles is 0.10% and the external magnetic field is 280 mT; the effective absorption ratio reaches a maximum of 1.0812 ± 0.0001 under adiabatic conditions. They suggested that several factors such as an increased heat transfer, an increased bubble rupture, and disturbances in the absorption fluid contributed to the absorption of ammonia.
3.1.3 Falling film absorption system
The absorption performance of CO2 in different nanofluids adding Al2O3, TiO2, and Fe3O4 nanoparticles through a wet-walled column was investigated by Samadi et al. [32]. The mass transfer was found to be enhanced in the presence of Al2O3 nanoparticles, whereas that of the TiO2 / H2O nanofluid decreased. The mass transfer rate of nanofluids containing Fe3O4 nanoparticles increased with increasing volume fraction of nanoparticles, but that is lower than that of water for all experimental conditions explored. A downward magnetic field also resulted in higher mass flux and mass-transfer coefficients than experiments without a magnetic field.
Taheri et al. [33] prepared nanofluids with diethanolamine (DEA) as the base fluid and performed experiments concerning the effect of SiO2 and Al2O3 nanoparticles on the absorption of CO2 and H2S in a wet-walled column system. The influence of some parameters such as particle type and concentration on this absorption was also investigated. The absorption of CO2 increased by 33% in the Al2O3 / DEA nanofluids with mass fraction of 0.05% and increased by 40% in SiO2 / DEA nanofluids with mass fraction of 0.05%. The mass transfer of H2S did not increase in the presence of silica nanoparticles but increased by 14% in Al2O3 / DEA nanofluids with a mass fraction of 0.1%.
Based on the preparation of stable α-Al2O3 and γ-Al2O3 nanofluids, Yang et al. [34] performed experiments regarding ammonia absorption, with and without nanoparticles. The type of nanoparticle and surfactant and the concentration of ammonia were considered to be the key factor affecting absorption. Ammonia absorption is weakened by α-Al2O3 nanofluids but enhanced by γ-Al2O3 nanofluids, and the efficiency of γ-Al2O3 nanofluids with PAA as surfactant is higher than that of SDBS. The absorptive capacity decreases with increasing ammonia solution concentrations, but the enhancement arising from the nanofluid is more pronounced than that without the nanoparticle.
Zhang et al. [35] prepared the H2O / LiBr-based nanofluids with Fe3O4 nanoparticle mass fractions of 0.01%, 0.05%, and 0.1%, and nanoparticle sizes of 20 nm, 50 nm, and 100 nm. They performed an investigation of the influence of the flow rate of the nanofluid, and the size and mass fraction of Fe3O4 nanoparticles on the absorption. The water vapor absorption rate was enhanced in the presence of nanoparticles. The reaction of heat and mass transfer is faster with smaller size of nanoparticles. The absorption enhancement ratio increases sharply with increasing mass fraction of nanoparticles in the lower range of mass fractions, after which there is a slow rising trend and even a decrease. The maximum mass transfer enhancement of the nanofluids with mass fraction of 0.05% and particle size of 20 nm is about 2.28 at a flow rate of 100 L·h−1. Zhang et al. [36] had also used numerical analysis software to analyze the absorption process of water vapor. The results showed that the rate of mass transfer rose with increasing nanoparticle concentration and decreasing nanoparticle diameter.
3.1.4 Gas–liquid hollow fiber membrane system
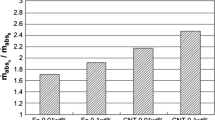
Peyravi et al. [37] prepared suspensions of Fe3O4, CNT, SiO2, and Al2O3 nanoparticles and conducted an investigation of the effect of nanoparticle on the mass transfer rate of CO2 in a gas–liquid hollow-fiber membrane contactor. The liquid flow rate and nanoparticle concentration have the greatest effect on CO2 absorption. The nanofluids stability and hydrodynamic diameter of the nanoparticles in the base fluid, both of which were considered as key factors in the choice of nanoparticles, were studied by UV-visible spectroscopy and the dynamic light scattering method. The experiment results showed that the maximum absorption rate of CO2 in nanofluids is enhanced by 43.8% at 0.15 wt% Fe3O4, 38.0% at 0.1 wt% CNT, 25.9% at 0.05 wt% SiO2, and 3.0% at 0.05 wt% Al2O3 compared with the base fluid.
Golkhar et al. [38] used nanofluids containing nano-silica and carbon nanotube to separate CO2 in a gas–liquid hollow fiber membrane contactor. The effects of type and concentration of nanoparticles, liquid, gas flow rates, liquid temperature, and CO2 inlet concentration on CO2 removal were investigated. The experimental results showed that the removal efficiency of nanofluid with 0.5 wt% silica increased by 20% at low flow rates and by about 9% at high rates, and that of CNT nanofluids is much better, increasing by 40% and 20% at low and high flow rates, respectively. The carbon dioxide removal efficiency of silica nanofluids was found to decrease slightly with increasing carbon dioxide inlet concentration because of fluid saturation. However, that of nanofluids with carbon nanotubes was almost constant and even increased.
Darabi et al. [39] developed a two-dimensional mathematical model to simulate the enhancement process of CO2 absorption by nanoparticles. They anticipated that the mass transfer enhancement was attributable to Brownian motion and the grazing effect. The absorption rate of nanofluids with 0.05 wt% of silica nanoparticles increased by 16%, whereas the corresponding value for CNT nanoparticles with high adsorption and hydrophobicity was 32%.
3.2 Microcosmic experimental study
Krishnamurthy et al. [5] observed the diffusion of dye droplets by microphotography in pure solution and nanofluids adding Al2O3 nanoparticles, and characterized the mass transfer enhancement of nanofluids through their diffusion area. The experimental results showed that the diffusion rate of the dye in Al2O3 / H2O nanofluids is much faster than that in pure water, and there is a peak when the volume fraction of nanoparticles is 0.5%. During the experiment, the molecular diffusion rate of fluorescein in all directions is consistent in pure water and the diffusion image area shows a regular circle whereas the diffusing surface of fluorescein is irregular in the nanofluids (Fig. 1). The main reason for this experimental phenomenon is the Brownian motion of Al2O3 nanoparticles in the fluid. The particles undergoing Brownian motion strike the diffusion frontal surface; the local micro-convection arising from the movement of nanoparticles influence the state of flow at this surface. Both ensure the diffusion of dye droplets in nanofluids is irregular in the different directions.
Based on the absorptive fluid with 8% mass fraction of ammonia, nanofluids with Cu, CuO, and Al2O3 added were prepared by Kim et al. [40]. The surface dispersant was added to ensure the dispersion stability of the nanofluids, as well as to enable the formation of ammonia bubbles at the end of the nozzle and their rise in the pure ammonia and nanofluids in the absorber to be imaged using a high-speed camera (Fig. 2). The experiment showed that the diameter of ammonia bubbles is the same in the nanofluid as in pure ammonia water once separated from the nozzles; the ammonia can be absorbed completely as they rise. Therefore, the absorption rate may characterized by the rise height of the bubble. The rising elevation of the ammonia bubble in nanofluids is less than that in pure ammonia water, which indicates that the absorption rate of ammonia in nanofluids is greater than that of pure ammonia. Zhao et al. [41] studied the state of a single bubble absorbed in the sulfite absorption process and analyzed the critical concentration of sulfite ions based on the double mode theory.
Setoura et al. [42] investigated Marangoni convection around stationary bubbles induced by CW laser heating of a single gold nanoparticle. Stationary bubbles were generated by irradiating each Au nanoparticle with a CW laser beam. The spatial distribution and velocity distribution of fluid convection around the stationary bubble were visualized from wide-field fluorescence microscopy of stained beads (Fig. 3). The Brownian motion of the beads was observed before laser irradiation. Once a bubble is generated by CW laser irradiation, a single bead begins to move toward the bubble and after a few seconds of bubble formation, the beads exhibited a ring-like structure of diameter 3 μm near the focal point of the laser. After the ring-like structure is formed, the number of beads at the interface between water and air bubbles gradually increases while keeping the diameter of the ring-shaped structure almost constant.
Jiang et al. [43] prepared nanofluids with Na2SO3 solution as base fluid and conducted a visualization study of nanoparticles undergoing mass transfer based on the total internal reflection fluorescence microscopy. The distribution of nanoparticle concentration at the gas–liquid interface and the bulk liquid phase, as well as the relationship between Brownian motion and particle size, solid content, and base fluid were analyzed. In the experiment with deionized water as base liquid, the concentration and Brownian velocity of the nanoparticles at the gas–liquid interface is higher than that in the bulk liquid (Fig. 4). Compared with different types of nanoparticles, the movement of TiO2 is faster than SiO2 nanoparticles, due mainly to the different densities for the nanoparticles, TiO2 > SiO2, resulting in SiO2 nanoparticles influenced more significantly by the liquid around it. In the experiments with Na2SO3 solution as base liquid, the velocity of the nanoparticle performing Brownian motion varies greatly and the dynamics of Brownian motion is different between the gas–liquid interface and the bulk liquid region when the solid content is relatively small. There is a best solid content for nanoparticle in regard to gas–liquid mass transfer enhancement; the optimal value from their experiments was 0.6 g/L. Micro-convective mass transfer is used to explain the enhancement of nanoparticles; the Sherwood number (Shp) is used to characterize the relative effect of micro-convection mass transfer and diffusion mass transfer. Considering the various factors affecting mass transfer such as nanofluid properties, the correlation between Shp and the diffusion coefficient were obtained based on their experimental results.
4 Mechanism study of mass transfer performance for nanofluids
4.1 Enhancement mechanism for nanofluids
The major enhancement mechanisms of gas–liquid mass transfer in nanofluids have been widely accepted, including the shuttle effect, the mixing of the gas–liquid boundary layer, and the inhibition of bubble coalescence.
-
(1).
Shuttle effect
The shuttle mechanism, also the called skimming effect, is based on the assumption that particles enter the mass transfer boundary layer and dwell for a short period if the particle size is smaller than the mass transfer film thickness in the liquid phase. During this process, particles adsorb a certain amount of mass transfer components and then return to the bulk liquid phase with the mass transfer microelement. Because the concentration of mass transfer components is around the particles, desorption of them occurs so that particle regeneration is achieved and their transport realized. In this way, the process of gas–liquid mass transfer is strengthened by its cyclical character [44,45,46,47,48]; see Fig. 5.
Kim et al. [40] studied the ammonia bubble absorption enhanced using three different ammonia nanofluids (Cu-NH3 / H2O CuO-NH3 / H2O and Al2O3-NH3 / H2O) By measuring and analyzing the change in transmissivity of a helium neon laser beam through the nanofluids during absorption, they concluded that nanoparticles moved to the gas–liquid interface to absorb gas and released the gas on returning to the bulk liquid. This “skimming effect” may be used to explain the behavior of the different ammonia nanofluids in enhancing the absorption of ammonia bubbles. The absorption enhancement effect of spherical functionalized MCM41 used in the nanofluid for CO absorption, studied by Zhu et al. [7], is one example explained by the shuttle effect. From their experimental observations, the surface hydroxyl of MCM41 was implicated in transporting CO from the bubble to the liquid, and therefore active in promoting the mechanism behind the shuttle effect. Suitable hydrophobic groups adhering to nanoparticles increase the contact area between the hydroxyl group and the gas and enhance the amount of adsorption for each particle. Hence, the amount transported in each cycle would increase. However, if the adhered groups are too numerous, a large number of particles float on the liquid interface and obstruct the shuttling of particles passing through the two phases of gas and liquid, resulting in less recycling, which ultimately would influence the mass transfer enhancement effect by nanoparticles.
Jiang et al. [49] established a 3-D unsteady model to study the mass transfer for CO2 absorption enhanced by nanoparticles in monoethanolamine (MEA) solution. For the contact surface, it was considered that the absorption of CO2 by nanofluids was a mass transfer process in the half plane because bubbles were big enough compared with nanoparticles in bubble absorption experiment and there was a stable phase interface in the gas–liquid contact area based on the analysis of shuttle effect and related theories. For the gas/liquid films, they thought that gas film and liquid film were both on the sides of contact surface and the mass transfer component was passed through two films in a way of diffusion and achieved a balance of concentration at the gas–liquid interface. It was a round trip that the component went back-wards and forwards between the liquid film and the main liquid phase to accomplish the process of mass transfer. The component CO2 gas went into the liquid film through the interface and reacted with the component of MEA. As a result of concentration difference for CO2 and MEA, two components continued to migrate forward by diffusion effect. The mixture process of fluid element was more intense enhanced by the nanoparticles moving in irregular Brownian motion, which lead to the facilitation of mass transfer. In addition, nanoparticles in the vicinity of gas–liquid interface would stay for a while. During this time, nanoparticles would absorb reaction products and move to the main liquid phase with fluid elements. After that the reaction products would be separated from the nanoparticles since the reaction product concentration was very low in the main liquid phase. In the end the desorbed nanoparticles would move to the liquid film again with fluid element.
-
(2).
Mixing of the gas–liquid boundary layer
Nanoparticles may change the hydrodynamic state of the gas–liquid interface during mass transfer and promote additional interfacial turbulence and convective mass transfer at the gas–liquid interface. The nanoparticle behaviors that are the main cause of convective mass transfer and concentration gradient are: i) the continuous collisions of nanoparticles with the boundary layer, thereby reducing the thickness of the boundary layer and also the mass transfer resistance between gas and liquid; ii) nanoparticle mixing within the boundary layer, particularly if the particle size is smaller than the boundary layer thickness, thereby creating concentration gradients of the liquid phase through the Brownian motion of the nanoparticles; iii) the motion of larger-diameter particles may increase the frequency of surface renewal through collisions and other interactions at the gas–liquid interface, and thereby enhancing mass transfer; and iv) constant collisions of nanoparticles at the gas–liquid interface may induce large bubbles to fragment. With many smaller bubbles, the interfacial area increases, and hence the mass transfer rate between gas and liquid rises [50,51,52].
Lu et al. [22,23,24] investigated the effect of a suspension containing carbon nanotube (CNT) or micrometer-sized AC particles on the CO2 absorption in a stirred reactor with constant temperature. The experimental results show that both CNT and micrometer-sized AC could enhance the CO2 absorption significantly. They suggest that the Brownian motion of nanoparticles in the mass transfer boundary layer and the micro-convection arising from Brownian motion of the nanoparticles should be considered in the absence of a skimming effect.
-
(3).
Inhibition of bubble coalescence
The mechanism underlying the inhibition of bubble coalescence involves particles at the bubble surface increasing the bubble stiffness and hindering coalescence between bubbles; this would indirectly increase the gas–liquid mass transfer area. Furthermore, the drag on the bubbles in the fluid increases, prolonging the residence time of bubbles in the liquid phase, and thereby enhancing the mass transfer flux [53,54,55,56]. The observation of a microcosmic state of the bubbles around nanoparticles was performed through optical microscopy by Roizard et al. [57]. The experimental analysis indicates that particles gather on the gas–liquid surface during mass transfer (Fig. 6).
4.2 Weakening mechanism for nanofluids
Many studies have shown that nanofluids could strengthen the gas–liquid mass transfer, but some researchers have come to the opposite conclusion [58,59,60,61,62]. In their investigations the presence of particles in the liquid phrase weaken the mass transfer between gas and liquid. The possible mechanisms for this weakening are: the agglomeration of nanoparticles reducing the mass transfer; an increase in viscosity for the base liquid raising the thickness of the diffusion boundary layer and thereby reducing the mass-transfer coefficient of the liquid phase and blocking the contact between gas and liquid by the nanoparticles.
In general, although the enhancing and weakening mechanisms explain the experimental results reasonably well, they each have their respective scope and have not been proved definitively. While the various mechanisms may be used to explain the mass transfer process of nanofluids in some absorption systems, in others, determining which mechanism plays the key role is difficult. There is no unified theoretical understanding of the underlying mechanisms so far and further research is needed.
5 Conclusion
Experimental research in recent years has shown that nanoparticles enhance the gas–liquid mass transfer for most absorption systems but may also inhibit this transfer in some system. The irregular Brownian movement of nanoparticles and the perturbation of the fluid around particles arising from that movement are particular microscopic behaviors in nanofluids that researchers generally take into consideration to explain the unique experimental phenomena in gas–liquid mass transfer processes. However, little is known through direct experimental observation on how both Brownian motion and perturbation combine to affect mass transfer. Further study of the micro-phenomenon is necessary. While having a narrow range of applicability in regard to solid content of nanoparticles, the laser speckle method (LSV) is usually used to measure indirectly the velocity of nanoparticles. However, even at low particle concentrations, the error in measuring particle movements using this method is large because of interparticle interactions. The influence of nanoparticles on gas–liquid mass transfer is uncertain, often differing greatly in the mass transfer enhancement for the same nanoparticles in different gas–liquid absorption systems and under different experimental conditions. Moreover, a quantitative description of gas–liquid mass transfer is lacking because of experimental limitations such as nanoparticle preparation and stability, and visualization equipment. The current mechanisms were derived through physical reasoning based on experimental observations and results. Therefore the mechanism behind gas–liquid mass transfer enhancement by nanoparticles remains to be explored and confirmed.
Based on the current research, four areas for future study were identified:
-
1).
The stability of nanofluids suspension directly affects mass transfer during absorption. Improvement in the preparation process of nanofluids is needed to obtain more stable nanofluids.
-
2).
Because of differences in the experimental conditions, the enhancement factors of nanoparticles on gas–liquid mass transfer are also different. The relationship between these factors and macroscopic parameters needs to be studied more comprehensively.
-
3).
More microscopic and visualization experiments are needed to study the structure of nanoparticles and the microscopic characteristics during gas–liquid mass transfer. Such experiments will help in understanding the mechanism of mass transfer more clearly.
-
4).
Last but not least, combining the macro- and micro-experimental research would entail bridging different length scales that may underlie the enhancement mechanism and clarify the dominance of one influencing factor over another.
References
Bigdeli MB, Fasano M, Cardellini A, Chiavazzo E, Asinari P (2016) A review on the heat and mass transfer phenomena in nanofluid coolants with special focus on automotive applications. Renew Sustain Energy Rev 60:1615–1633
Dhuriya R, Dalia V, Sunthar P (2018) Diffusiophoretic enhancement of mass transfer by nanofluids. Chem Eng Sci 176:632–640
Saidur R, Leong KY, Mohammad HA (2011) A review on applications and challenges of nanofluids. Renew Sustain Energy Rev 15:1646–1668
Choi SUS, Eastman J. (1995) Enhancing thermal conductivity of fluids with nanoparticles
Krishnamurthy S, Lhattacharya P, Phelan PE, Prasher RS (2006) Enhanced mass transport in nanofluids. Nano Lett 6:419–423
Ma X, Su F, Chen R, Zhang Y (2007) Heat and mass transfer enhancement of the bubble absorption for a binary nanofluid. J Mech Sci Technol 21:1813–1818
Zhu H, Shanks BH, Heindel TJ (2008) Enhancing CO-Water Mass Transfer by Functionalized MCM41 Nanoparticles. Ind Eng Chem Res 47:7881–7887
Jeong M, Lee JW, Lee SJ, Kang YT (2017) Mass transfer performance enhancement by nanoemulsion absorbents during CO2 absorption process. Int J Heat Mass Transf 108:680–690
Pang C, Wu W, Sheng W, Zhang H, Kang YT (2012) Mass transfer enhancement by binary nanofluids (NH3/H2O + Ag nanoparticles) for bubble absorption process. International Journal of Refrigeration-Revue Internationale Du Froid 35:2240–2247
Wang T, Yu W, Liu F, Fang M, Farooq M, Luo Z (2016) Enhanced CO2 Absorption and Desorption by Monoethanolamine (MEA)-Based Nanoparticle Suspensions. Ind Eng Chem Res 55:7830–7838
Liu L, Wang M, Liu YF (2015) Experimental Investigation on Preparation and Stability of Al2O3/CuO-water Nanofluids. In: Carl J (ed) Proceedings of the 2015 Asia-Pacific Energy Equipment Engineering Research Conference. Atlantis Press, Paris, pp 99–102
Prakash V, Rai B, Tyagi VK, Niyogi UK (2015) Dispersion and characterizations of nanofluids prepared with CuO and CNT nanoparticle. J Indian Chem Soc 92:1245–1251
Yu W, Xie HQA (2012) Review on Nanofluids: Preparation, Stability Mechanisms. and Applications Journal of Nanomaterials 17
Ghadimi A, Saidur R, Metselaar HSC (2011) A review of nanofluid stability properties and characterization in stationary conditions. Int J Heat Mass Transf 54:4051–4068
Jama M, Singh T, Gamaleldin SM, Koc M, Samara A, Isaifan RJ et al (2016) Critical Review on Nanofluids: Preparation, Characterization, and Applications. J Nanomater
Zhu HT, Lin YS, Yin YS (2004) A novel one-step chemical method for preparation of copper nanofluids. J Colloid Interface Sci 277:100–103
Wu W, Liu S, Hong H, Chen S. (2012) Stability Analysis of Water-Based Nanofluids Prepared by Using Supersonic Dispersion Method. In: Manufacturing Science and Technology, Pts 1–8. Edited by Fan W. pp. 6174–6180
Yang L, Du K, Niu X, Li Y, Zhang Y (2011) An experimental and theoretical study of the influence of surfactant on the preparation and stability of ammonia-water nanofluids. International Journal of Refrigeration-Revue Internationale Du Froid 34:1741–1748
Hong TK, Yang HS, Choi CJ (2005) J Appl Phys 97:1–4
Lee S-Y, Mo K-S, Choi J-H, Hur NH, Kim Y-K, Oh B-K et al (2015) Enhancement of CH4-water mass transfer using methyl-modified mesoporous silica nanoparticles. Korean J Chem Eng 32:1744–1748
Olle B, Bucak S, Holmes TC, Bromberg L, Hatton TA, Wang DIC (2006) Enhancement of oxygen mass transfer using functionalized magnetic nanoparticles. Ind Eng Chem Res 45:4355–4363
Lu S, Xing M, Sun Y, Dong X (2013) Experimental and Theoretical Studies of CO2 Absorption Enhancement by Nano-Al2O3 and Carbon Nanotube Particles. Chin J Chem Eng 21:983–990
Lu S, Zhao Y, Song J, Li Y (2017) Experimental studies of CO2 absorption enhancement in water-based nanofluids of carbon nanotubes. Braz J Chem Eng 34:597–606
Lu S-m, Xing M, Li Y, Song J. (2014) Theoretical and Experimental Research of CO2 Absorption Enhancement by Carbon Nano-tube. In: Micro-Nano Technology Xv. Edited by Tang F; pp. 388–393
Lu S, Song J, Li Y, Xing M, He Q (2015) Improvement of CO2 absorption using AL(2)O(3) nanofluids in a stirred thermostatic reactor. Can J Chem Eng 93:935–941
Park S-W, Choi B-S, Kim S-S, Lee B-D, Lee J-W (2008) Absorption of carbon dioxide into aqueous colloidal silica solution with diisopropanolamine. J Ind Eng Chem 14:166–174
Zhang Y, Zhao B, Jiang J, Zhuo Y, Wang S (2016) The use of TiO2 nanoparticles to enhance CO2 absorption. International Journal of Greenhouse Gas Control 50:49–56
Faraj SHE, Esfahany MN, Jafari-Asl M, Etesami N (2014) Hydrogen Sulfide Bubble Absorption Enhancement in Water-Based Nanofluids. Ind Eng Chem Res 53:16851–16858
Kim JH, Jung CW, Kang YT (2014) Mass transfer enhancement during CO2 absorption process in methanol/Al2O3 nanofluids. Int J Heat Mass Transf 76:484–491
Pineda IT, Kang YT (2016) CO2 absorption enhancement by nanoabsorbents in Taylor-Couette absorber. Int J Heat Mass Transf 100:39–47
Wu W-D, Liu G, Chen S-X, Zhang H (2013) Nanoferrofluid addition enhances ammonia/water bubble absorption in an external magnetic field. Energy and Buildings 57:268–277
Samadi Z, Haghshenasfard M, Moheb A (2014) CO2 Absorption Using Nanofluids in a Wetted-Wall Column with External Magnetic Field. Chemical Engineering & Technology 37:462–470
Taheri M, Mohebbi A, Hashemipour H, Rashidi AM (2016) Simultaneous absorption of carbon dioxide (CO2) and hydrogen sulfide (H2S) from CO2-H2S-CH4 gas mixture using amine-based nanofluids in a wetted wall column. Journal of Natural Gas Science and Engineering 28:410–417
Yang L, Du K, Cheng B, Jiang Y, (2010) Ieee. The Influence of Al2O3 Nanofluid on the Falling Film Absorption with Ammonia-water. In: 2010 Asia-Pacific Power and Energy Engineering Conference
Zhang LY, Liu YY, Wang Y, Li HQ, Yang XH, Jin LW, et al. (2016) Experimental study on enhanced falling film absorption process using H2O/LiBr nanofluids
Zhang LY, Li Y, Wang Y, Cao LX, Meng XZ (2016) Asme. Effect of nanoparticles on H2O/LiBr falling film absorption process
Peyravi A, Keshavarz P, Mowla D (2015) Experimental Investigation on the Absorption Enhancement of CO2 by Various Nanofluids in Hollow Fiber Membrane Contactors. Energy Fuel 29:8135–8142
Golkhar A, Keshavarz P, Mowla D (2013) Investigation of CO2 removal by silica and CNT nanofluids in microporous hollow fiber membrane contactors. J Membr Sci 433:17–24
Darabi M, Rahimi M, Dehkordi AM (2017) Gas absorption enhancement in hollow fiber membrane contactors using nanofluids: Modeling and simulation. Chem Eng Process 119:7–15
Kim J-K, Jung JY, Kang YT (2007) Absorption performance enhancement by nano-particles and chemical surfactants in binary nanofluids. International Journal of Refrigeration-Revue Internationale Du Froid 30:50–57
Zhao B, Li Y, Tong HL, Zhuo YQ, Zhang L, Shi H et al (2005) Study on the reaction rate of sulfite oxidation with cobalt ion catalyst. Chem Eng Sci 60:863–868
Setoura K, Ito S, Miyasaka H (2017) Stationary bubble formation and Marangoni convection induced by CW laser heating of a single gold nanoparticle. Nanoscale 9:719–730
Jiang JZ, Zhao B, Cao M, Zhuo YQ, Wang SJ (2015) Effect of nanoparticles on oxygen absorption enhancement during sulfite forced oxidation. Int J Heat Mass Transf 90:1098–1104
Keshishian N, Esfahany MN, Etesami N (2013) Experimental investigation of mass transfer of active ions in silica nanofluids. International Communications in Heat and Mass Transfer 46:148–153
Lee JW, Pineda IT, Lee JH, Kang YT (2016) Combined CO2 absorption/regeneration performance enhancement by using nanoabsorbents. Appl Energy 178:164–176
Nagy E, Feczko T, Koroknai B (2007) Enhancement of oxygen mass transfer rate in the presence of nanosized particles. Chem Eng Sci 62:7391–7398
Pasieka J, Coulombe S, Servio P (2014) The Effect of Hydrophilic and Hydrophobic Multi-Wall Carbon Nanotubes on Methane Dissolution Rates in Water at Three Phase Equilibrium (V-L-w-H) Conditions. Ind Eng Chem Res 53:14519–14525
Pineda IT, Lee JW, Jung I, Kang YT (2012) CO2 absorption enhancement by methanol-based Al2O3 and SiO2 nanofluids in a tray column absorber. International Journal of Refrigeration-Revue Internationale Du Froid 35:1402–1409
Jiang J-Z, Liu L, Sun B-M (2017) Model study of CO2 absorption in aqueous amine solution enhanced by nanoparticles. International Journal of Greenhouse Gas Control 60:51–58
Yoon S, Chung JT, Kang YT (2014) The particle hydrodynamic effect on the mass transfer in a buoyant CO2-bubble through the experimental and computational studies. Int J Heat Mass Transf 73:399–409
Nedeltchev S (2017) Theoretical prediction of mass transfer coefficients in both gas-liquid and slurry bubble columns. Chem Eng Sci 157:169–181
Sheng W, Wu W, Zhang H, Pang C, Wu R. (2012) Mechanism Analysis on Performance Enhancement of Ammonia Bubble Absorption by Nanofluid. In: Materials Science and Information Technology, Pts 1–8. Edited by Zhang CS. pp. 195–201
Amani P, Amani M, Mehrali M, Vajravelu K (2017) Influence of quadrupole magnetic field on mass transfer in an extraction column in the presence of MnFe2O4 nanoparticles. J Mol Liq 238:145–154
Ghanadi AM, Nasab AH, Bastani D, Kordi AAS (2015) The Effect of Nanoparticles on the Mass Transfer in Liquid-Liquid Extraction. Chem Eng Commun 202:600–605
Sara ON, Icer F, Yapici S, Sahin B (2011) Effect of suspended CuO nanoparticles on mass transfer to a rotating disc electrode. Exp Thermal Fluid Sci 35:558–564
Kim K, Lee J, Seo K, Kim MG, Ha KS, Kim C (2016) Enhancement of methane-water volumetric mass transfer coefficient by inhibiting bubble coalescence with electrolyte. J Ind Eng Chem 33:326–329
Roizard C, Poncin S, Lapicque F, Py X, Midoux N (1999) Behavior of fine particles in the vicinity of a gas bubble in a stagnant and a moving fluid. Chem Eng Sci 54:2317–2323
Jung J-Y, Lee JW, Kang YT (2012) CO2 absorption characteristics of nanoparticle suspensions in methanol. J Mech Sci Technol 26:2285–2290
Ma X, Su F, Chen J, Bai T, Han Z (2009) Enhancement of bubble absorption process using a CNTs-ammonia binary nanofluid. International Communications in Heat and Mass Transfer 36:657–660
Pineda IT, Choi CK, Kang YT (2014) CO2 gas absorption by CH3OH based nanofluids in an annular contactor at low rotational speeds. International Journal of Greenhouse Gas Control 23:105–112
Turanov AN, Tolmachev YV (2009) Heat- and mass-transport in aqueous silica nanofluids. Heat Mass Transf 45:1583–1588
Yang L, Du K, Niu XF, Cheng B, Jiang YF (2011) Experimental study on enhancement of ammonia-water falling film absorption by adding nano-particles. International Journal of Refrigeration-Revue Internationale Du Froid 34:640–647
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51606065). We thank Richard Haase, Ph.D., from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, JZ., Zhang, S., Fu, XL. et al. Review of gas–liquid mass transfer enhancement by nanoparticles from macro to microscopic. Heat Mass Transfer 55, 2061–2072 (2019). https://doi.org/10.1007/s00231-019-02580-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-019-02580-7