Abstract
Nanofluids, the fluid suspensions of nonmaterials, have shown many interesting properties and the unique features offer unprecedented potential for many applications. Research on nanofluids has progressed rapidly since its enhanced thermal conductivity was first noted, about a decade ago, though much debate and inconsistency have been reported. Insufficient understanding of the formulation, mechanism of nanofluids further limits their applications [1–34]. Inconsistent data have been presented in the literature on the effect that nanofluids have on the boiling heat-transfer coefficient; however, almost all researchers [35–43] have noted an enhancement in the critical heat flux during nanofluid boiling. Some researchers have observed nanoparticle deposition at the heater surface, which they have related back to the critical heat flux augmentation. In the review, the future developments of these technologies are discussed. In order to be able to put the nanofluid heat transfer technologies into practice, fundamental of these studies are greatly needed to comprehend the physical mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Increasing interests have been paid to nanofluids as they are potential fluids for heat transfer enhancement. A performance shown by nanofluids makes them a candidate for enhanced heat transfer media. The base fluids used for study of heat transfer are deionised water (DW), ethylene glycol (EG), glycerol, silicone oil, and the binary mixture of DW and EG. Various nanoparticles (NPs) involving Al2O3, NPs with different sizes, SiC, NPs with different shapes. MgO NPs, ZnO NPs, SiO2 NPs, Fe3O4 NPs, TiO2 NPs and carbon nanotubes with different pre-treatment are used as additives. The thermal conductivity enhancements of nanofluids could be influenced by multi-faceted factors including the volume fraction of the dispersed NPs, the tested temperature, the thermal conductivity of the base fluid, the size of the dispersed NPs, the pre-treatment process and the additives of the fluids. The thermal transport mechanisms in nanofluids are further discussed, and the capable approaches for optimizing the thermal conductivity of nanofluids have been anticipated.
Nanofluids are used to improve heat transfer during single-phase, nucleate boiling, flow boiling and critical heat flux in various thermal systems. The fundamental mechanism of nanofluid heat transfer has not yet well established so far. Thus, the applications of these technologies are significantly limited.
1.1 The nano fluid-based approach
During past years, a significant effort has been devoted to research in well-known applications such as chemical processing, general manufacturing and energy conversion devices with general power systems heat exchangers and high performance gas turbines. In addition, a major number of research papers speak to topics that are at the frontiers of both fundamental research and important promising technologies, including nanoscale structures.
1.1.1 Methods of preparation of nanofluids
-
a.
One step method
To decrease the agglomeration of nanoparticles, Eastman et al. [44] developed a one-step physical vapour condensation method to prepare Cu/ethylene glycol nanofluids. The one-step process consists of at the same time making and dispersing the particles in the fluid. In this method, the processes of drying, storage, carrying and distribution of nanoparticles are avoided, so the agglomeration of nanoparticles is minimized and the stability of fluids is increased [45]. The one-step processes can prepare homogeneously scattered nanoparticles, and the particles can be stably overhanging in the base fluid. The vacuum (Submerged Arc Nanoparticle Synthesis System) SNASS is another efficient method to prepare nanofluids using different dielectric liquids [46, 47]. The different morphologies are mainly subjective and determined by various thermal conductivity properties of the dielectric liquids. The nanoparticles prepared exhibit needle-like, polygonal, square and circular morphological shapes. The method avoids the undesired particle aggregation quite well. One-step physical method cannot synthesize nanofluids in large scale and the cost is also high, so the one-step chemical method is developing rapidly. Zhu et al. [48] presented a step chemical method for preparing copper nanofluids by reducing with in ethylene glycol under microwave irradiation. Well-dispersed and stably suspended copper nanofluids were obtained. Mineral oil-based nanofluids containing silver nanoparticles with a narrow-size distribution were also prepared by this method [49]. The particles could be stabilized by Korantin, which coordinated to the silver particle surfaces via two oxygen atoms forming a dense layer around the particles. The silver nanoparticle suspensions were stable for about one month. Stable ethanol-based nanofluids containing silver nanoparticles could be prepared by microwave-assisted one-step method [50]. In the method, poly vinyl pyrrolidone (PVP) was employed as the stabilizer of colloidal silver and reducing agent for silver in solution. The cationic surfactant octadecylamine (ODA) is also an efficient phase-transfer agent to synthesize silver colloids [51]. The phase transfer of the silver nanoparticles arises due to coupling of the silver nanoparticles with the ODA molecules present in organic phase via either coordination bond formation or weak covalent interaction. Phase transfer method has been developed for preparing homogeneous and stable graphene oxide colloids. Graphene oxide nanosheets (GONs) were successfully transferred from water to n-octane after modification by oleylamine, and the schematic illustration of the phase transfer process [52].
-
b.
Some other methods
Wei and Xie [53] developed a continuous-flow micro fluidic micro reactor to amalgamate copper nanofluids. By this method, copper nanofluids can be continuously synthesized, and their microstructure and properties can be varied by adjusting parameters such as reactant concentration, flow rate and additive. CuO nanofluids with high solid volume fraction (up to 10 vol%) can be synthesized through a novel precursor transformation method with the help of ultrasonic and microwave irradiation [54]. The precursor Cu(OH)2 is completely transformed to CuO nanoparticle in water under microwave irradiation. The ammonium citrate prevents the growth and aggregation of nanoparticles, resulting in a stable CuO aqueous nanofluid with higher thermal conductivity than those prepared by other dispersing methods. Phase-transfer method is also a facile way to obtain monodisperse noble metal colloids [55]. In a water–cyclohexane two-phase system, aqueous formaldehyde is transferred to cyclohexane phase via reaction with dodecylamine to form reductive intermediates in cyclohexane. The intermediates are capable of reducing silver or gold ions in aqueous solution to form dodecylamine-protected silver and gold nanoparticles in cyclohexane solution at room temperature.
Feng et al. [56] used the aqueous organic phase-transfer method for preparing gold, silver and platinum nanoparticles on the basis of the decrease of the PVP’s solubility in water with the temperature increase. Phase-transfer method is also applied for preparing stable kerosene-based Fe3O4 nanofluids. Oleic acid is successfully grafted onto the surface of Fe3O4 nanoparticles by chemisorbed mode, which lets Fe3O4 nanoparticles have good compatibility with kerosene [1]. The Fe3O4 nanofluids prepared by phase-transfer method do not show the previously reported “time dependence of the thermal conductivity characteristic”. The preparation of nanofluids with controllable microstructure is one of the key issues. It is well known that the properties of nanofluids strongly depend on the structure and shape of nonmaterial’s. The recent research shows that nanofluids synthesized by chemical solution method have both higher conductivity enhancement and better stability than those produced by the other methods [2]. This method is distinguished from the others by its controllability. The nanofluids microstructure can be varied and manipulated by adjusting synthesis parameters such as temperature, acidity, ultrasonic and microwave irradiation, types and concentrations of reactants, additives and the order in which the additives are added to the solution.
-
c.
The stability of nanofluids
The agglomeration of nanoparticles results in not only the settlement and clogging of micro channels but also the decreasing of thermal conductivity of nanofluids. So, the exploration on stability is also a key issue that influences the properties of nanofluids for application. It is necessary to study and analyze influencing factors to the dispersion stability of nanofluids. (a) The stability evaluation methods for nanofluids. (b) The ways to enhance the stability of nanofluids and (c) The stability mechanisms of nanofluids.
2 Measurement of thermal conductivity
Thermal conductivity is the most vital parameter accountable for enhanced heat transfer, many experimental work been reported on this characteristic as shown in the Table 1.
The transient hot wire method [8], the steady-state parallel-plate technique [11] and the temperature oscillation technique [9] have been employed to measure the thermal conductivity of nanofluids. Among them the transient hot wire method has been used most extensively. Because in general nanofluids are electrically conductive, it is difficult to apply the ordinary transient hot-wire technique directly. A modified hot-wire cell and electrical system was proposed by Nagasaka and Nagashima [10] by coating the hot wire with an epoxy adhesive which has excellent electrical insulation and heat conduction. However, Das et al. [9] pointed that possible concentration of ions of the conducting fluids around the hot wire may affect the accuracy of such experimental results. The oscillation method was proposed by Roetzel et al. [11] and further developed by Czarnetski and Roetzel [12]. This method is purely thermal and the electrical components of the apparatus are separate from the test sample. Hence ion movement should not affect the measurement. Alumina (Al2O3) and copper oxide are the most common and economical nanoparticles used by many researchers in their experimental investigations. All the experimental results have established the enhancement of the thermal conductivity by adding of nanoparticles.
Eastman et al. [44] measured the thermal conductivity of nanofluids containing Al2O3, CuO and Cu nanoparticles with two different base fluids: water and HE-200 oil. A 60 % improvement of the thermal conductivity was achieved as compared to the ensuing base fluids for only 5 % volume of nanoparticles. They also showed that the use of Cu nanoparticles (using the one-step method) results in larger enhancement than that of CuO (using the two-step method).
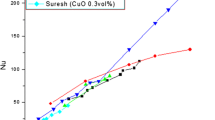
Lee et al. [3] suspended CuO and Al2O3 (18.6 and 23 nm, 6, 24.4 and 38.4 nm for them, in that order) with two different base fluids: water and ethylene glycol (EG) and obtained four combinations of nanofluids: CuO in water, CuO in EG, Al2O3 in water and Al2O3 in EG. Their experimental grades showed that nanofluids have considerably higher thermal conductivities than the same liquids without nanoparticles. The CuO/EG mixture showed enhancement of more than 20 % at 4 vol% of nanoparticles. In the low volume fraction range (<0.05 in test), the thermal conductivity ratio increase almost linearly with volume fraction. Although the size of Al2O3 particle is smaller than that of CuO, Copper nanofluids exhibited improved thermal conductivity values than Al2O3-nanofluids; no clarification is to be had for this study at this time refer Fig. 1.
Improved thermal conductivity of oxide nanofluids systems as measured by Lee et al. [3]. k/ko denotes the ratio of thermal conductivity of nanofluid to that of the base fluid
Wang et al. [4] measured the capable thermal conductivity of nanofluids by a steady-state parallel-plate technique. The base fluids (water, ethylene glycol (EG), vacuum pump oil and engine oil) contained suspended Al2O3 and CuO nanoparticles of 28 and 23 nm of average diameters, respectively. Experimental results demonstrated that the thermal conductivities of all nanofluids were higher than those of their base fluids. Also, comparison with various data indicated that the thermal conductivity of nanofluids increases with decreasing particles size. Results demonstrated 12 % improvement of the effective thermal conductivity at 3 vol% of nanoparticles as compared to 20 % improvement reported by Masuda et al. [13] and 8 % reported by Lee et al. [3] at the same volume fraction of particles. Xuan et al. [6] improved the thermal conductivity of water using Cu particles of comparatively large size (100 nm) to the same size as has been found using CuO particles of much lesser dimension (36 nm). A suitable mixture dispersants may develop the stability of the suspension. They used oleic acid for transformer oil–Cu nanofluids and laureate salt for water–Cu suspension in their study and found that Cu particles in transformer oil had superior characteristics to the suspension of Cu particles in water.
Xie et al. [14] investigated the effects of the pH value of the suspension, the specific surface area (SSA) of the spread Al2O3 particles, the crystalline phase of the solid phase, and the thermal conductivity of the base fluid on the thermal conductivity of nanofluids. They found that the increase in the difference between the pH value and isoelectric point (the pH at which a molecule carries no net electrical charge) of Al2O3 resulted in improvement of the effective thermal conductivity. Also, the thermal conductivity enhancements were highly reliant on the specific surface area (SSA) of the nanoparticles. The crystalline phase of the nanoparticles did not appear to have any noticeable effect on the thermal conductivity of the suspensions.
Eastman et al. [44] used pure Cu nanoparticles of less than 10 nm size and achieved 40 % increase in thermal conductivity for only 0.3 % volume fraction of the solid dispersed in ethylene glycol. They indicated that the increased ratio of surface to volume with decreasing size should be vital factor. Also, they showed that the additive acid may stabilize the suspension and thus increase the effective thermal conductivity. A Fe-nanofluid was prepared by Hong et al. [15] with ethylene glycol; Fe nanoparticles with mean size of 10 nm were formed by chemical vapour condensation process. It is found that Fe nanofluids exhibited higher improvement of thermal conductivity than Cu nanofluids. Their result indicated that the material with high thermal conductivity is not always the best candidate for the suspension to improve the thermal characteristics of base fluids. Also, they concluded that the thermal conductivity of nanofluids increased non-linearly with the solid volume fraction.
Hong et al. [16] also investigated the effect of the clustering of Fe nanoparticles on the thermal conductivity of nanofluids. They found that the thermal conductivity of nanofluids is directly related to the agglomeration of Fe nanoparticles, which caused the nonlinear relation between the Fe volume fraction and thermal conductivity of nanofluids due to rapid clustering of nanoparticles in condensed nanofluids (Fig. 2).
Murshed et al. [5] investigated TiO2 nanoparticles in rod shape (Φ10 × 40) and spherical shape (Φ15) dispersed in deionised water. They observed that nearly 33 and 30 % enhancement of the effective thermal conductivity occurred for TiO2 particles of Φ10 × 40 and Φ15, respectively. Their results showed that both particle size and shape influence the thermal conductivity of nanofluids.
Xie et al. [17] Prepared and measured the thermal conductivities of 26 nm and 0.6 μm SiC suspensions in deionised water and EG using a transient hot-wire method. Different from experimental results of Lee et al. [3] they found that the nanofluids with the same solid particles in different base fluids had the same improvement in the effective thermal conductivity. Furthermore, results showed that HC model [19] is capable of predicting the thermal conductivity of 0.6 μm SiC suspensions, while it under-predict that of 26 nm particles [17, 18].
Das et al. [9] as shown in Fig. 3, examined the effect of temperature on thermal conductivity enhancement for nanofluids containing Al2O3 (38.4 nm) or CuO (28.6 nm) through an experimental investigation using temperature oscillation method. They observed that a two to fourfold increase in thermal conductivity can take place over the temperature range of 21–52 °C. The results suggest the application of nanofluids as cooling fluids for devices with high energy density where the cooling fluid is likely to work at a temperature higher than the room temperature. They also mention that the inherently stochastic motion of nanoparticles could be a probable explanation for the thermal conductivity enhancement since smaller particles show greater enhancements of thermal conductivity with temperature than do larger particles.
Comparison between selected theoretical models and experimental data on thermal conductivity for Al2O3/water nanofluids [9]
Li et al. [20] conducted an experimental investigation to examine the effects of variations in the temperature and volume fraction on the effective thermal conductivity of CuO (29 nm) and Al2O3 (36 nm) water suspensions. Results demonstrated that nanoparticle material, diameter, volume fraction and bulk temperature have significant effects on the thermal conductivity of the nanofluids. For example, for Al2O3/water suspension, increase in the mean temperature from 27 to 34.7 °C results in the enhancement of nearly three times. They also derived two simple two-factor linear regression for the discussed nanofluids (Al2O3/water: (keff − kb)/kb = 0.764φ + 0.0187(T − 273.15) − 0.462, CuO/water: (keff − kb)/kb = 3.761φ + 0.0179(T − 273.15) −0.307). However, additional investigations are necessary to verify the impact of the temperature on the effective thermal conductivity of nanofluids.
Patel et al. [21] studied gold (Au) and silver (Ag) nanoparticles with thoriate and citrate as coatings in water- and toluene based fluids. The nanofluids were prepared to check the conductivity enhancement effect at low concentrations they found 5–21 % enhancement of the thermal conductivity of nanofluids for water with citrate in the temperature range 30–60 °C at a very low loading of 0.00026 vol% of Ag particles. For a loading of 0.011 % of Au particles, the improvement of thermal conductivity was around 7–14 %. Such interesting phenomena indicate that, except for particle dimension, there exist important (Table 2).
3 Boiling heat transfer enhancement
-
a.
Micro-engineered surfaces: The impact of surface on pool boiling heat transfer. Promoting steady onset of nucleate boiling at low wall superheat levels for electronic cooling
-
b.
Enhancing nucleate boiling
-
c.
Enhancing critical heat flux
Continuous advances in semiconductor smallness and manufacturing are bringing power densities to progressively higher levels. For example, at the upper border of future applications, high-end military and aerospace band-gap amplifier will produce waste heat flux on the order of 1 × 104 kW/m2. Only two-phase (boiling) liquids are suitable for such high dissipation rates. Faulkner et al. [57] tried to achieve 1 × 104 kW/m2 cooling flux using boiling with ceramic/water nanofluids. Their maximum heat flux dissipation was only 0.125 × 104 kW/m2 for saturated boiling and 0.280 × 104 kW/m2 for sub-cooled boiling. From their results it is observed that the high potential of boiling of nanofluids in cooling systems.
Witharana [35] investigated the boiling heat transfer coefficients (HTC) of Au (not mentioned size)/water, SiO2 (30 nm)/water, and SiO2/ethylene glycol nanofluids in a cylindrical vessel with 10 cm in diameter and 10 cm in height. The bottom of the vessel was supplied by fixed heat flux and the top was open to the atmosphere. Results of Au/water nanofluids (φ = 0.0002 and 0.001 wt%) showed that the HTC of nanofluids was higher than that of pure water, and increased with increasing gold particle concentrations. For example, the enhancement of HTC was above 11 % in the intermediate heat flux (3 × 104 W/m2) and as high as 21 % in the extreme case (4 × 104 W/m2). However, the SiO2/water and SiO2/ethylene glycol nanofluids recorded decreased HTC as compared to the base fluids, which was somewhat contrary to expectations. The author had not explained such odd phenomena. Possibly re-examination of the experiments should be a good option.
Li et al. [36] also observed deteriorated pool boiling heat transfer for CuO/water nanofluids. They attributed the reason to the decreasing of active nucleation sites from nanoparticle sedimentation.
Das et al. [58] carried out an experimental study of pool boiling characteristics of Al2O3 nanofluids under atmospheric conditions on a tube having diameter in 20 mm. They found that the addition of nanoparticles degraded the boiling performance by increasing the wall superheat for a given heat flux. The deterioration in boiling performance increased with increasing particle concentration and surface roughness. This means there should be additional effects to degrade the boiling characteristics such as the changed surface features of the nanoparticles. Since the surface tension and latent heat were unchanged and the only inauspicious change was the increased viscosity, heat transfer character during pool boiling was expected to be improved considering the important, increase of thermal conductivity, which had active effects on the major factors in heat transfer during pool boiling such as the micro-layer evaporation and reorganization of thermal boundary layer. They attributed it to the affected surface roughness during pool boiling of nanofluids. For higher particle concentration and higher surface roughness, the uneven surface can trap the particles more easily and make the surface smoother, which can cause the degradation of the boiling performance.
Das et al. [37] also studied the pool boiling performance under tubes with small diameter (4, 6.5 mm) where the bubble size and tube diameter are in the same order. They observed that deterioration for the narrow tubes was lower than that in the large tube (D = 20 mm). The small tube results in a large curvature of the surface to induce direct departure rather than sliding of larger bubbles. It is conformed, apart from the increased effective thermal conductivity; there should be some other factors that affect the boiling performance of nanofluids.
Bang et al. [38, 39] studied boiling of Al2O3–water nanofluids on a 100 mm square surface at high heat fluxes and it is observed that the surface roughness after boiling increased with nanoparticle concentration. However, the critical heat flux (CHF) (the peak heat flux, under which a boiling surface can stay in nucleate boiling regime) performance has been enhanced to 32 and 13 % for both horizontal flat surface and vertical flat surface in the pool, respectively. They observed that the increased roughness caused by the deposition of nanoparticles will cause a fouling effect to deteriorate the boiling heat transfer performance. On the other hand, however, noteworthy pool boiling heat transfer enhancement was found for Al2O3/water nanofluids.
Tu et al. [40] and You et al. [41] investigated the boiling curve and the CHF of Al2O3/water nanofluids in pool boiling with various nanoparticle concentrations ranging from 0 to 0.05 g/l. They found that the boiling heat transfer coefficients of all various concentrations as well as pure water were the same, which demonstrated that the nucleate boiling heat transfer effectiveness was not affected by the inclusion of nanoparticles. They also found that the size of bubbles increased with addition of nanoparticles to water. Correspondingly, the incidence of bubble departure degraded substantially. They claimed that there were some unknown key factors to increase the CHF in nanofluids, which need further study. On the other hand, however, significant pool boiling heat transfer improvement was found for Al2O3/water nanofluids.
Vassallo et al. [42] confirmed that the CHF increases for nanofluids (silica–water). They conducted experiments for both nano- and micro-solutions at the same solid volume fraction on a 0.4 mm diameter horizontal Ni–Cr wire at the atmospheric pressure. The heat transfer enhancements were not found in the nucleate boiling regime, but the CHF was increased significantly for both nano- and micro-particles. Addition of nanoparticles resulted in a maximum heat flux of about three times that of pure water and almost twice that of micro-particle/water mixture.
Zhou [43] investigated experimentally the heat transfer characteristics of copper/acetone based nanofluids with and without acoustic cavitations. Results showed that the copper nanoparticles and acoustic cavitation had significant influence on heat transfer in the fluid. However, the addition of nanoparticles did not affect the dependence of the heat transfer on acoustic cavitation and fluid sub-cooling. As compared to the experimental results of Das et al. [37, 58], the pool boiling heat transfer did not reduce with increased particle volume fractions in absence of acoustic field. While in an acoustic field, the boiling heat transfer of nanofluids was enhanced and the boiling hysteresis disappeared.
Wen et al. [59] conducted experiments on pool boiling heat transfer using γ-Al2O3–water nanofluids, which were produced through an electrostatic stabilization method with the aid of a high shear homogeniser. They found that presence of alumina in the nanofluid can enhance the boiling heat transfer significantly, by 40 % for a 1.25 wt% concentration of the particles. Considering the controversies from previous studies, they proposed some possible reasons such as the extra thermal resistance to the boiling surface caused by the sedimentation of nanoparticles, effect of surfactant, and interaction between boiling surface and nanofluids. The aggregation of nanofluids should be an important factor to affect the boiling performance, which need to be clarified quantitatively further. The currently available experimental data on boiling heat transfer of nanofluids (as shown in Table 3) are limited. However, conflicting results were observed from these limited data as far as the effect of nanoparticles on the boiling heat transfer performance is concerned. The inconsistencies indicate that our understanding of the thermal behavior of nanofluids related to the boiling heat transfer is still poor. Further detailed and valuable investigations are necessary to understand the phenomena of boiling of nanofluids the pool boiling will be affected by the surface properties such as surface roughness, surface wettability, and surface contamination. In the reviewed studies, however, only the surface roughness is the most often considered parameter. The systematic studies should have been carried out to include the interaction between the surface and nanofluids (wettability).
4 Enhancing nucleate boiling and critical heat flux
The occurrence enhancement nucleate boiling heat transfer can be achieved by creation of re-entrant type cavities with poorly wetted walls. Nucleate boiling heat transfer can be enhanced by roughening the surface or by impregnating the surface with artificial nucleation cavities.
Re-entrant cavities promote nucleation by vapour trapping and evaporation.
Size of dispersed nanoparticles seems to be important. With reducing nanoparticle size, the dispersion behavior improves and the surface-to-volume ratio increases. Thermal transport in nanofluids involves the heat transfer in the vicinity of the nanoparticle-fluid interfaces, so the increased surface area will improve the heat transfer rate between nanoparticles and fluids.
Nanoparticle geometry has influence on the effectiveness. At the current stage, spherical nanoparticles and long carbon nanotubes are the most used nanostructures for nanofluids. Other nanostructures, such as nanorods, nanowires, nanoplates and complex-shaped nanoparticles are less investigated.
Nanoparticle deposition on the heater surface has been observed by nearly all the researchers who have conducted nanofluid boiling, both pool and convective. This is thought to be the main reason behind the critical heat flux enhancement. This nanoparticle layer increases the surface roughness, the surface area, and the surface wettability. The mechanisms underlying this CHF enhancement have still not been clarified, and they remain under discussion and investigation. Water has been the most commonly used working fluid with nanoparticles so far in the literature.
Within consequently, liquid convection is increased resulting in enhanced heat transfer rates. Although roughening a surface increases pool boiling heat transfer rates, the performance of the surface deteriorates with time. Long term stability of nucleation sites is an important parameter in developing commercially viable surfaces for enhanced nucleate boiling heat transfer. An enhanced structure consisting of six layers of grooved copper plates capable of being stacked was employed by Ramaswamy et al. [60] they used this structure to investigate the effects of sub-cooling, pressure and height on its boiling performance. They observed that an increase in sub cooling and pressure caused an increase in the heat transfer rate. The effect of varying pore size and pitch on boiling performance was investigated in the same study. An increase in pore size and reduction in pitch augmented the heat transfer rate (Figs. 4, 5).
Boiling curves at different concentration of Al2O3–water nanofluids during pool boiling [41]
Coursey et al. [61] investigated the performance of graphite foam in nucleate boiling at various chamber pressures, working fluids and liquid levels. They concluded that heat fluxes approaching 50 × 104 W/m2 were attainable with wall temperatures maintained below 85 °C (Fig. 6).
Li et al. [9] studied pool boiling of water over copper surfaces with and without copper nano-rods. Significant enhancement in pool boiling heat transfer rates was observed. The pool boiling curves shifted to the left i.e. lower wall superheats were required to sustain a given level of heat flux on the surface with nano-rods compared to the plain surface. The magnitude of peak heat fluxes however, was relatively constant (140–160 W/cm2). Many bubbles of smaller diameter were observed on the nano-rod coated surface compared to the plain surface. This was similar to the photographic observations of Sathyamurthi et al. [62] for boiling over MWCNT coated surfaces. The increased nucleation was attributed to the micro-scale defects with the nano-scale pores between the nano-rods acting as ‘feeders’ to the larger cavities. Aging of the test surface caused a decrease in heat transfer rates but these were higher than the heat transfer rates over the plain copper surface. Bubble release frequencies were 2–4 times the release frequencies of the plain surface which varied from 2 to 30 Hz for wall superheats ranging from 0 to 25 K. Bubble departure diameters however, were 2.5–3.5 times higher for the plain surface. Chang et al. [11] investigated the impact of micro porous coatings on pool boiling heat transfer rates using FC-72 as the working fluid. Five different coatings ranging between 3 and 50 nm in size were evaluated. Three coating materials comprised of aluminium (1–20 nm), copper (1–50 nm) and diamond (8–12 nm) powders with brushable ceramic as binder and methyl ethyl ketone as the carrier. The other two coating materials were diamond (8–12 nm) and silver (3–10 nm) with Omega bond as the binder and alcohol as the carrier. All five coatings showed similar heat transfer rates (30 W/cm2) at CHF with the excess temperature ranging between 10 and 20 Therefore, a heat transfer enhancement of 200 % was obtained over that shown by a reference surface. Additionally, the microstructured surfaces showed early incipience of boiling (ONB) at significantly lower heat flux levels (2,000–4,000 W/m2) and at significantly lower excess temperatures (2.5–9.). By contrast, corresponding heat flux at boiling incipience was 0.0003 W/m2 and the excess temperature was 45° for the reference surface. One of the obstacles in employing phase change cooling of electronic components is the high excess temperatures required to initiate the incipience of boiling. This study demonstrates that micro porous surfaces can be employed to lower incipience.
Honda et al. [12] studied pool boiling of FC-72 over three micro-fabricated surfaces (10 x 10 x 0.5 mm). Three different surfaces, two consisting of micro pin fins (50, 50 to 60, 100 nm pitch) with and without sub-micron scale roughness (32 nm) and a third surface with sub-micron scale roughness of 25 nm and no pin fins. Experiments were conducted at subcooling levels of 0, 3, 25 and 45 K. The micro-pin fins enhanced the heat transfer in the nucleate boiling regime and in increasing CHF. The chip with sub-micron scale roughness showed an increase in heat transfer rates at low heat fluxes whereas the chips with micro-pin fins enhanced heat fluxes in the high heat flux regime. Photographic observations revealed the presence of vapor trapping between the micro-pin fins after bubble departure leading to enhanced nucleation rates and possibly, higher heat transfer rates. Critical heat flux was observed to increase linearly with subcooling. Heat transfer rates at CHF were 1.8–2.3 times the CHF for a smooth surface, similar to the increase in surface area of 2.2 times for the micro-fabricated surfaces.
4.1 Boiling on CNT coated surfaces
Endo et al. [63] CNT were the first to produce and analyze carbon nanotubes (CNT) using high resolution electron microscopy in 1975. The rolled-up lamellar sheets of `carbon produced by pyrolysis of Benzene and Ferrocene Were analyzed. Single walled and multi-walled carbon nanotubes (SWCNT and MWCNT) were imaged in this study. In Iijima et al. [23] reported the formation of microtubules of graphitic carbon. Capped, needle-like MWCNT 4–30 nm in diameter and 1 nm in length were produced by using D.C. arc discharge evaporation in an inert argon filled chamber. CNT have inspired numerous research activities due to their interesting physical properties. Physical properties of CNT are highly dependent on its length, diameter and hilarity. The thermal properties of CNT are dependent upon its phonon dispersion relation and phonon density states [13]. Phonons are quantized vibration modes occurring in crystalline materials. The thermal conductivity of CNT varies with temperature and the phonon mean free paths. The estimation of thermal conductivity of SWCNT has been the subject of numerous studies and is a subject of debate. CNT may possess high thermal conductivity values (3,000–6,000 W/mK) according to studies [17–19]. However, another study [22] shows thermal conductivity of CVD-grown MWCNT possesses thermal conductivities of the order of 25 W/mK. However, these MWCNT were grown by CVD at temperatures as low as 600 K resulting in many defects. It must be mentioned here, that the MWCNT used in this study are made using a similar technique and similar temperatures. Therefore, it is reasonable to expect similar thermal properties for the CNT used in this study. Crystalline, defect-free CNT are produced at elevated temperatures of the order of 1,100 K or more. The impact of CNT on pool boiling heat transfer rates has been the subject of few studies.
Independent investigations by Ahn et al. [64] and Ujereh et al. [65] were amongst the first in this area. Ujereh et al. [65] reported a 60 % enhancement at CHF employing silicon substrates covered with multi-walled carbon nanotubes (MWCNT) of 30 nm diameter and 20–30 microns length with FC-72 as the working fluid. The study was restricted to the nucleate boiling regime. Almost simultaneously, Ahn et al. [64] reported a 25–28 % enhancement in heat transfer (within the heating block) at CHF using two silicon substrates coated with MWCNT 9 and 25 nm in height with PF-5060 as the working fluid. The 25 nm tall CNT surface showed a 57 % increase in heat transfer rates at the Leiden frost point while the 9 nm tall CNT substrate showed no perceptible enhancement in the film-boiling regime.
Pool boiling experiments were conducted using PF-5060 as the working fluid on silicon substrates with (9 and 25 nm) and without nanotubes. The enhancement in heat flux was weakly dependent on the height of the MWCNT layer in the nucleate boiling regime. The enhancement in heat transfer rates for nanotube coated substrates decreased with increase in subcooling. This was attributed to the change in morphology of the bubbles with increase in subcooling. Additionally, the nucleation site density increased on the nanotube coated surfaces compared to the bare substrate. In nucleate boiling, MWCNT coated substrates yielded higher wall heat fluxes under saturated and subcooled conditions compared to a bare silicon surface. The 25 nm tall MWCNT array augmented CHF by 2 % compared to a bare silicon surface under saturated; the heat flux was sensitive to the height of the MWCNT coating. For Type-B MWCNT (25 nm height) the wall heat flux values were enhanced under saturated (62 %), 5. sub cooling (62–124 %), 10. Subcooling conditions (66–148 %), (compared to control experiments performed on a bare silicon surface). However, for Type-A MWCNT (9 nm thickness) the wall heat flux values are similar to the corresponding values for the bare silicon surface during film boiling, for low subcooling. At high subcooling the film boiling heat flux values were enhanced significantly and by similar magnitudes for both bare silicon and Type-A MWCNT coated heaters.
Venkatachalapathy et al. [66] worked on the pool boiling characteristics, critical heat flux and heat transfer co-efficient have been studied on polished surface and sand blasted surface with Al2O3–water nanofluids in the study showed that the critical heat flux of sand blasted surface with deionised water was 1.4 % higher than that of polished surface. Figures 4 and 5 low concentrations of alumina, 1, 2 and 4 g/l were used for further study of polished surface and sand blasted surface (SBS). The maximum critical heat flux (CHF) of sand blasted surface for 4 g/l of alumina–water nanofluid was 19.3 % higher than deionised water as seen from Figs. 7 and 8.
Comparison of CHF for DI water and various nanofluid concentrations for polished surface [66]
Comparison of CHF for DI water and various nanofluid concentrations for sand blasted surface [66]
Whereas the boiling heat transfer co-efficient (BHT) deteriorated for 4 g/l, i.e. maximum concentration. The increase in nucleation sites was the reason for the enhancement in CHF of sand blasted surface as compared to polished surface and the particle deposition on the heater surface during the boiling process was the reason for deterioration of boiling heat transfer co-efficient. Hence pool boiling characteristics of alumina nanofluids depend on the size of nanoparticles, volume concentrations and surface roughness. The boiling heat transfer coefficient of sand blasted surface for 1, 2, and 4 g/l of Al2O3–water nanofluids were 1.5, 6.5, and 6.8 % lower than that of DI water whereas the polished surface showed 2.3, 6.0, and 11.7 % respectively as seen from Figs. 9 and 10. A maximum heat transfer coefficient of 55 kW/m2 K for polished surface with 1 g/l of nanofluid concentration whereas for the sand blasted surface, it was around 52 kW/m2 K. The BHT was deteriorated with the increase in volume concentration of nanofluids mainly due to the particle deposition on the heater surface.
Effect of nanofluid concentration on heat transfer coefficient for polished surface [66]
Effect of nanofluid concentration on heat transfer coefficient for sand blasted surface [66]
Huang et al. [67] carry out the experiment on TiO2 nanoparticle-coated nickel wires were produced by electrical heating in various nanofluid concentrations ranging from 0.01 to 1 wt% with various processing heat fluxes from 0 to 1,000 kW/m2. The experimental results confirmed up to 82.7 % enhancement on critical heat flux (CHF) in condition of coated nickel wire (processed in 1 wt% with 1,000 kW/m2) boiling in pure water. The contact angle measurement revealed that the hydrophilic porous coating formed by vigorous vaporization of TiO2 nanofluid in nucleate boiling regime enormously modified the wettability of heating surface consequently improving the CHF.
The coverage of nanoparticle deposition tended to become more complete as concentration and processing heat flux increased based on SEM and EDS analysis. The nanoparticles dispersed in base fluid exhibited little effect on CHF enhancement and could even hinder the percentage of CHF augmentation from boosting, which demonstrated that one could enhance CHF by using only small amount of nanoparticles just sufficient to form surface coatings instead of preparing working fluid with great bulk (Figs. 11, 12).
Boiling curves represented by data points for bare nickel wires in pure water and in TiO2 nanofluids of various concentrations [67]
Average CHF versus TiO2 nanofluid concentrations [67]
Sharma et al. [69] pool boiling experiments were conducted for sandblasted stainless steel (grade 316) plate heaters submerged in deionised water and water-based zinc–oxide nanofluid, for transient heat flux conditions with power through the heaters increasing quadratically with time. Heat flux in the experiments was increased from zero to CHF in short time frames of 1, 10 and 100 s. Consistent with previous studies, transient CHF for DI water was higher than steady state CHF, and CHF increased with decreasing duration of the transient. Additionally, it was observed that for nanofluid tests, a porous and hydrophilic nanoparticle layer started to deposit on the heater surface in short time frames of 10 and 100 s, and this layer was responsible for the enhanced CHF compared to DI water. However, for the 1 s tests, nanoparticle deposition did not occur and consequently the CHF was not enhanced. Finally, experiments with heaters precoated with nanoparticles were performed and it was found that CHF was enhanced for all transient durations down to 1 s, establishing firmly that the CHF enhancement occurs due to surface modifications by the deposited nanoparticles, and not by nanoparticles suspended in solution (Figs. 13, 14, 15).
a Post-test SEM images for heaters used in steady state experiments showing a clean surface for heaters used with deionised water (b) deposit made of nanoparticles on heaters boiled in nanofluid [69]
Post-test SEM images for heaters used in transient experiments with nanofluids. Showing nanoparticle deposition for t0 = 100 s (a), 10 s (b) and a clean surface for t0 = 1 s (c) [69]
Test results for various transient tests, both for DI water and nanofluid. Also shown are the DI water and nanofluid CHF obtained for steady state tests. The error bars show the standard deviation in measured CHF data [69]
Hsu et al. [70] in this study efforts taken to investigates the effects of surface wettability on pool boiling heat transfer. Nano-silica particle coatings are used to vary the wettability of the copper surface from superhydrophilic to superhydrophobic by modifying surface topography and chemistry. Experimental results show that critical heat flux (CHF) values are higher in the hydrophilic region. Conversely, CHF values are lower in the hydrophobic region. The experimental CHF data of the modified surface do not fit the classical models. Therefore, in this study proposes a simple model to build the nexus between the surface wettability and the growth of bubbles on the heating surface.
Duangthongsuk et al. [71] in this research involved conducting an experiment on pool boiling characteristics of Al2O3–water nanofluid. The experimental concentration ranged between 0.00005 and 0.03 vol%. The pressure used was at 1 and 2 at The boiling surface was a horizontal copper cylinder with a diameter of 28.5 mm, a length of 90 mm, and surface roughness of 3.14 µm. The main purpose of this research was to study the effect of the nanofluid’s concentration and pressure on the heat transfer coefficient and on heat flux, by comparing with water. Results from the experiment on pool boiling of nanofluids indicated that the heat transfer coefficient of Al2O3–water nanofluid was lower than that of water and tended to decrease when the concentration was higher (Figs. 16, 17).
Boiling curves for Al2O3–water nanofluid at 101 kPa [71]
Boiling heat transfer coefficient for Al2O3–water nanofluid at 101 kPa [71]
5 Discussions on nanofluid heat transfer technologies
As a new research important frame, nanofluid two-phase flow and thermal physics have the potential to develop heat transfer and energy efficiency in thermal management systems for many applications, such as microelectronics, power electronics, transportation, nuclear engineering, heat pipes, refrigeration, air-conditioning and heat pump systems. So far, the study of nanofluid two-phase flow and thermal physics is still in its formative years. This field of research provides many opportunities to study new frontiers but also poses great challenges. Recently, Cheng et al. [72] summarized the current status of research in this newly developing interdisciplinary field. In broad, the use of nanofluids appears promising in several aspects of thermal physics, but still faces several challenges: (1) the lack of agreement between experimental results from different research groups and (2) the lack of theoretical appreciative of the fundamental mechanisms with respect to nanoparticles.
Thermo-physical and transport properties of nanofluids are very important in nanofluid heat transfer technologies on single- and two-phase flow (boiling, flow boiling and condensation). So far, most studies on nanofluid thermal properties have persistent on thermal conductivity and limited studies have concerned viscosity [72–75]. For single-phase convective heat transfer, it is clear that single-phase heat transfer coefficient can be enhanced by nanofluid due to its higher thermal conductivity compared to the base fluid. It should also be noted that the viscosity of a nanofluid is generally higher than that of the base fluid. Therefore, frictional pressure drops of single phase flow of nanofluid are generally higher than those of the base fluid. Although several patents claim that the invented nanofluid heat transfer technologies may be used in evaporation and condensation processes, it should be realized that there are many challenges in the study of nanofluid two-phase flows [76]. The available experimental data on nucleate pool boiling heat transfer of nanofluids are quite limited and many conflicts exist between different studies on the heat transfer. The inconsistencies indicate that the understanding of the thermal behaviours of nanofluids related to nucleate pool boiling heat transfer is still poor. The results on CHF enhancement by nanofluids are consistent with each other in the literature; however, the mechanism responsible for this is not yet clear. Advanced physical models are required to explain and predict the influence of nanoparticles on nucleate pool boiling and CHF. Nanofluids may be used to cool electronic chips using evaporation of nanofluid in multi-microchannels shown in Fig. 7 due to its enhanced CHF performance. Although some patents also mentioned the use of nanofluids in computer chip cooling, there is still much fundamental research to do for this purpose.
The heat transfer coefficients associated with liquids are usually an order of magnitude higher than those associated with gases. Liquid cooling systems can be classified as direct cooling and indirect cooling systems. In direct cooling systems, the electronic components are in direct contact with the liquid, and thus the heat generated in the components is transferred directly to the liquid. In indirect cooling systems, however, there is no direct contact with the components. Liquid cooling systems are also classified as closed-loop and open-loop systems, depending on whether the liquid is discharged or recirculated after it is heated. Only dielectric fluids can be used in immersion or direct liquid cooling. High-power electronic components can be cooled effectively by immersing them in a dielectric liquid and taking advantage of the very high heat transfer coefficients related with boiling (Fig. 18).
5.1 Future developments
The study of flow boiling and two-phase flow of nanofluids is very limited in the literature so far. None of the available reviews [72–75] has specifically mentioned this new important research edge although all have presented studies of nucleate pool boiling heat transfer of nanofluids with very brief descriptions. There are many challenges in the study of nanofluid two phase flow and thermal physics as mentioned in future technology and development. Explanations and new theories are also needed to take into account all the important characteristics of nanofluids on flow boiling. So far, no systematic knowledge of their effects is available. Therefore, it is recommended that two-phase flow and thermal physics (pool boiling, flow boiling and condensation with nanofluids) should also be investigated in the future. Based on the experimental results, new theoretical work should be developed to achieve an advanced knowledge in modelling of the evaporation of nanofluids. Flow boiling compared to nucleate pool boiling can greatly enhance the cooling performance of a micro-channel heat sink by increasing the heat transfer coefficient. Furthermore, since flow boiling relies to a great degree on latent heat transfer, better temperature axial uniformity is realized both in the coolant and the wall compared to a single-phase heat sink. The question here is whether nanoparticles could further improve an already superior heat transfer performance. It would be very valuable certainly to see if the increase in nucleate pool boiling CHF also occurs in flow boiling CHF, for which there may be abundant high heat dissipation applications in micro devices and the new generation of CPU chips, just to name a few. On the other hand, it must be pointed out that nanoparticles should only be used for processes whose exit quality is less than that of the onset of dry, in order to minimize the deposition of the nanoparticles on the channel wall.
6 Conclusion
A review of the state-of-the-art nanofluids research for heat transfer application was conducted in this work, which showed that in progress understanding on nanofluids is still fairly limited. There are a number of challenges facing the nanofluids society ranging from formulation, practical application to method understanding. Engineering suitable nanofluids with controlled particle size and morphology for heat transfer applications is still a challenge. The current uncertainties on the content of nanofluids, including both solid phase and liquid phase, could be one of the main reasons accountable for the controversy and inconsistency reported. Besides thermal conductivity effect, future research should consider other properties, especially viscosity and wettability and study systematically their influence on flow and heat transfer.
To properly present the experimental results and to understand the physical mechanisms related to the two phase and thermal phenomena, the nanofluid physical properties should be systematically investigated to set up a consistent database of physical properties in addition to thermal conductivity.
References
Yu W, Xie H, Chen L, Li Y (2010) Enhancement of thermal conductivity of kerosene-based Fe3O4 nanofluids prepared via phase-transfer method. Colloids Surf A 355(1–3):109–113
Wang L, Fan J (2010) Nanofluids research: key issues. Nanoscale Res Lett 5(8):1241–1252
Lee S, Choi SUS, Li S, Eastman JA (1999) Measuring thermal conductivity of fluids containing oxide nanoparticles. J Heat Transf 121:280–289
Wang X, Xu X, Choi SUS (1999) Thermal conductivity of nanoparticle-fluid mixture. J Thermophys Heat Transf 13(4):474–480
Murshed SMS, Leong KC, Yang C (2005) Enhanced thermal conductivity of TiO2–water based nanofluids. Int J Therm Sci 44(4):367–373
Xuan Y, Li Q (2000) Heat transfer enhancement of nanofluids. Int J Heat Fluid Transf 21:58–64
Hwang YJ, Ahn YC, Shin HS, Lee CG, Kim GT, Park HS, Lee JK (2009) Investigation on characteristics of thermal conductivity enhancement of nanofluids. Curr Appl Phys
Kestin J, Wakeham WA (1978) A contribution to the theory of the transient hot-wire technique for thermal conductivity measurements. Phys A 92:102–116
Das SK, Putta N, Thiesen P, Roetzel W (2003) Temperature dependence of thermal conductivity enhancement for nanofluids. ASME Trans J Heat Transf 125:567–574
Nagasaka Y, Nagashima A (1981) Absolute measurement of the thermal conductivity of electrically conducting liquids by the transient hot-wire method. J Phys E Sci Instrum 14:1435–1440
Roetzel W, Prinzen S, Xuan Y (1990) Measurement of thermal diffusivity using temperature oscillations. In: Cremers C, Fine H (eds) Thermal conductivity, vol 21. Plenum Press, New York, pp 201–207
Honda H, Takamastu H, Wei JJ (2002) Enhanced boiling of FC-72 on silicon chips with micro-pin fins and sub-micron scale roughness. J Heat Transf 124:383–390
Masuda H, Ebata A, Teramae K, Hishinuma N (1993) Alteration of thermal conductivity and viscosity of liquid by dispersing ultra-fine particles (dispersion of 7-AI2O3, SiO2, and TiO2 ultra-fine particles). Netsu Bus-sei (Japan) 7(4):227–233
Xie H, Wang J, Xi T, Liu Y, Ai F, Wu Q (2002) Thermal conductivity enhancement of suspensions containing nanosized alumina particles. J Appl Phys 91(7):4568–4572
Hong T-K, Yang H-S, Choi CJ (2005) Study of the enhanced thermal conductivity of Fe nanofluids. J Appl Phys 97(6):1–4
Hong K, Hong T-K, Yang H-S (2006) Thermal conductivity of Fe nanofluids depending on the cluster size of nanoparticles. Appl Phys Lett 88(3):31901
Xie H, Wang J, Xi T, Liu Y (2001) Study on the thermal conductivity of SiC nanofluids. J Chin Ceram Soc 29(4):361–364
Xie H, Wang J, Xi T, Liu Y (2002) Thermal conductivity of suspensions containing nanosized SiC particles. Int J Thermophys 23(2):571–580
Hamilton RL, Crosser OK (1962) Thermal conductivity of heterogeneous two component systems. Ind Eng Chem Fundam 1:182–191
Li CH, Peterson GP (2006) Experimental investigation of temperature and volume fraction variation on the effective thermal conductivity of nanoparticle suspension (nanofluids). J Appl Phys 99(8):08431
Patel HE, Das SK, Sundararagan T, Nair AS, Geoge B, Pradeep T (2003) Thermal conductivities of naked and monolayer protected metal nanoparticle based nanofluids: manifestation of anomalous enhancement and chemical effects. Appl Phys Lett 83:2931–2933
Putnam SA, Cahill DG, Braun PV, Ge Z, Shimmin RG (2006) Thermal conductivity of nanoparticle suspensions. J Appl Phys 99(8):084308
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354(6348):56–57
Choi SUS, Zhang ZG, Yu W, LockWood FE, Grulke EA (2001) Anomalous thermal conductivity enhancement in nano-tube suspensions. Appl Phys Lett 79:2252–2254
Xie H, Lee H, Youn W, Choi M (2003) Nanofluids containing multiwalled carbon nanotubes and their enhanced thermal conductivities. J Appl Phys 94(8):4967–4971
Biercuk M, Llaguno M, Radosavljevic M, Hyun J, Johnson A, Fischer J (2002) Carbon nanotube composites for thermal management. Appl Phys Lett 80(15):2767–2769
Llaguno M, Hone J, Johnson A, Fischer J (2001) Thermal conductivity of single-wall carbon nanotubes: diameter and annealing dependence. In: Kuzmany H, Fink J, Mehring M, Roth S (eds) AIP conference proceedings, vol 591. Woodbury, New York
Choi ES, Brooks JS, Eaton DL, Al-Haik MS, Hussaini MY, Garmestani H, Li D, Dahmen K (2003) Enhancement of thermal and electrical properties of carbon nanotube polymer composites by magnetic field processing. J Appl Phys 94(9):6034–6039
Wen D, Ding Y (2004) Effective thermal conductivity of aqueous suspensions of carbon nanotubes (carbon nanotube nanofluids). J Thermophys Heat Transf 18(4):481–485
Ding Y, Alias H, Wen D, Williams RA (2005) Heat transfer of aqueous suspensions of carbon nanotubes (CNT nanofluids). Int J Heat Mass Transf 49(1–2):240–250
Assael MJ, Chen CF, Metaxa IN, Wakeham WA (2003) Thermal conductivity of suspensions of carbon nanotubes in water. In: 15th symposium on thermophysical properties. National Institute of Standards, University of Colorado, Boulder, USA
Assael MJ, Chen CF, Metaxa IN, Wakeham WA (2004) Thermal conductivity of suspensions of carbon nanotubes in water. Int J Thermophys 25(4):971–985
Assael MJ, Metaxa IN, Arvanitidis J, Christofilos D, Lioutas C (2005) Thermal conductivity enhancement in aqueous suspensions of carbon multiwalled and double-walled nanotubes in the presence of two different dispersants. Int J Thermophys 26(3):647–664
Liu M-S, Ching-Cheng M, Ching-Cheng Lin M, Huang IT, Wang C-C (2005) Enhancement of thermal conductivity with carbon nanotube for nanofluids. Int Commun Heat Mass Transf 32(9):1202–1210
Witharana S (2003) Boiling of refrigerants on enhanced surfaces and boiling of nanofluids. Ph.D. thesis, The Royal Institute of Technology
Li CH, Wang BX, Peng XF (2003) Experimental investigations on boilingof nano-particle suspensions. In: 2003 Boiling heat transfer conference, Jamica, USA
Das SK, Putra N, Roetzel W (2003) Pool boiling of nano-fluids on horizontal narrow tubes. Int J Multiph Flow 29(8):1237–1247
Bang IC, Chang SH (2004) Boiling heat transfer performance and phenomena of Al2O3–water nanofluids from a plain surface in a pool. In: Proceedings of ICAPP, Pitterburgh, USA
Bang IC, Chang SH (2005) Boiling heat transfer performance and phenomena of Al2O3–water nano-fluids from a plain surface in a pool. Int J Heat Mass Transf 48(12):2420–2428
Tu JP, Dinh N, Theofanous T (2004) An experimental study of nanofluid boiling heat transfer. In: Proceedings of 6th international symposium on heat transfer, Beijing, China
You SM, Kim JH, Kim KH (2003) Effect of nanoparticles on critical heat flux of water in pool boiling heat transfer. Appl Phys Lett 83:3374–3376
Vassallo P, Kumar R, Amico SD (2004) Pool boiling heat transfer experiments in silica–water nano-fluids. Int J Heat Mass Transf 47:407–411
Zhou DW (2004) Heat transfer enhancement of copper nanofluid with acoustic cavitations. Int J Heat Mass Transf 47:3109–3117
Eastman JA, Choi SUS, Li S, Yu W, Thomson LJ (2001) Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl Phys Lett 78(6):718–720
Li Y, Zhou J, Tung S, Schneider E, Xi S (2009) A review on development of nanofluid preparation and characterization. Powder Technol 196(2):89–101
Lo CH, Tsung TT, Chen LC (2005) Shape-controlled synthesis of Cu-based nanofluid using submerged arc nanoparticle synthesis (SNASS). J Cryst Growth 277(1–4):636–642
Lo CH, Tsung TT, Chen LC, Su CH, Lin HM (2005) Fabrication of copper oxide nanofluid using submerged arc nanoparticle synthesis system (SNASS). J Nanopart Res 7(2–3):313–320
Zhu HT, Lin YS, Yin YS (2004) A novel one-step chemical method for preparation of copper nanofluids. J Colloid Interface Sci 277(1):100–103
Bönnemann H, Botha SS, Bladergroen B, Linkov VM (2005) Monodisperse copper- and silver-nanocolloids suitable for heat-conductive fluids. Appl Organomet Chem 19(6):768–773
Singh AK, Raykar VS (2006) Microwave synthesis of silver nanofluids with polyvinylpyrrolidone (PVP) and their transport properties. Colloid Polym Sci 286(14-15):1667–1673
Kumar A, Joshi H, Pasricha R, Mandale AB, Sastry M (2003) Phase transfer of silver nanoparticles from aqueous to organic solutions using fatty amine molecules. J Colloid Interface Sci 264(2):396–401
Yu W, Xie H, Wang X, Wang X (2011) Highly efficient method for preparing homogeneous and stable colloids containing graphene oxide. Nanoscale Res Lett 6:47
Yu W, Xie H (2012) A review on nanofluids: preparation, stability mechanisms, and applications. J Nanomater 2012, Article ID 435873
Zhu HT, Zhang CY, Tang YM, Wang JX (2007) Novel synthesis and thermal conductivity of CuO nanofluid. J Phys Chem C 111(4):1646–1650
Chen Y, Wang X (2008) Novel phase-transfer preparation of monodisperse silver and gold nanoparticles at room temperature. Mater Lett 62(15):2215–2218
Feng X, Ma H, Huang S et al (2006) Aqueous-organic phase-transfer of highly stable gold, silver, and platinum nanoparticles and new route for fabrication of gold nanofilms at the oil/water interface and on solid supports. J Phys Chem B 110(25):12311–12317
Faulkner D, Khotan M, Shekarriz R (2003) Practical design of a 1,000 W/cm2 cooling system. In: Annual IEEE semiconductor thermal measurement and management symposium. Institute of Electrical and Electronics Engineers Inc., San Jose, CA, USA, pp 223–230
Das SK, Putra N, Roetzel W (2003) Pool boiling characteristics of nano-fluids. Int J Heat Mass Transf 46(5)851–862
Wen D, Ding Y (2005) Experimental investigation into the pool boiling heat transfer of aqueous based γ-alumina nanofluids. J Nanopart Res 7(2):265–274
Ramaswamy C, Joshi Y, Nakayama W, Johnson W (2003) Effects of varying geometrical parameters on boiling from micro fabricated enhanced structures. J Heat Transf 125:103
Coursey J, Roh H, Kim J, Boudreaux P (2002) Graphite foam thermosyphon evaporator performance parametric investigation of the effects of working fluid, liquid level and chamber pressure. In: Proceedings of IMECE200 1
Sathyamurthi V, Ahn H, Banerjee D, Lau S (2009) Subcooled pool boiling experiments on horizontal heaters coated with carbon nanotubes. J Heat Transf 131:071501
Endo M et al (1993) The production and structure of pyrolytic carbon nanotubes. J Phys Chem Solid 54(12):1841–1848
Ahn H, Sinha N, Zhang M, Banerjee D, Fang S, Baughman R (2006) Pool boiling experiments on multiwalled carbon nanotubes (MWCNT) forests. J Heat Transf 128:1335
Ujereh S, Fisher T, Mudawar I et al (2007) Carbon nanotube arrays on nucleate pool boiling. Int J Heat Mass Transf 50(19–20):4023–4038
Venkatachalapathy S et al (2013) Effect of surface modifications on the enhancement of critical heat flux in saturated pool boiling. In: International conference on advance research in mechanical aeronautical and civil Sep 7, 2013 Pattaya
Huang C-K et al (2011) Boiling enhancement by TiO2 nanoparticle deposition. Int J Heat Mass Transf 54:4895–4903
Song SL et al (2013) CHF enhancement of SiC nanofluid in pool boiling experiment. Exp Therm Fluid Sci (in press)
Sharma Vivek I et al (2013) Experimental investigation of transient critical heat flux of water-based zinc–oxide nanofluids. Int J Heat Mass Transf 61:425–431
Hsu C-C et al (2012) Surface wettability effects on critical heat flux of boiling heat transfer using nanoparticle coatings. Int J Heat Mass Transf 55:3713–3719
Duangthongsuk W et al (2013) Pool-boiling heat transfer characteristics of Al2O3–water nanofluids on a horizontal cylindrical heating surface. Curr Nanosci 9:56–60
Cheng L, Bandarra FEP, Thome JR (2008) J Nanosci Nanotechnol 8:3315
Peterson GP, Li CH (2006) Recent patents on engineering nanofluid heat transfer technologies. Adv Heat Transf 39:275
Wang XQ, Majumdar AS (2007) Heat transfer characteristics of nanofluids: a review. Int J Therm Sci 46(1):1-19
Das SK, Choi SUS, Patel H (2006) Heat transfer in nanofluids -a review. Heat Transf Eng 27(10):3-19
Cheng L, Mewes D (2006) Review of two-phase flow boiling of mixtures in small and mini channels. Int J Multiph Flow 32(2):159-284
Kathiravan R, Ravikumar (2011) Pool boiling characteristics of multi walled carbon nanotube (CNT) based nanofluids over a flat plate heater. Int J Heat Mass Transf 54(2011):1289-1296
Hegde RN et al (2012) Flow visualization and study of critical heat flux enhancement in pool boiling with Al2O3-water nanofluids. Int J Therm Sci 16(2)445–453
Lee JH et al (2012) Experimental study on the pool boiling CHF enhancement using magnetite-water nanofluids. Int J Heat Mass Transf 55:2656–2663
Mourgues A et al (2013) Boiling behaviors and critical heat flux on a horizontal and vertical plate in saturated pool boiling with and without ZnO nanofluid. Int J Heat Mass Transf 57:595–607
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kshirsagar, J.M., Shrivastava, R. Review of the influence of nanoparticles on thermal conductivity, nucleate pool boiling and critical heat flux. Heat Mass Transfer 51, 381–398 (2015). https://doi.org/10.1007/s00231-014-1412-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-014-1412-3