Abstract
CdS/PS and ZnS/PS nanocomposites have been prepared by solution casting method with different wt% of cadmium sulphide (CdS) and zinc sulphide (ZnS) nanoparticles and characterized through X-ray diffraction and transmission electron microscope measurements. The effective thermal conductivity of polymer nanocomposites has been measured by transient plane source method over the temperature range from room to 150 °C. The experimental results showed that the thermal conductivity has been found to increase up to 4 wt% of CdS/ZnS nanoparticles and then decrease for 6 and 8 wt% of nanoparticles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the past few years, the studies of composite of polymer—inorganic nanoparticles materials have drawn enormous attention in the field of nonmaterials because they show a variety of applications in the fields of optics, electronics, magnetics, and biology [1–4] with unique mechanical, photoelectric and thermal properties. They combine the advantages of both inorganic materials (rigidity, high thermal stability) and organic polymers (flexibility, dielectric, ductility and processability) [5–7]. Due to the interesting properties of chalcogenide nonmaterials, many works on the chalcogenide/polymer nanocomposites have been reported [8–10]. Among them, cadmium sulphide (CdS) and zinc sulphide (ZnS) are the most assuring compound materials because of their wide range of applications in optoelectronic [11], piezoelectronic [12] and semiconducting devices [13].
Thermal stability of CdS (ZnS)/polymer nanocomposites is a key importance aspect from the application point of view. Polymers generally have the low stability which restrain them from applications in higher temperature region. Thermal stability of polymer can also be improved by the dispersion of CdS and ZnS nanoparticles. Kuljanin et al. [14] have reported the influence of CdS-filler particles in the micrometer size range on the thermal stability of polystyrene (PS) matrix. Improvement of the thermal stability of the PS matrix for about 50 K was found in the presence of the CdS-filler. The thermal stability of nanocomposites ZnS/PS with various compositions (0, 5, 10, 15 and 20 wt% ZnS) has been investigated by Jakovljevic et al. [15] using nonisothermal thermogravimetric analysis with different temperature programs. Improvement of the thermal stability of the PS in composites with respect to the pure PS matrix is demonstrated for all compositions. The thermogravimetric analysis of the CdS nanocomposite with PMMA [16] and PS [14] were carried out. An improved thermal stability of the CdS/PMMA (or PS) nanocomposites with respect to the pure matrix can be noticed.
Besides the thermal stability, thermal conductivity of polymer nanocomposite is also a very important parameter from the applications point of view. The continuous increase in component densities being packed onto an electronic circuit requires more power to run it. The high power density of electronic components results in rapid heat generation, which adversely affects reliability of the device [17]. Therefore, it is necessary to dissipate heat away from the components sufficiently fast enough in order to keep their temperatures below critical values and prevent the device from permanent damage. As the heat dissipation requirements increase, improved thermally conducting packaging composite materials are required [18]. To overcome these obstacles, polymers are filled with particles that enable them to fulfill the application-specific requirements while keeping their low density, easy manufacturability, and low cost. In this context, thermally conductive polymer composites have become increasingly important for the industry [19].
Polymers typically have very low thermal conductivity (~0.1–0.5 W/mK). However, thermally conductive polymers have been pursued in recent years, this pursuit driven by important applications such as electronics cooling and energy storage. One approach to obtain polymer composites with higher effective thermal conductivity is by dispersing highly thermally conductive fillers in the polymer matrix. However, Bigg et al. [20] reported that there is no additional improvement when the ratio of thermal conductivity of the fillers and polymer matrix is greater than 100 times. Therefore, for fillers with thermal conductivities that are equal or greater than 100 times of the conductivity of polymer matrix, the filler’s volume fraction may become a critical factor governing the thermal conductivity of the composite. In general, composites with low loading of filler particles will allow the heat flow uniformly through the composite since all the particles are well dispersed. However, low content of filler particles limits the interconnection among the filler particles. Increasing the filler content promotes the formation of preferential heat conducting paths for dissipating heat through the composites. Therefore, it is necessary to study the effect of concentration of higher thermally conductive fillers on the thermal conductivity of polymer composite. In view of this, present paper deals with the composition as well as temperature dependence of thermal conductivity of polystyrene filled with CdS and ZnS nanoparticles. A simplified solution casting method has been used for preparation of CdS/PS and ZnS/PS nanocomposites, based on mixing the CdS (ZnS) filler in nanometer range with the polymer matrix, already developed and described in the previous papers [21, 22]. The prepared nanocomposites have been structurally characterized through X-ray diffraction (XRD) and transmission electron microscope (TEM). Composition and temperature dependence of the thermal conductivity of CdS (ZnS)/PS nanocomposites has been experimentally studied using the transient plane source (TPS) method in order to understand and describe the heat transfer through the composites.
2 Experimental details
2.1 Sample preparation
The cadmium sulfide (CdS) and zinc sulfide (ZnS) nanoparticles have been synthesized by chemical precipitation method using dimethylformamide and thio-glycerol as stabilizing agent, respectively as described earlier [21, 22].
CdS (ZnS)/PS nanocomposites have been prepared by solution casting method. Firstly, PS was dissolved in tetrahydrofurane (THF) solution by magnetic stirrer for 3 h and then CdS (ZnS) nanoparticles with different (0, 2, 4, 6 and 8) wt% were added into the THF solution containing PS. The obtained solutions were stirred for 24 h and then agitated by ultrasonicator for 20 min to get the uniform dispersion of CdS/ZnS nanoparticles. These solutions were poured in the petri-dishes to obtain the CdS (ZnS)/PS nanocomposite films. After 2 days, the nanocomposite films were taken out from the petri-dishes and dried in vacuum (10−2 torr) for 6 h to remove the solvent. The thickness of the prepared samples was ≈0.12 mm. These four (2, 4, 6 and 8) wt% of CdS (ZnS) have been chosen for preparing the nanocomposites so as to correlate the thermal behavior of these materials with their earlier studied mechanical [21, 23] as well as thermal properties [22, 23].
2.2 Structural characterization
The XRD spectra of the nanocomposites have been recorded using Bragg–Brentano geometry on a Panalytical X’pert Pro diffractometer with a Cu Kα radiation source (λ = 1.5406 Å). The X-ray tube was operated at 45 kV and 40 mA.
The TEM measurements of nanocomposites have been performed on TECNAI G2 30 U-TWIN system operating at an accelerating voltage of 300 kV. The samples for TEM measurement have been prepared by dissolving CdS (ZnS)/PS nanocomposite films in THF solvent using ultrasonicator. A drop of prepared solution was placed on the carbon coated copper grid and solvent removed by evaporation at room temperature. The thickness of the thin layer for TEM measurements should be approximately 80–100 Å.
2.3 Thermal conductivity measurement
The thermal conductivities of CdS/PS and ZnS/PS nanocomposites have been determined using TPS technique. In this method, the transient plane source element behaves both as heat source and temperature sensor. TPS sensor (Fig. 1) consists of an electrically conducting pattern of thin nickel foil (10 μm) in the form of double spiral embedded in an insulating layer made of kapton (50 μm). Sensor is sandwiched between the two pieces of the samples having perfectly smooth surface so as to ensure perfect thermal contact. Details of TPS technique have been given elsewhere [24, 25]. For the measurement of effective thermal conductivity of thin film with thickness of the order of microns, the experiment was performed in two steps. In the first step, sensor is sandwiched between the two pieces of the sample-each one with a plane surface facing the sensor. This arrangement was placed between the two auxiliary metal (stainless steel) pieces as shown in the Fig. 2. Data for the temperature increase over a given time was collected using the software available with the Hot Disk Thermal Constant Analyser TPS (model 2500S). In the second step, the experiment was repeated with the Hot Disk sensor sandwiched between the same two pieces of the metal and data for the temperature increase was again collected taking the same experimental conditions as mentioned above. Employing these two temperature increase, thickness of the film and power delivered to the sample, the effective thermal conductivity (λe) of thin sample can be determined through the following relation:
where P is the total output of power given to the sensor, A is the area of conducting pattern of sensor, Δx is the thickness of thin samples and ΔT is the temperature difference across the sample. The factor two is due to the symmetrical distribution of heat flux on both sides of the sample. The numbers of specimen taken for the measurement were two and the results of their thermal conductivities have a deviation of about 5 % which is in the range of experimental error of thermal conductivity.
3 Results and discussion
3.1 Structure and morphology
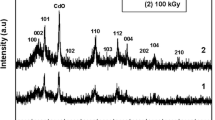
The XRD specta of CdS/PS and ZnS/PS nanocomposites are shown in Fig. 3a, b, respectively. Figure 3a, b show one broad hump and three broad peaks in the XRD spectrum of the all nanocomposites. The first broad hump at lower diffraction angle around 18o–20o is due to the amorphous nature of PS. It is also observed from Fig. 3a, b that other three XRD peaks at the angles 26.4o, 43.5o, 52.7o corresponding to (111), (220), (311) planes and at angles 28.8o, 47.8o, 56.6o corresponding to (101), (103), (004) planes indicate the cubic phase of CdS nanoparticles and the hexagonal phase of ZnS nanoparticles [22], respectively. The XRD peaks are relatively broad due to the small crystalline domains in the materials.
Figure 4a, b shows the TEM micrographs of CdS/PS and ZnS/PS nanocomposites, respectively. The average particle diameter of CdS and ZnS nanoparticles is approximately 15 and 5 nm, respectively. It can be seen from the Fig. 4a, b that the nanoparticles are well dispersed at low concentration (4 wt%), whereas the particles are agglomerated with the increased nanoparticles content in polymer. This indicates that particle–particle interaction dominates over the particle-polymer interaction.
3.2 Effective thermal conductivity of nanocomposites
The knowledge of thermal properties like thermal conductivity is required prior to any potential technological application as the environmental and thermal stability of a material should be well characterized to identify the optimal condition for performance of a device. The effect of temperature in polymer nanocomposites is of practical importance because most polymers are processed at relatively high temperature and have applications in a wide temperature range. The thermal property that is required to describe the heat transfer through material is thermal conductivity. The variation of effective thermal conductivity for PS nanocomposites with CdS and ZnS nanoparticles has been studied from room temperature to 150 °C. The experimentally obtained value of effective thermal conductivity for the polystyrene matrix at room temperature is 0.14 W/mK. The variation of effective thermal conductivity (λe) of CdS/PS and ZnS/PS nanocomposites with temperature are shown in Fig. 5a, b, respectively. It is seen that the effective thermal conductivity of all the nanocomposites show a similar trend of increasing almost linearly up to a temperature which is characteristic to each sample, beyond which effective thermal conductivity shows approximately constant value for all the samples. This temperature is called glass transition temperature and has been determined through dynamic mechanical analyzer (Tritec 2000 DMA) [26]. The variation of glass transition temperature (Tg) with CdS/ZnS concentration in PS matrix is shown in Fig. 6. The above mentioned behavior of effective thermal conductivity is explained on the basis of structural changes occurring in the PS and CdS(ZnS)/PS nanocomposites during the heating and also on the basis of various phonon scattering mechanisms.
In the amorphous polymers like PS the thermal conductivity in low temperature region is controlled by variation of phonon mean free path. For PS the mean free path of phonons is very small [27], because in amorphous state number of defects are present at room temperature due to the fact that during polymerization of polymers, certain defects such as bends in chains, gap between two chains in line, chains of smaller lengths than the others, etc. are created in the system. Therefore, in the temperature region below Tg, the temperature dependence of thermal conductivity is controlled [28, 29] by the variation of phonon free path and structure scattering and chain defect scattering are the main phonon scattering below Tg. As the temperature increases from room temperature up to Tg, the polymer chains straighten out more and more, increasing the corresponding mean free path. This results in minimization of the chain defects and thus the contribution to the corresponding thermal resistance decreases linearly with the rise in temperature and as a result thermal conductivity increases.
Above the glass transition temperature region, scattering by micro-voids (vacant site scattering) contributes to the thermal resistance besides structural scattering. As the temperature increases the polymer passes gradually from glassy to rubbery state. During this transformation individual units of polymer, atomic groups and small chain segments undergo intensive thermal motion and large torsional rotations and the sliding of chain segments starts to play a dominant role to control the thermal conductivity above the glass transition temperature. This has two fold effects on the structure of the system; initially the dominant chain movements create some vacant sites or micro-voids which scatter phonon in the similar way to the point defects [30]. With the increase of temperature, the number and size of these micro-voids increases and consequently, the contribution of vacant site scattering to the thermal resistance would increase linearly with temperature. Thus, the structure scattering and vacant site scattering are responsible to control the thermal conductivity above the glass transition temperature and hence the thermal conductivity becomes constant [23].
It is observed that the thermal conductivity of polymer/CdS (ZnS) nanocomposites is relatively low compared with the intrinsic thermal conductivity of CdS (42.7 W/mK for 13 nm) [31] and ZnS (17.4 W/mK for 4 μm) [32]. This is because of large interfacial thermal resistance between CdS (ZnS) and the surrounding polymer matrix which hinders the transfer of phonons dominating heat conduction in polymer and CdS (ZnS). It is also observed from Fig. 5a, b that the thermal conductivity increases for 2 and 4 wt% of CdS (ZnS) nanocomposites. This behavior can be explained on the basis of compact structure of composites. When the nanoparticles are introduced into the matrix, they acquire the position of voids and reduce the free volume (voids filled with air) resulting into compact structure of composites, which improves the thermal conduction or thermal conductivity of nanocomposites over the pure PS. Also, the amount of air convection due to the temperature gradient, through voids, is negligibly small because of the presence of very little amount of air filled in the pores. This is due to the addition of filler nanoparticles to the voids already existing in the polymer matrix. Therefore, the air convection is ignored during the heat transfer in nanocomposite. As, the concentration of CdS and ZnS fillers increases to 4 wt%, the free volume further decreases and makes the nanocomposites more compact than the composite with 2 wt% of CdS and ZnS. This seems to be the optimum compact structure for which the thermal conductivity is maximum in the entire studied range of temperature. It is also seen from Fig. 5 that beyond 4 wt% of CdS and ZnS nanoparticles the thermal conductivity decreases in the entire range of temperature. This behavior of thermal conductivity can be explained on the basis of the structural changes occurring in the composite due to the change in dispersion of nanoparticles from uniform to agglomeration, as indicated by the TEM images (Fig. 4a, b). It is interesting to note from Fig. 5 that the thermal conductivity of the nanocomposites containing 8 wt% of CdS (ZnS) into polymer have got the thermal conductivity even less than the thermal conductivity of pure polymer for all the temperatures in the study. This could be explained on the basis of the existence of excess free volume over the pure polymer obtained due to strong agglomeration of CdS and ZnS nanoparticles into the polystyrene polymer. Moreover, this phenomenon is also explained [33] by very low efficiency of heat transfer due to interfacial thermal resistance between particles and matrix, so that the higher thermal conductivity of the filler cannot be taken into advantage and the composite behaves like a hollow material, thus reducing its conductivity compared to the dense reference matrix.
4 Conclusions
A systematic study of prepared samples of CdS/PS and ZnS/PS nanocomposites leads to the following conclusions:
-
The variation of effective thermal conductivity of CdS/PS nanocomposites is similar to the variation of effective thermal conductivity of ZnS/PS nanocomposites.
-
The increase of effective thermal conductivity of CdS/ZnS-PS nanocomposites with the increase of temperature up to glass transition temperature is attributed to the straightening of the chains and removal of defects. Almost constant value of thermal conductivity beyond glass transition temperature (Tg) is explained on the basis of structure and vacant site scattering of phonons.
-
Thermal conductivity of nanocomposites increases up to 4 wt% of the nanoparticles and beyond this wt%, it decreases. This is suggestive of the fact that compact structure of nanocomposites up to 4 wt% and agglomeration for 6 and 8 wt% of the CdS and ZnS nanoparticles, respectively are responsible for the observed variation.
References
Wang ZL (2000) Characterizing the structure and properties of individual wire-like nanoentities. Adv Mater 12:1295–1298
Duan XF, Huang Y, Agarwal R, Lieber CM (2003) Single-nanowire electrically driven lasers. Nature 421:241–245
Tokio N, Keisuke F, Akio K (1999) High-efficiency cadmium-free Cu(In, Ga)Se2 thin-film solar cells with chemically deposited ZnS buffer layers. IEEE Trans Electron Devices 46:2093–2097
Colvin VL, Schlamp MC, Alivisatos AP (1994) Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature 370:354–357
Rathi S, Dahiya JB (2012) Polyamide 66/nanoclay composites: synthesis, thermal and flammability properties. Adv Mater Lett 3:381–387
Luccio TD, Laera AM, Tapfer L (2006) Controlled nucleation and growth of CdS nanoparticles in a polymer matrix. J Phys Chem B 110:12603–12609
Yang Y, Xue S, Liu S, Huang J, Shen J (1996) Fabrication and characteristics of ZnS nanocrystals/polymer composite doped with tetraphenylbenzidine single layer structure light-emitting diode. Appl Phys Lett 69:377–379
Matsuo Y, Tahara K, Sugie Y (1996) Synthesis of poly(ethylene oxide)-intercalated graphite oxide. Carbon 34:672–674
Matsuo Y, Tahara K, Sugie Y (1997) Structure and thermal properties of poly(ethylene oxide)-intercalated graphite oxide. Carbon 35:113–120
Ding Y, Jones DJ, Torres PM (1995) Two-dimensional nanocomposites: alternating inorganic-organic polymer layers in zirconium phosphate. Chem Mater 7:562–571
Vaccaro K, Davis A, Dauplaise HM, Spaziani SM, Martin EA, Lorenzo JP (1996) Cadmium sulfide surface stabilization for InP-based optoelectronic devices. J Electron Mater 25:603–609
Lin YF, Song J, Ding Y, Lu SY, Wang ZL (2008) Piezoelectric nanogenerator using CdS nanowires. Appl Phys Lett 92:022105-1–022105-3
Salata OV, Dobson PJ, Sabesan S, Hull PJ, Hutchison JL (1996) Preparation of nanoparticulate CdS films suitable for opto-electronic device applications. Thin Solid Films 288:235–238
Kuljanin J, Vuckovic M, Comor MI, Bibic N, Djokovic V, Nedeljkovic JM (2002) Influence of CdS-filler on the thermal properties of polystyrene. Eur Polym J 38:1659–1662
Jakovljevic JK, Cincovic MM, Stojanovic Z, Krkljes A, Abazovic ND, Comor MI (2009) Thermal degradation kinetics of polystyrene/cadmium sulfide composites. Polym Degrad Stab 94:891–897
Jakovljevic JK, Stojanovic Z, Nedeljkovic JM (2006) Influence of CdS-filler on the thermal properties of poly(methyl methacrylate. J Mater Sci 41:5014–5016
Rymaszewski EJ, Tummala RR, Watari T (1997) Microelectronic packaging—an overview. In: Tummala RR, Rymaszewski EJ, Klopfenstein AG (eds) Microelectronic packaging handbook. Part I. Chapman & Hall, New York, pp 1–128
Wong CP, Bollampally S (1999) Thermal conductivity, elastic modulus, and coefficient of thermal expansion of polymer composites filled with ceramic particles for electronic packaging. J Appl Polym Sci 74:3396–3403
Lee GW, Park M, Kim J, Lee JI, Yoon HG (2006) Enhanced thermal conductivity of polymer composites filled with hybrid filler. Compos A Appl Sci Manuf 37:727–734
Big DM (1986) Metal filled polymers. Marcel Dekker, New York
Agrawal S, Patidar D, Saxena NS (2011) Investigation of temperature dependent mechanical properties of CdS/PMMA nanocomposites. J Compos Mater 45:2507–2514
Agrawal S, Patidar D, Saxena NS (2011) Glass transition temperature and thermal stability of ZnS/PMMA nanocomposites. Phase Transitions 84:888–900
Saxena NS (2010) Thermal and mechanical behavior of polymer nanocomposite. J Polym Eng 30:575–586
Gustafsson SE (1991) Transient plane source techniques for thermal conductivity and thermal diffusivity measurements of solid materials. Rev Sci Instrum 62:797–804
Mangal R, Saxena NS, Sreekala MS, Thomas S, Singh K (2003) Thermal properties of pineapple leaf fiber reinforced composites. Mater Sci Eng A 39:281–285
Menard KP (1999) Dynamic mechanical analysis: a practical introduction. CRC Press, Boca Raton
Choy CL (1977) Thermal conductivity of polymers. Polymer 18:984–1004
Perpechoko II (1981) An introduction to polymer physics. Mir, Moscow
Saxena NS, Pradeep P, Mathew G, Thomas S, Gustafsson M, Gustafsson SE (1999) Thermal conductivity of styrene butadiene rubber compounds with natural rubber prophylactics waste as filler. Eur Polym J 35:1687–1693
Lee TCP, Millens W (1977) US Patent 834: 4046
Raji P, Sanjeeviraja C, Ramachandram K (2004) Thermal properties of nano-crystalline CdS. Cryst Res Technol 39:617–622
Every AG, Tzou Y, Haselman DPH, Raj R (1992) The effect of particle size on the thermal conductivity of ZnS/diamond composites. Acta Metall Mater 40:123–129
Han Z, Fina A (2011) Thermal conductivity of carbon nanotubes and their polymer nanocomposites: a review. Prog Polym Sci 36:914–944
Acknowledgments
Authors are thankful to Council of Scientific and Industrial Research (CSIR), New Delhi for providing financial assistance through Emeritus Scientist Scheme during the course of this work. Authors would also like to thank Dr. Mahesh Baboo for his help in different ways.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agarwal, S., Patidar, D. & Saxena, N.S. Effective thermal conductivity of CdS/ZnS nanoparticles embedded polystyrene nanocomposites. Heat Mass Transfer 49, 947–953 (2013). https://doi.org/10.1007/s00231-013-1138-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-013-1138-7