Abstract
In literature it is generally supposed that under terrestrial conditions the driving force in natural, nucleate boiling heat transfer is namely buoyancy caused by earth gravity, which is expressed in the empirical correlations for technical applications. However, experiments in microgravity performed during the past three decades demonstrate unanimously that up to a medium level heat flux the overall heat transfer in pool boiling is nearly independent from gravity. We refer and discuss in this paper on results of experiments performed with various liquids and liquid states and also using various heater geometries on mission platforms which provide low gravity for short and long periods. Beside the measurements of the experimental parameters to determine the heat transfer, we observed the macroscopic boiling process itself with movie films and videos in order to study the bubble dynamics. From these records we learned about the mechanisms of heat and vapour bubble transport, about the interaction between solid heater, superheated liquid, and vapour without gravity or other external force only generated by the bubbles themselves, and we observed significant details about the boiling process not recognized so far. These findings are essential for a better understanding of the complex physical process; and therefore they are important for the formulation of empirical correlations, and in future for numerical simulations to predict properly boiling heat transfer for technical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The overall boiling process is known to be very complex due to the combination of various physical phenomena occurring in a very transient manner. Because it consists in sequences of nucleation or bubble activation at onset of boiling, followed by bubble growth up to detachment from the heated wall, the bubble departure, and transport of liquid and vapour away from the heater. At pool boiling under terrestrial conditions these processes are influenced more or less on buoyancy effects caused by earth gravity and it is generally assumed that their interaction is responsible for the overall heat transfer expressed in the applied empirical correlations for pool boiling. In spite of the numerous experimental investigations of boiling heat transfer the question of the influence of gravity was not studied. Only few experiments of limited quality with lower or higher gravity values have been performed up to the 1980s with partially contradictory results.
At the beginning of our research the objective was to answer the following key questions:Is boiling an appropriate mechanism of heat transfer in space applications for heating, or cooling purposes, and energy conversion. From a scientific point of view it was interesting, how heat transfer and bubble dynamics would occur without buoyancy, or generally, without any external force have an effect on them like shear, electrical or magnetic field forces? Is phase change and bubble dynamics itself able to maintain stable heat transfer in pool boiling? Most analytical correlations used today to calculate heat transfer coefficients for practical applications in pool boiling are more or less based on the assumption that buoyancy detaches the bubbles from the heating surface and carry the bubbles, respectively the vapour and its latent heat away. If according to this basic model the bubbles would not detach, they would form rapidly a vapour layer at the heat exchanger surface and thermally insulate it, consequently heat transfer would break down in absence of gravity. In contrast to this assumption that buoyancy is the driving force for bubble departure and stable heat transfer, it was surprising that in the experiments performed in a microgravity environment the bubbles basically still detach and depart from the heater surface, they did not form an insulating vapour film which leads to heater burnout, and the heat transfer did not collapse. On the contrary the experiments in microgravity show that the boiling heat transfer coefficients up to about 50–60% of the critical heat flux achieved under earth gravity stay nearly identical compared with the terrestrial values. At lower heat fluxes and low system pressures they are even up to 15% higher compared to terrestrial conditions and show a slow decrease with increasing heat flux. Most used analytical model correlations for pool boiling heat transfer fail completely when extrapolated to the actual low gravity conditions. Thus gravity in these correlations must be practically treated as a constant and can not be regarded as a physical parameter. Furthermore the theoretical treatment of heat transfer between the gravity-free and terrestrial data indicates that the basic physical mechanisms in both cases must be identical and more or less independent from gravity. Today there is general agreement that the evaporation at the liquid–vapour interface at the bubble base at the heater is the dominating process for the heat transfer with very high values at the contact line of the three phases solid–liquid–vapour. But just as important as the bubble generation is the bubble transport away from the heater surface.
The basic questions arise how the bubbles eventually detach, depart and move away from heater without buoyancy or other external forces, and what are the mechanisms able to replace the buoyancy driven effects? Therefore we concentrated our research on pool boiling only, without applying any external forces [1], in order to find out what are the heat and mass transport mechanisms the bubbles develop. That’s why a microgravity environment itself is an outstanding environment to study boiling in order to gain a better detailed understanding of the complex interrelated thermo-physical mechanisms for correct application under space conditions but rather at earth too. We like to refer to flow boiling studies performed under microgravity by H. Ohta [2].
2 Experiments
Various carrier systems that allow the simulation of microgravity could be used, such as the Drop Tower at ZARM, Bremen, Germany (5 s duration of microgravity, 10−4 g), Drop Shaft at JAMIC, Hokkaido, Japan (10 s), parabolic flight trajectories with NASA’s aircraft KC-135, Houston, USA (20 s), ballistic sounding rockets TEXUS at Kiruna, Sweden (6 min), and as highlights finally three Space Shuttle missions (several hours/days). As far as the experimentation possibilities of the respective missions allowed, a systematic research program was developed and realized which was always adjusted to the possibilities of the respective mission platform, and the mission boundary conditions itself; and it was continuously updated to the scientific findings of preceding experiments, and to new experimental parameters. Likewise appropriate experiment assemblies were developed, designed and adopted to the mission platforms to obtain optimum results. The mean features of the experiment configuration consisted of a hermetically sealed liquid cell with control of the bulk temperature, and the fluid pressure was controlled by means of an elastic metallic bellows with adjustable pressure setting applied from a gaseous pressure bottle [3, 4, 7]. With this system the pressure during boiling could be kept at constant level, and with liquid temperature variation the saturation pressure of the liquid could be changed easily, as well as a wide range of subcooled liquid conditions could be established and kept. Various heater geometries were used like platinum wires with 0.05–0.2 mm diameter, semiconductor thermistors with a shape of small spheres of 0.3–1.4 mm diameter, and a flat gold coated surface of 20 × 40 mm2 in order to study also the influence of geometry. These heaters could be employed simultaneously both for heat flux control and as resistance thermometers to measure the average temperature at the heater surface. Moreover the experiment assemblies also comprised special electronics for process control, data recording, film and video cameras. It should be mentioned that in all cases certain limitations existed in terms of available power, size and mass, and experimental time, therefore we could not apply technical heater systems.
3 Results and the gravity efficiency
As mentioned the most important result of the boiling experiments performed was the study of the influence of gravity as a parameter [1, 3]. As usual the heat flux, the heater wall temperature, the liquid bulk temperature, and the liquid pressure (at saturation state or sub cooling) have been measured. Simultaneously visual observation of the boiling process and bubble dynamics are recorded by video cameras. Reference measurements to all microgravity experiments are performed on earth under terrestrial conditions with exactly the same experimental facility, the same liquid and liquid conditions before and after each mission. With it the influence of gravity can be best described by the gravity efficiency ɛ μg represented as the ratio of heat transfer coefficients or heat fluxes determined under microgravity (μg) to those under earth (1 g) performed at identical experimental conditions.
As an example in Fig. 1 the gravity efficiency at nucleate pool boiling for a saturated refrigerant R123 in microgravity 10−4 g at a small circular plan heater is shown versus the heat flux at various system pressures (saturation states). It is interesting to note, as already mentioned above, that the heat transfer under microgravity conditions is in the same order as at 1 g, and that at low system pressures and low heat fluxes the efficiency is even enhanced by up to 15%. This trend is decreasing with increasing heat flux and with increasing saturation pressure. The last assessment indicates the great effect of surface tension, which may be shown that with increasing saturation temperature the surface tension decreases and the heat transfer efficiency decreases too. We achieved similar results with other heaters and other liquids too, thus the results according to Fig. 1 can be regarded to be representative for all of our microgravity experiments [1, 3, 5, 6]. In order to avoid the destruction of the heaters by thermal burnout the heat flux could not be increased above a certain value, however, the general observation was that nucleate boiling could be maintained up to about 50–60% of the maximum heat flux values achieved on earth before transitioning to the critical regime of film boiling.
Gravity efficiency at saturated boiling, R123, BTPU, circular plane heater of 3 mm diameter [6]
4 Gravity efficiency of some extrapolated correlations
In the same way we analysed pool boiling correlations [8–10]. If except the actual gravity all other experimental and liquid parameters are regarded to be identical, the efficiency of gravity can be formed as ratio of actual gravity a over earth gravity g and a power law eq. (2) can be developed.
The gravity efficiency is plotted in Fig. 2 versus the gravity ratio from 102 to 10−4 g. It can be seen that no common trend concerning the gravity dependence could be deduced from existing correlations. The exponent n of the power law in Eq. (2) vary in the range between 0.5 and −0.35. However derived from microgravity experiment results the exponent should be close to unity ± 10% over a wide range of pressures and heat fluxes. Certainly this exponent can not be a constant number; it will be itself a function of liquid state and heat flux. The correlation of Rohsenow [8], especially most used in USA, with n = 0.5 drops drastically down to 1% at microgravity. The uncertainty resulting from the extrapolated equations may be the reason that pool boiling systems have not been used for technical space applications. It should be mentioned that in agreement with the μg results also hyper-gravity experiments in centrifuges do not show an increase of the heat transfer efficiency as some correlations predict, but more a decrease of the efficiency of about 5–10% by an increase of gravity to 100 g [11].
Of course all these correlations are based on experimental results achieved on earth, and they were never thought for extrapolation to microgravity; but they are based on the physical model that buoyancy force acts on the bubbles for detachment and departure, and determine the heat transfer under these assumptions; this is even the case when the departure diameter is used as a characteristic length scale in the correlations. From our microgravity results these buoyancy dependent boiling models are questionable. In the following we will discuss the detailed thermo-physical phenomena events which are influenced by gravity, and which mechanisms still maintain heat transfer in microgravity, and what are the relevant driving forces if buoyancy effects go to zero.
5 Onset of boiling
Let us begin to elaborate on the boiling regime with nucleation at the onset of boiling. According to the nucleation theory the onset of boiling doesn’t depend on gravity. Rather it depends on the geometry of the nucleate sites, the heater material, on the liquid and its thermodynamic state and most on the local temperature at the nucleate site. The latter one is influenced by the heat flux and by natural convection at 1 g. It was observed that incipience of boiling occurs at μg at a lower heat flux than at 1 g but nearly at the same average temperature. After heater power on at 1 g buoyancy driven natural convection sets in developing liquid flow patterns, and cools the heater not evenly, but according to the formation of the convection patterns. Nucleation occurs where locally the temperature fits a nucleation site to become active. In Fig. 3 the temperature course versus time at μg after power on is compared with 1 g under the same experimental conditions: (platinum wire 0.05 mm diameter, heat flux 69 kW/m2, liquid is refrigerant R134a at saturation state, p/pc = 0.21). In both cases the temperature increase of the wire follows first according to heat conduction. Under μg conditions onset of boiling occurs at about 60°C, it follows a rapid temperature decrease to 45°C within 0.5 s. Under 1 g conditions the average temperature seems to be 1°–2° lower at onset of boiling, and then within 3 s the temperature slowly decrease to reach the same value than at μg. In the further course of ramping up the boiling curve both temperatures remain almost at the same level which means, that at the same heat flux also the same heat transfer coefficients are adapted, that means the gravity efficiency ε μg . = 1. At the onset of boiling the slow decrease of the heater temperature at 1 g compared to μg indicates that a large part of the wire is still cooled by buoyancy convection, and the temperature necessary for nucleation spreads only slowly over the entire heater wire and activates boiling. Under μg the onset of boiling occurs more evenly along the whole heater due to the preceding thermal diffusion regime and is like a small phase change explosion. These heater temperature evolutions are well in line with the evolution of the bubbles spreading along the wire as recorded by the video observation.
6 Bubble growth
The bubble growth rate depends on the heat flux and temperature at the heater surface. We may assume that in boiling the temperature at the interface on the vapour side of the bubble is nearly at saturation and small gradients lead to strong evaporation. From our experimental observations we conclude that the major part of the evaporation results from the thin liquid micro layer at the bubble base in direct contact with the solid heater, at this location high temperatures are calculated at the interface [12]. Further we observed [3] that smaller bubbles move with a liquid flow toward the wedge formed contour of larger bubbles, this supports new boiling models [13, 14]. As far as the bubble interface is in direct contact with the superheated liquid of the boundary layer at the heater surface additional evaporation comes along the bubble interface. The size of this boundary layer is gravity dependent, and has so far influence on the growth rate, but this part of evaporation is very small compared with the strong evaporation rate from the micro layer at the bubble base; its influence is hard to ascertain experimentally.
7 Bubble detachment and departure
Of course steady state boiling in microgravity needs even mechanisms that the bubbles can be transported away from heater. Since the heat transfer coefficients are practically identical for microgravity and earth gravity, these transport mechanisms in μg must replace gravity, and they are responsible for bubble detachment and departure from heater. After nucleation and during growth static and dynamic forces hold the bubbles attached at the heater wall, and there are forces which provide them a first push to detach and depart them. In the following we will describe some observations to understand these mechanisms without consisting on completeness.
7.1 Inertia forces
In some video recordings we could observe under microgravity after incipience of boiling on heater wires a swarm of very tiny bubbles shooting from the heater. These first nucleated bubbles are growing in the very high superheated liquid boundary layer at the heater; due to the high superheat they grow very fast, and push the liquid away. The inertia forces of this liquid act on these bubbles and detaches them and carries them along. But this happens only during the first event of boiling onset at the very fast bubble growth rate. This event is well known as a mechanism for bubble detachment due to inertia forces, but calculations show, that this effect happens only at the first phase of boiling onset where a high superheat of the liquid boundary layer is necessary for bubble nucleation resulting in a fast bubble growth. This was even the reason that the temperature of the thin heater wire dropped down fast as observed in Fig. 3. This swarm of tiny bubbles move slowly away, while subsequently at the heater wire the usual bubble boiling appears.
7.2 Oscillation of heater wall temperature (Ripening)
It is often observed that the temperature of the solid surface below a bubble oscillates with the frequency of bubble generation and departure. The heat for evaporation during bubble growth is mainly taken from the small, thin circular area of the heater wall at the bubble base, where the bubble is attached at the wall. In the centre of it a dry spot is formed, as recent numerical and experimental studies demonstrate [14]. The reaction force resulting from bubble growth and the recoil force caused by the strong evaporation at the bubble base holds the bubble at the surface and deforms it, forming an obvious wetting angle. This strong evaporation consumes the heat from the solid wall, and reduces that way very locally the wall temperature. Also a very thin slice of the solid wall is affected by the local heat sink, the temperature recovery by thermal diffusion in the solid material is a slow process compared with the spontaneous evaporation process. Therefore temperature oscillation is caused. At the low temperature phase of this oscillatory process the evaporation rate is highly reduced and further bubble growth practically stops, and just at that moment the bubble holding forces vanish. Now the bubble gets released and detaches from the heater and reforms itself to spherical shape, and the dry spot below the bubble rewets by superheated liquid flowing from the side. The bubble lifts itself by the formation to sphere and the inertia of the following liquid pushes it further off. This bubble departure mechanism is confirmed in all our boiling experiments at microgravity by the observation that when the power of the heater was switched off suddenly all bubbles depart immediately. They jump from the heater surface and immediately the liquid fills the empty space and rewets the solid surface. The bubble is like a spring which is kept at the wall by the holding forces caused by evaporation and dynamic bubble growth, and gets released, when evaporation and therefore bubble growth is stopped. The energy for such a bubble departure process is stored in the deformed bubble by surface tension energy and released by reshaping from the deformed shape to a sphere. The bubble departure is also supported by the inertia of the moved liquid during bubble growth, see Sect. 7.1. That process of temperature oscillation for bubble detachment can also be compared in a simplified way with an apple hanging at a tree, when the apple is getting ripe the sap (heat flux) to the apple is interrupted, the apple falls down; therefore we may call it also “bubble ripening”, but this biological oscillation process in nature occurs only once a year.
7.3 Bubble coalescence mechanisms
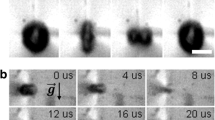
Very strong transport mechanisms are bubble coalescence processes, Fig. 4. We observed during boiling vigorous lateral and vertical bubble coalescence processes, which push the bubbles from the surface and further away. There are three effects: (i) even regarding only the steady state case before and after unification of bubbles, the centre of the joint bubbles moves away from the heater surface, the released volume is replaced by following liquid, it’s inertia pushes off the bubbles; (ii) after coalescence the unified bubble oscillates alternately in lateral and orthogonal direction, in perpendicular direction it hits the wall and pushes it selves off, (iii) the momentum and inertia of the following liquid push the bubbles further off.
8 Bubble removal at saturation state
As described before the mechanisms of bubble detachment and departure are observed to be identical both for saturated and subcooled liquids. However, the mechanisms of bubble or vapour removal differ. In saturated liquids the removal is mainly caused by coalescence processes, which depend on the size of the coalesced bubbles. From videos we observed that the bubbles still grow and therefore get larger after detachment from the heater. If two bubbles of same diameter freely coalesce in liquid the centre of the joint bubble will be at the contact point, when the oscillation comes to rest. The released surface energy caused by the reduced surface of the joint bubble is dissipated by oscillatory motion. If this coalescence happens parallel to the heater surface, the joint bubble will move away, Fig. 4. After detachment the bubbles at saturated boiling hover above the heater surface, and newly generated bubbles grow below, touch, and join them, and lift the bubble by the dynamics of the coalescence process. If a small bubble contacts a larger one, the vapour of the small bubble flows fast into the larger bubble by the pressure difference due to Laplace equation. The velocity of this vapour flow is accelerated with the decreasing diameter of the small bubble. The empty space of the vanishing small bubble is filled up by following liquid, which becomes accelerated with the decreasing bubble size, and pushes by its inertia the large bubble. In Fig. 5 we observed on the 0.2 mm diameter heater wire the bubble removal by coalescence processes. In the upper picture row on the left the two bubbles 1 and 2 contact each other, followed to the right by the coalescence process between them. In the second row the united bubble 3 oscillates lengthwise and crosswise. If we follow the position of the two bubbles from the first row to the last picture for bubble 3, we register a remarkable movement within the period of 300 ms, the frame sequence is 20 ms, the frame width is 6.4 mm. We must regard that no external forces act on the bubble. As we observed in the video, all bubbles grow with increasing distance from the heater by continuous coalescence processes, each bubble coalescence pushing the joint bubbles step by step by the inertia of the following liquid. These basic hydrodynamic processes maintain the vapour transportation in microgravity. Certainly the boiling process at saturated liquid state comes to rest when the pool is filled up with vapour.
9 Heat and vapour removal at subcooled liquid state
In case of subcooled boiling the bubbles generate a strong flow as long as they are attached to the heater. This flow is perpendicular to the heater surface and causes a reaction force which presses the bubbles together with the recoil and bubble growth force towards the heater surface. However, as described before even at subcooled boiling bubbles detach from the heater by temperature oscillation (ripening) and coalescence processes. If the bubbles detach also the reaction force of the flow around the bubble is stopped. Such detached bubbles move away along with the flow, they are taken along by the inertia and shear of their own produced flow, and by the flow generated by neighbouring bubbles. They become smaller with distance from the heater due to condensing in the subcooled liquid, Fig. 6.
This flow can be identified as a certain type of thermocapillary flow or surface tension driven flow (Marangoni convection), caused by temperature gradients along the surface of the bubbles between base and top. The origin of this thermocapillary flow will be discussed below. After departure in some distance from the heater these bubbles come to rest in the subcooled bulk liquid, condense, and disappear. A detailed view shows Fig. 7. Bubble 1 jumps from the surface after coalescence with the two small neighbouring bubbles, the joint bubble stops in some distance from the heater and condenses. The bubble set 2 coalesces, the joint bubble lifts up from the surface and returns to it. This behaviour may be due to the fact that this bubble penetrates with its top into colder liquid layers, developing a thermocapillary flow along its surface which drives the bubble backwards towards the heater. In the same way we may explain the sudden stop and stand still of bubbles in the middle of the overall flow. They penetrate with their top into the subcooled bulk liquid, and develop around them a thermocapillary flow. Even this flow is in the same direction as the overall flow, a reaction force is generated on the bubbles in the opposite direction which stops the bubbles and holds them in place, if the reaction force of the thermocapillary flow on the bubbles surface is of the same order as the shear force on the bubbles of the overall flow. This opinion is supported by the observation that stagnant bubbles condense much slower than moving ones.
10 Liquid jet stream generated on large bubbles
A further observation [1, 6] was noted on small plane circular heaters and on spherical beads (glass-coated semiconductor thermistors); under certain conditions of liquid state and heat flux large bubbles were formed, they are attached and remain at the heater and grow to a size much larger, than the size of the heater itself (up to 10 times). These bubbles produce a strong thermocapillary jet flow at the top, Fig. 8, visualised by diffraction interferometry applied to a dedicated nucleation and boiling experiment operated in the Bubble, Drop and Particles Unit (BDPU, ESA facility) during the LMS Spacelab mission (1996). In this case the energy and mass balance is covered by the evaporation at the base, the vapour flow through the bubble, the condensation of the vapour at the upper part, and the thermocapillary flow on the liquid side of the bubble, which takes heat and mass with it into the bulk liquid. If the heat flux was changed these large bubbles adjust their size until heat, respectively, mass transfer from bubble base to top is balanced. These bubbles act quasi as steady state adjustable heat pipes between the heater and liquid.
In order to study this surface tension driven jet flow we measured the radial velocity distribution with particle image velocimetry (PIV) which was provided in the optical diagnostic system of BDPU as mentioned above. For example at a subcooling of ΔT sub = 4.8 and 9.9 K the Gaussian velocity distributions are shown in Fig. 9, the parameter Δx indicates the distance from the heater surface. The maximum velocity in the centre of the jet flow is increasing with the heat flux and decreasing with the distance from the heater. Surprising was that at higher sub cooling the velocities are getting lower, for example at ΔT sub = 4.8 K the maximum velocity is 8.5 m/s, and at 9.9 K it is 4.6 m/s, even at a higher heat flux. This result is against the common understanding of thermocapillary convection. That’s why in this case the temperature difference between heater and the bulk liquid, generally used to describe thermocapillary convection by Marangoni number, can not be applied, and is no measure to describe heat transfer in subcooled boiling.
Radial velocity distribution from the jet flow of Fig. 8, Δx distances from heater
11 Origin of thermocapillary convection in subcooled liquid
It must be emphasised that in the case of boiling in saturated liquids we could never observe any flow in the liquid which would indicate the existence of surface tension driven flow, however, at subcooled boiling, as demonstrated, always convection was observed in all of our experiments. Up to now, it is not clear whether thermocapillary convection starting in the micro wedge region at the bubble base may occur or not. Indeed, there exist high temperature gradients [14] along the interface due to the high evaporation rates there. However, the dimension of this area is so small that either a flow doesn’t develop or could not be observed with present applicable optical methods. Therefore, we exclude the micro-wedge area from our further consideration concerning thermocapillary convection.
We repeat the statement expressed before: no convection could be observed at saturated boiling, but a strong convection appears always at subcooled boiling, see Figs. 6, 7, 8. The first is understandable by the general experience that the interfacial heat and mass exchange is a strong process, already very small temperature differences lead immediate to a strong mass exchange either through evaporation or condensation, so that deviations from equilibrium at the interface are immediately balanced. This leads to nearly uniform temperature along the interface, so that no temperature gradients along the bubble interface appear strong enough to generate a thermocapillary flow.
The question arises: what generates the liquid flows at subcooled boiling? Following the arguments discussed above we should assume, that the bubble interface in subcooled boiling is also near equilibrium at saturation temperature. Also any bubble at subcooled boiling is generated in the superheated liquid at the heater wall, and grow within it, but if it grows beyond this superheated layer, and penetrates with it’s top into the subcooled liquid, by that the interface may get only a little below saturation temperature, and the vapour molecules will condense immediately. At the subcooled liquid side the heat of the condensing molecules is transported into the subcooled liquid. However, non-condensable gases, inert gases (like air), first dissolved in the liquid, are released on the bubble base with the evaporating molecules, transported with them to the upper part, but when the vapour molecules condense; the non condensable molecules remain at the interface and accumulate there. The total pressure inside the bubble can be assumed to be constant; it consists of the partial pressure of the accumulated non condensable gas and the partial pressure of the vapour. At the top of the bubble interface where the non-condensable gas is most accumulated the partial vapour pressure is reduced, and according with it the saturation pressure, and with it the saturation temperature. The partial vapour pressure determines the saturation temperature at the interface, which is lower at the bubble top than at its base; consequently at the interface a temperature gradient from bubble top to base is established, which generates a thermocapillary flow in opposite direction from base to top Fig. 10. Calculations show that also very small impurities of non-condensable gases in the liquid are sufficient to generate a layer of inert gas inside the bubble interface which is thick enough to cause thermocapillary flow [15, 16, 17]. The difference of the partial vapour pressure between bubble base and top drives also the vapour and with it the non-condensable gases to the top. The shear force of the thermocapillary flow at the bubble interface on the liquid side supports this transport, and promotes on the top the accumulation of non-condensable gases. There could be a limit of the thickness of the accumulated non-condensable gas layer, because the vapour molecules must diffuse across this layer, but then the bubble will grow and the surface for condensation will increase. This could be the reason that large bubbles adapt their size for optimal heat transport, as described in Sect. 10.
It must be emphasized that for our experiments we have used liquids as pure as commercially available and we further purified and de-gassed them further by multiple distillations under high vacuum conditions.
12 Pumping at high liquid subcooling, cavitation boiling
At high liquid subcooling a higher heat flux is necessary to raise the wall temperature so far up to nucleation temperature, that bubbles may be generated. Otherwise heat conduction at μg or convection at 1 g is sufficient to cool the heater below nucleation temperature. If boiling occurs, the high heater surface superheat causes a rapid bubble growth, which consumes the surrounding superheat energy immediately. The bubble grows, the interface extends, and gets in touch with subcooled liquid, and then the vapour condenses spontaneously, the bubble collapse. The rapid periodic bubble expansion and collapse acts like the piston of a pump pushing with high frequency hot liquid away. During the short period in which the bubble and its interface exist, thermocapillary flow cannot be developed. Because of the microscopic size of the bubbles and the high frequency of this bubble growth, and condensation process the details of the events could not be visualized with the available optical instrumentation, but by in situ diagnostic inspection with an interferometer a slow moving plume of hot liquid could be detected in microgravity, which is identified with the pumped liquid, whereas at 1 g strong convection prevails and cover the whole process. This process is similar to the mechanism according them ink jet printers are working.
13 Conclusions
We achieved unexpected scientific results at boiling experiments in microgravity. At low system pressures and medium heat flux nearly the same and partially even higher heat transfer coefficients compared to earth conditions were measured. We must draw the conclusion that the key mechanisms for heat transfer in boiling are the same in 1 g and μg. The standard analytical correlations describing the heat transfer in boiling fail, if they are extrapolated to microgravity or hyper-gravity.
-
1.
The evaporation at the bubble base is the most important mechanism which determines heat transfer in boiling, because this process in μg is up to a certain heat flux nearly identical to that at 1 g.
-
2.
In absence of gravity the bubble detachment, departure and transport is due to the momentum and inertia generated by the bubbles and their coalescence processes. They maintain the vapour transport away from the heater. Various coalescence mechanisms could be detected; they replace gravity related buoyancy effects and are the responsible mechanisms for the detachment of the bubbles from the heater surface in saturated and subcooled state. In order to understand the heat transfer and the bubble behaviour optical observations in direct correlation with the heat transfer measurements were performed. These mechanisms are discussed.
The origin of the flow observed around bubbles in subcooled liquids can be identified as surface tension driven flow (thermocapillary), which in case of subcooled boiling is due to accumulation of non-condensable gases in the upper part of the bubbles. This conclusion results from the fact that in saturated boiling no such flow could be observed.
It is astonishing that the periodical removal of the vapour bubbles from the heater surface either at 1 g by gravity or at μg the different bubble dynamics has less influence on the overall heat transfer as generally assumed, and even at flow boiling the heat transfer does not noticeably change at the conditions of microgravity.
We describe in this paper the physical processes and mechanisms of the complex boiling system which appear at μg and definitely as well at 1 g, but in this case it is covered by buoyancy driven convection and bubble movement. The detailed knowledge of these mechanisms is important and essential for numerical studies, but even so for the formulation of empirical correlations with a wider range of validity as most of the present correlations allow.
References
Straub J (2001) Boiling heat transfer and bubble dynamics in microgravity. Advances in heat transfer. In: Hartnett JP, Irvine TF, Cho YI, Greene GA (eds) Academic Press, San Diego, San Francisco, New York, Boston, London, Sydney, Tokyo, vol 35. p 57
Ohta H (2003) Microgravity heat transfer in flow boiling. Advances in heat transfer.In: Hartnett JP, Irvine TF, Cho YI, Greene GA (eds) Academic Press, San Diego, San Francisco, New York, Boston, London, Sydney, Tokyo, vol 37. p 1
Zell M (1991) Untersuchung des Siedevorgangs unter reduzierter Schwerkraft. Dissertation, TU München
Zell M, Straub J, Vogel B (1989) Pool boiling under microgravity. J Physico-Hydrodyn 11:813–823
Micko S (2000) Sieden am Heizdraht unter reduzierter Schwerkraft. Dissertation, TU München
Steinbichler M (2000) Experimentelle Untersuchung des gesättigten und unterkühlten Siedens an Miniaturheizflächen unter Mikrogravitation. Dissertation, TU München
Steinbichler M, Micko S, Straub J (1998) Nucleate boiling heat transfer on small hemispherical heaters and a wire under microgravity. In: Heat Transfer, Proceedings of the 11th IHTC,Kyongju, Korea, vol 2. pp 539–544
Rohsenow WM (1952) A method of correlating heat transfer data for surface boiling of liquids. Trans ASME, Ser C, J Heat Transfer 74:969–976
Kutatelatse JJ (1963) Fundamentals of heat transfer, Edward Arnold Ltd., translated from Russian
Stephan K, Abdelsalam M (1980) Heat transfer correlation for natural convection boiling. Int J Heat Mass Transfer 23:73–87
Merte H, Liersch GF, Straub J, Keller RB (2009) Nucleate pool boiling with increased gravity and subcooling near the critical heat flux. Proceedings of ITP, Interdisciplinary Transport Phenomena VI, October Volterra, Italy
Stephan P, Hammer J (1994) A new model for nucleate boiling. Heat transfer. Heat Mass Transfer 30:119–125
Straub J (1995) The micro wedge model, a Physical description of nucleate boiling without external forces. Materials and fluids under low gravity In: Ratke L (ed) European Symposium on gravity dependent phenomena in physical sciences, Berlin, Springer Berlin, 1996
Kern J, Stephan P (2003) Theoretical model for nucleate boiling heat and mass transfer of binary mixtures. Transactions of the ASME. J Heat Transfer 125:1106
Marek R, Straub J (2001) The origin of thermocapillary convection in subcooled nucleate boiling. Int J Heat Mass Transfer 44:39–53
Marek R (1996) Einfluß thermokapillarer Konvektion und inerter Gase beim Blasensieden in unterkühlter Flüssigkeit. Dissertation, TU München
Straub J (2002) Origin and effect of thermocapillary convection in subcooled boiling: observations and conclusions from experiments performed in microgravity. In: Sadhal SS, Dhir VK, Ohta H, Smith RW, Straub J (eds) Microgravity transport processes in fluid, thermal, biological, and materials, sciences. Annals of the New York Academy of Sciences. The New York Academy of Sciences, New York, vol 974. p 348
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Dr.-Ing., Dr.h.c.mult. Karl Stephan on the occasion of his 80th birthday.
Rights and permissions
About this article
Cite this article
Straub, J., Zell, M. Transport-mechanisms in natural nucleate boiling in absence of external forces. Heat Mass Transfer 46, 1147–1157 (2010). https://doi.org/10.1007/s00231-010-0689-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-010-0689-0