Abstract
Variation in degree of surface wettability is presented through the application of Cooper’s correlative approach (h ∝ M −0.5 q ″0.67 w ) for computing enhancement (ϕ) in nucleate pool boiling of aqueous solutions of SDS and Triton X-100 and its presentation with Marangoni parameter (χ) that represents the dynamic convection effects due to surface tension gradients. Dynamic spreading coefficient defined as σ dyn N a , which relates spreading and wetting characteristics with the active nucleation site density on the heated surface and bubble evolution process, represents cavity filling and activation process and eliminates the concentration dependence of nucleate pool boiling heat transfer in boiling of aqueous surfactant solutions. Using the dynamic spreading coefficient (σ dyn N a = 0.09q ″0.71 w ), correlation predictions within ±15% for both SDS and Triton X-100 solutions for low heat flux boiling condition (q ″w ≤ 100 kW/m2) characterised primarily by isolated bubble regime are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The addition of small amounts of surfactants in water has been found to enhance the nucleate boiling heat transfer coefficient of water significantly [4, 16]. Because of their low concentration, the presence of surfactants in water causes no significant change in the physical properties of the aqueous solution except for surface tension [16]. The heat transfer coefficient is considered to be related to the equilibrium or static surface tension of the fluid as h α σ n in the nucleate boiling regime. However, as pointed out by Lowery and Westwater [8], there is no unanimity regarding the value of exponent n, and it ranges from −2.5 to +1.275. This is also illustrated by Wasekar and Manglik [16], where variations in the value of n from 0.4 to −11.87 are presented.
Boiling being inherently dynamic, dynamic and not the equilibrium surface tension should be a relevant correlation parameter. Dynamic surface tension is characterised by the adsorption of surfactants at the vapour–liquid interface under dynamic conditions and is primarily dependent on the bulk diffusivity of surfactant molecules, the rate of expansion/contraction of the interface, and the kinetics including the surface activity of the surfactants at the interface. The role of dynamic surface tension was pointed out by Morgan et al. [10] for the first time as the surface tension of “young surface” that may govern the nucleate boiling behaviour of aqueous surfactant solutions. Later, Jontz and Myers [7] carried out dynamic surface tension measurements to predict the boiling behaviour. They could not however satisfactorily predict the nucleate boiling performance of surfactants: Tergitol and Aerosol in their aqueous solutions. Yang [18] attributed dynamic surface tension affecting the boiling process through the rapid extension of vapour-liquid interface during growth and coalescence of vapour bubbles in the vicinity of the boiling surface. With the consideration that the same value of dynamic surface tension yields similar heat transfer performances, the importance of dynamic surface tension under boiling conditions as one of the primary determinants of nucleate boiling heat transfer characteristics of aqueous surfactant solutions was established by Wasekar and Manglik [15]. However there was no conclusive expression presented to correlate the experimental data using dynamic surface tension.
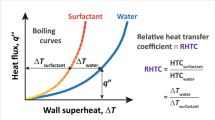
Significance of dynamic surface tension measured at near boiling conditions is presented by Wasekar [12] for the surfactant combinations independent of ionic nature and molecular weight using a correlation based purely on dynamic surface tension alone. It covers heat flux range of 30–225 kW/m2 and the normalised dynamic surface tension (normalised with water value (σ dyn/σ w)80°C) range of 0.65–0.96. Heat transfer coefficient ratios (h A /h B ) of lower A to higher B molecular weight surfactants are correlated within ±25% accuracy as shown in Fig. 1. Furthermore, the correlation predicts negligible dependence of comparative saturated, nucleate pool boiling heat transfer performances on the dynamic surface tension at high heat flux levels of 200–225 kW/m2. The results clearly explain the importance of concentration boundary layer influence through dynamic surface tension values under boiling conditions.
Dynamic surface tension based correlation predictions [12]
The correlation by Wasekar [12] however provides comparative heat transfer performances amongst various surfactants and calculation of heat transfer coefficient values for a given aqueous surfactant system is not possible using this correlation. It is therefore necessary to predict the heat transfer coefficients in nucleate boiling of aqueous surfactant solutions for the effective design of cooling systems. This paper presents influence of surface wettability during the nucleate pool boiling of aqueous SDS (sodium dodecyl sulphate, an anionic surfactant) and Triton X-100 (Octylphenol ethoxylate with 9–10 EO, a non-ionic surfactant) solutions and provides a correlative approach based on the dynamic spreading coefficient for the prediction of nucleate boiling heat transfer coefficient under the conditions of low heat flux boiling.
2 Theory
2.1 Surface wettability
Diffusion of surfactant molecules from the bulk towards the vapour–liquid interface depends primarily on the molecular weight of the surfactant such that heavier surfactant molecules, which represent higher molecular weight surfactant tend to diffuse slowly compared to faster moving lower molecular weight surfactant molecules. This diffusion process has important role in determining the dynamic surface tension at the interface mainly because of the adsorption at the interface taking place from the concentration sublayer at the interface. Thus, molecular weight of surfactant can impact significantly the nucleate boiling heat transfer of aqueous surfactant solutions.
Cooper [5] presented molecular weight dependence on saturated nucleate pool boiling through an accurate reduced pressure correlation based on extensive experimental data. For a given surface roughness value and the operating pressure conditions, the nucleate boiling heat transfer coefficient for a pure fluid is shown to be dependent on the fluid molecular weight as,
This correlation has wide ranging applicability in terms of operating conditions, heater geometries and fluids. The correlation covers reduced pressures from 0.001 to 0.9 and molecular weights from 2 to 200. The correlation however does not account for the variations in degree of surface wettability [6].
As defined by Bernardin et al. [2], the interaction of liquid with a solid is referred to as wettability. When the liquid spreads spontaneously out as a thin film across the surface, it is said to “wet” the surface. When the interactions are weak, the liquid beads up on the solid surface and the liquid partially wets the surface. The wetting characteristics of a vapour-liquid-solid system can be described by the interfacial energies or tensions. Surfactants have great effect on the wetting properties of water and wetting and surfactant diffusion and adsorption at interfaces may also lead to surface tension gradients on the free surface [11]. These gradients are caused by local variations in surfactant concentration. For evaporation of a droplet, Sefiane [11] has observed that there are two evaporation regimes. At low surfactant concentrations, the droplets are pinned, they then depin with a sudden rise or jump in contact angle and the sharp decrease in the base width. At higher surfactant concentrations however the droplets are pinned for most of the droplet lifetime. It is only near the end of the droplet lifetime that the base width decreases. The depining of the evaporating drop at low surfactant concentrations and not at higher concentrations was explained by a maximum in the force generated by surface tension gradient acting to pull triple line.
The importance of surface tension gradients in boiling of aqueous surfactant solutions is presented through suitably defined parameter, which represents the Marangoni convection due to the gradients of dynamic surface tension with bulk concentration. Correlative approach of Cooper [5] for the nucleate boiling heat transfer coefficient with molecular weight and heat flux is used to compute enhancement, ϕ for the experimental boiling data of SDS and Triton X-100 [15]. Figure 2 gives the variation of ϕ with respect to this Marangoni parameter, χ. The variables ϕ and χ are given below.
here, σ water = 58.91 mN/m [3] and represents pure water boiling under saturated atmospheric conditions.
The gradients of dynamic surface tension are computed using the following equations obtained from the curve fits for experimentally measured values under boiling conditions [15].
For SDS:
and for Triton X-100:
Figure 2 covers concentrations up to near micellar concentrations and heat flux levels from 30 to 225 kW/m2. The equations for curve fits depicted are given below.
For SDS:
For Triton X-100:
This depiction clearly presents relevance of Marangoni parameter, which tends to establish the physical picture, where non-uniform rates of evaporation and bubble growth at the interface lead to variations in surfactant concentrations along the interface. The representation in the form of ϕ and χ is independent of heater surface and geometry and is for the given operating conditions of saturation pressure for both the nucleate pool boiling of pure water and aqueous surfactant solutions. As seen from Fig. 2, the higher the Marangoni parameter, the greater is the nucleate boiling heat transfer coefficient. The correlations for SDS and Triton X-100 (Ref. Eqs. 6 and 7), present the effect of degree of surface wettability, which is not accounted by Cooper’s correlation (Ref. Eq. 1). This effect of degree of surface wettability is represented by the ratio of constants associated with the Marangoni parameter and is characterised by a constant value of 1.234 for the degree of enhancement as seen from Fig. 2 for Triton X-100 over SDS.
Furthermore, as pointed out by Carey [3], contact angle θ is a direct index of the wettability of liquid. By convention, a liquid for which θ = 0° is said to completely wet the solid surface, a liquid with a value of θ between 0° and 90° is termed a wetting liquid, for 90° < θ < 180° the liquid is said to be nonwetting, and for θ = 180° it is completely nonwetting. As presented by Wu et al. [17], the equilibrium or static contact angle values for Triton X-100 decreases significantly with the surfactant concentration and are lower than the values for SDS. Thus, the contact angle values for Triton X-100 show better wetting characteristics of Triton X-100 over SDS, which is also reflected through the effect of degree of surface wettability, which is characterised by a constant value of 1.234 for Triton X-100 over SDS. A value of less than 1 will therefore represent poor wetting characteristics with a value of 1 representing equality in wetting behaviour.
2.2 Dynamic spreading coefficient
To predict nucleate boiling heat transfer coefficient in boiling of aqueous surfactant solutions, Wasekar [14] introduced the concept of dynamic spreading coefficient, which primarily relates the spreading and wetting characteristics with the active nucleation site density on the heated surface and the bubble evolution process, the theory for which is described below.
Young’s equation,
Spreading coefficient,
Equation 10 indicates that S must be negative, which indicates the tendency of the liquid to wet and spread into a thin film. A positive value of S indicates that the liquid will wet and spontaneously spread into a thin film whereas a negative value of S indicates that the liquid will partially wet the solid and establish an equilibrium or static contact angle θ [3].
Based on the experimental findings, Basu et al. [1] have shown that
Now, combining Eqs. 10 and 11 and using liquid-vapour surface tension under dynamic conditions of boiling, expression for dynamic spreading coefficient S dyn can be given as,
Boiling bubbles as observed in the nucleate pool boiling of aqueous solutions of SDS and Triton X-100 are more regularly shaped and have smaller sizes as compared to the pure water boiling bubbles [4, 16]. Furthermore, the bubble growth behaviour in nucleate pool boiling of aqueous SDS and Triton X-100 solutions show absence of microlayer evaporation at the bubble base Wasekar [13]. With the consideration of area of influence within a circle around each active nucleation site with a diameter equal to the bubble departure diameter for microconvection regions, computations for N a were carried out using microconvection model of Mikic and Rohsenow [9]. For low heat flux boiling (q ″w ≤ 100 kW/m2) characterised by isolated bubble regime, Eqs. 13 and 14 respectively, give correlation fit for the dynamic spreading coefficient and correlation for the prediction of nucleate boiling heat transfer coefficients for both the surfactants SDS and Triton X-100, as presented in Figs. 3 and 4.
Dynamic spreading coefficient [Eq. 13] for q ″ w ≤ 100 kW/m2 [open symbols—Triton X-100]
Correlation predictions using Eq. 14 for low heat flux boiling (q ″ w ≤ 100 kW/m2)
As seen from Fig. 3, dynamic spreading coefficient, which represents cavity filling and activation process, not only eliminates the concentration dependence of nucleate pool boiling heat transfer but also represents generalised nucleate boiling performance of aqueous surfactant solutions for low heat flux boiling conditions (q ″ w ≤ 100 kW/m2). Figure 4 presents the correlation predictions given by Eq. 14, which correlates the experimental data of Wasekar and Manglik [15] using dynamic spreading coefficient given by Eq. 13 within ±15%.
3 Conclusions
Application of Cooper’s correlative approach (h ∝ M −0.5 q ″0.67 w ) for computing enhancement (ϕ) and its presentation with Marangoni parameter (χ) lead to the insights about the effect of degree of surface wettability, which is represented by the ratio of constants associated with the Marangoni parameter and is characterised by a value of 1.234 for the degree of enhancement for Triton X-100 over SDS. Prediction of the nucleate pool boiling heat transfer coefficient in aqueous surfactant solutions is carried out using the dynamic spreading coefficient defined as σ dyn N a , which represents cavity filling and activation process and eliminates the concentration dependence of nucleate pool boiling heat transfer in boiling of aqueous surfactant solutions. In larger sense, it represents force per unit volume, which can be used to evaluate the nucleate boiling performance of a given surfactant-surface combination. The correlation predictions using the dynamic spreading coefficient (σ dyn N a = 0.09q ″0.71 w ) for low heat flux boiling (q ″ w ≤ 100 kW/m2) characterised by isolated bubble regime correlates the experimental data of Wasekar and Manglik [15] for both the SDS and Triton X-100 surfactants within ±15%. This correlation can also be used for the prediction of heat transfer coefficients during boiling in confined spaces, which has significant importance in the development of compact and micro thermal components such as micro heat exchangers, cooling technology for electronic cooling and others, in addition to the processing of materials such as steel where accelerated or ultra fast cooling is applied to improve the controlled-rolled thick plate and strip properties such as tensile strength, toughness and weldability, among others.
Abbreviations
- C :
-
Surfactant bulk concentration (wppm)
- c p :
-
Water specific heat capacity (J/kg K)
- d d :
-
Bubble departure diameter (m)
- f :
-
Bubble departure frequency (1/s)
- h :
-
Heat transfer coefficient (kW/m2 K, W/m2 K)
- k :
-
Water thermal conductivity (W/m K)
- M :
-
Molecular weight
- N a :
-
Active nucleation site density (1/m2)
- n :
-
Exponent
- q ″w :
-
Wall heat flux (kW/m2, W/m2)
- S :
-
Spreading coefficient (N/m)
- S dyn :
-
Dynamic spreading coefficient (N/m3)
- χ :
-
Marangoni parameter (Eq. 3)
- ϕ :
-
Defined in Eq. 2
- θ :
-
Contact angle (deg)
- ρ :
-
Density of water (kg/m3)
- σ :
-
Equilibrium or static surface tension (m N/m, N/m)
- σ lv :
-
Liquid–vapour surface tension (N/m)
- σ sv :
-
Solid–vapour surface tension (N/m)
- σ sl :
-
Solid–liquid surface tension (N/m)
- σ dyn :
-
Dynamic surface tension @50 ms, 80°C (mN/m, N/m)
References
Basu N, Warrier GP, Dhir VK (2002) Onset of nucleate boiling and active nucleation site density during subcooled flow boiling. J Heat Trans 124:717–728
Bernardin JD, Mudawar I, Walsh CB, Franses EI (1997) Contact angle temperature dependence for water droplets on practical aluminum surfaces. Int J Heat Mass Trans 40(5):1017–1033
Carey VP (1992) Liquid-vapor phase-change phenomena. Hemisphere Washington, pp 63–638
Cheng L, Mewes D, Luke A (2007) Boiling phenomena with surfactants and polymeric additives: a state-of the-art review. Int J Heat Mass Trans 50:2744–2771
Cooper MG (1984) Heat flow rates in saturated nucleate pool boiling—a wide-ranging examination using reduced properties. In: Hartnett JP, Irvine TF Jr (eds) Advances in heat transfer, vol 16. Academic press, Orlando, pp 157–239
Dhir VK (1999) Nucleate boiling. In: Kandlikar SG, et al. (eds) Handbook of phase change, boiling and condensation, Ch. 4, Section 4.1–4.6
Jontz PD, Myers JE (1960) The effect of dynamic surface tension on nucleate boiling coefficients. AIChE J 6(1):34–38
Lowery AJ Jr, Westwater JW (1957) Heat transfer to boiling methanol—effect of added agents. Ind Eng Chem 49(9):1445–1448
Mikic BB, Rohsenow WM (1969) A new correlation of pool boiling data including the effect of heating surface characteristics. J Heat Trans 91:245
Morgan AI, Bromley LA, Wilke CR (1949) Effect of surface tension on heat transfer in boiling. Ind Eng Chem 41(12):2767–2769
Sefiane K (2004) Effect of nonionic surfactant on wetting behaviour of an evaporating drop under a reduced pressure environment. J Coll Interface Sci 272:411–419
Wasekar VM (2008a) On the significance of dynamic surface tension in nucleate pool boiling of aqueous surfactant solutions. In: Sharma KV (ed) Proceedings of the 19th national and 8th ISHMT-ASME heat and mass transfer conference, JNTU Hyderabad, India, January 3–5, Paper TPH-18
Wasekar VM (2008b) Transient bubble growth and thermocapillary in nucleate boiling of water and aqueous surfactant solutions. In: Gaitonde UN, et al. (eds) A festschrift in honour of Prof Suhas P Sukhatme, Research publishing, pp 101–108
Wasekar VM (2006) Role of dynamic surface tension and surface tension gradients in pool boiling of aqueous SDS solutions. In: Proceedings of the 18th national and 7th ISHMT-ASME heat and mass transfer conference, HMT-2006-C090, pp 641–648
Wasekar VM, Manglik RM (2002) The influence of additive molecular weight and ionic nature on the pool boiling performance of aqueous surfactant solutions. Int J Heat Mass Trans 45:483–493
Wasekar VM, Manglik RM (1999) A review of enhanced heat transfer in nucleate pool boiling of aqueous surfactant and polymeric solutions. J Enhanc Heat Trans 6:135–150
Wu WT, Yang YM, Maa JR (1999) Pool boiling incipience and vapor bubble growth dynamics in surfactant solutions. Int J Heat Mass Trans 42:2483–2488
Yang YM (1990) Dynamic surface effect on boiling of aqueous surfactant solutions. Int Comm Heat Mass Trans 17:711–727
Acknowledgments
The work on ϕ-χ was carried out during author’s doctoral research. Management support at TATA STEEL LIMITED is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wasekar, V.M. Heat transfer in nucleate pool boiling of aqueous SDS and triton X-100 solutions. Heat Mass Transfer 45, 1409–1414 (2009). https://doi.org/10.1007/s00231-009-0517-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-009-0517-6