Abstract
Purpose
This systematic review aims to evaluate the existing evidence associating linezolid to serotonin toxicity when used as monotherapy or when co-administered with other serotonergic agents.
Methods
A systematic literature search using PubMed (till March 2023), IDWeek meetings (2003–2023), the European Congress of Clinical Microbiology and Infectious Disease Annual Meetings (2001–2023), and the American College of Clinical Pharmacy (1999–2023) identified studies and abstracts related to linezolid and serotonin toxicity.
Results
A total of 84 studies were included. The data collected in retrospective/observational studies compared the incidence of serotonin toxicity with linezolid monotherapy at 0.0050% and linezolid combination therapy at 0.0134%. All cases which discontinued linezolid and serotonergic agent/s at signs and symptoms of toxicity found symptom resolution; 75% of cases reported serotonin toxicity resolution within 24–48 h after discontinuation.
Conclusion
Linezolid therapy when optimal should not be deferred due to the risk of serotonin syndrome. The data collected reveals a low prevalence of serotonin toxicity in both linezolid monotherapy and linezolid concurrent with other serotonergic agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oxazolidinones were first discovered and developed in the 1970s for the treatment of plant diseases [1]. In the 1990s and in response to emerging gram-positive resistance, further research eventually led to the development of linezolid [2, 3]. Linezolid was approved by the Food and Drug Administration (FDA) for human use in the year 2000 as the first antibiotic from the oxazolidinone class [2]. It is currently FDA-approved for the treatment of uncomplicated and complicated skin and skin-structure infections, community-acquired pneumonia, nosocomial pneumonia, and infection caused by methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE) [4]. In registry trials, the most commonly reported drug-related adverse effects were nausea, diarrhea, headache, vomiting, and duration-related myelosuppression (thrombocytopenia, anemia, leukopenia). Serious adverse effects were rare and not significantly different from comparator groups [5]. Prior to FDA approval, it was found to be a weak competitive (reversible) inhibitor of monoamine oxidase (MAO), the enzyme responsible for serotonin, epinephrine, norepinephrine, and dopamine deactivation and metabolism [6]. These findings implied linezolid may burden an increased risk for serotonin syndrome when combined with other serotonergic agents. Although a novel microtiter-plate assay demonstrates that linezolid is a weak competitive inhibitor of human MAO, no clinical evidence of MAO A inhibition in clinical trials has been observed [7]. Additionally, there were no reports of serotonin toxicity in clinical trials [5, 8]. Despite this, the manufacturer advised caution with the co-administration of serotonergic or adrenergic agents [9] due to the potential for serotonin-related toxicity.
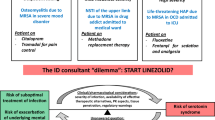
Serotonin toxicity, also referred to as serotonin syndrome, is the over activation of both the peripheral and central postsynaptic 5HT-1A and 5HT-2A receptors [10]. While excess serotonin is likely to be the major cause of effects, other neurotransmitters may contribute [10]. Toxicity can be manifested in a wide spectrum of clinical manifestations ranging from asymptomatic to potentially life-threatening (Fig. 1). Diagnosis of serotonin toxicity is typically aided by the use of the Hunter Serotonin Toxicity Criteria (HSTC) which is a more sensitive and specific test than the older Sternbach’s Criteria [11]. Serotonin toxicity is most commonly reported with the use of serotonergic agents, often when two agents are used concomitantly. In approximately 60% of patients with serotonin toxicity, the onset occurs within 6 h after starting a new drug, a dosage increase, or overdosing. Diagnosis is a limiting factor in treatment as symptoms can be mistaken for comorbidities or other disease states (e.g., neuroleptic malignant syndrome). If appropriately diagnosed, serotonin toxicity can be treated by discontinuation of the offending agent and supportive care [10]. In more severe cases, an anti-serotonergic medication, such as cyproheptadine, may be administered [10].
Since the approval of linezolid in the year 2000, reports of serotonin toxicity have emerged. In 2011, the FDA released a warning of serotonin toxicity if co-administered with serotonergic psychiatric medications based on case reports and information from the FDA Adverse Event Reporting System (AERS). These agents along with other commonly prescribed medications with serotonergic properties are listed in supplementary table 1 (Table 1S) [12]. However, robust studies supporting this association are lacking [13].
The objective of this systematic review was to evaluate the existing evidence associating linezolid to serotonin toxicity when used as monotherapy or when co-administered with another serotonergic agent. In addition, the pharmacology and mechanism of monoamine oxidase inhibition of linezolid are reviewed.
Methods
Search strategy and selection criteria
The Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) reporting guidelines were followed [14]. Studies were retrieved from PubMed from inception to March 2023 using the terms “linezolid” AND “serotonin.” In addition, abstracts from annual IDWeek meetings from 2003 to 2023, the European Congress of Clinical Microbiology and Infectious Diseases Annual Meetings from 2001 to 2023, and the American College of Clinical Pharmacy (ACCP) meetings from 1999 to 2023 were searched using “linezolid.” As some of the conference abstract databases were not searchable using keywords, a manual search was conducted. Case reports, case series, retrospective cohorts, and prospective studies were eligible for inclusion if they reported on patients that received linezolid and were screened for or developed serotonin toxicity.
Examining the incidence of serotonin toxicity with linezolid monotherapy may best be examined in large randomized controlled trials. As many randomized controlled trials examining linezolid may have excluded co-administration of serotonergic agents, we further searched PubMed for large (> 50 patients per arm) randomized controlled trials from 2011 to 2023 that included linezolid as part of a treatment regimen. This search was conducted by using the search term “linezolid” and the randomized controlled trial filter in PubMed. We excluded trials conducted prior to 2011 as many of these randomized trials were included in another publication that was identified for inclusion in the systematic review [6]. All randomized controlled studies that collected and reported adverse events were included.
Studies written in languages other than English were included if relevant information could be confidently extracted. Abstracts of all studies were screened by the two investigators. The reference list of all retrieved articles and any reviews were screened for additional studies. Discordant decisions for inclusion between the two screening investigators were subsequently discussed to achieve consensus.
Data extraction and analysis
Data from case reports included patient age, gender, complete medication therapy, time to diagnosis, and time to resolution of serotonin toxicity. Data extracted from retrospective or prospective studies included methodology, number of participants, serotonin toxicity diagnosis criteria, concomitant serotonergic agents, and duration of linezolid therapy.
Results
Chemistry and pharmacology of linezolid and monoamine oxidase inhibition
The structure of linezolid and other MAO inhibitors with similar chemical structures can be found in Fig. 2. The C5 acetyl amino-methyl group, phenyl morpholine group, and the fluorine atom on the phenyl group are essential for good antimicrobial activity [15]. The structure of linezolid lacks similarities with traditionally marketed non-selective MAOIs, except moclobemide which is a selective MAO-A inhibitor (Fig. 2). Linezolid has few structural similarities to newly developed potent MAO inhibitors.
The morpholine ring is essential for the MAO inhibitory effects as it implants in a large lipophilic pocket of MAO [16]. Compound #1 (Fig. 2) is a potent nicotinamide-based inhibitor for MAO-A isoform. In addition to the morpholine ring, the presence of the pyridine ring is essential for MAO inhibitory effect [16, 17]. Although linezolid has the morpholine ring, it lacks the pyridine group. The molecular docking studies of oxazolidinone derivatives created compound #2 (Fig. 2) as a potent, reversible, and selective MAO-A inhibitor and a promising anti-depressant [18]. The oxazolidinone group is fundamental for its MAO inhibitory effect; however, the potency is dependent on the presence of allyl substituent at the pyrrole N1-position. This structural feature is absent on the structure of linezolid. Linezolid concentration that causes 50% inhibition (IC50) of MAO-A or MAO-B was found to be 3 orders of magnitude less potent than clorgyline, and more than 2 orders of magnitude less potent than deprenyl in inhibiting MAO-A and MAO-B, respectively [15].
Considering both its chemistry and in vitro activity, linezolid has some structural features that enables the inhibition of MAO isoforms. However, it lacks important functional groups that cause potent inhibition compared to marketed MAO inhibitors.
Systematic review literature search
A total of 84 studies were included (Fig. 3): 14 randomized controlled trials, 1 summary of previously conducted randomized controlled trials, 57 case reports, 10 retrospective/observational studies, and 2 abstracts from ACCP meetings.
Linezolid monotherapy
The studies that evaluated linezolid use without co-administration of other serotonergic agents include a single case report, 5 retrospective/observational studies, 14 randomized controlled trials (published in 2011 or later), and 1 analysis of previous randomized controlled trials. The single case report was of a 65-year-old woman who was readmitted 5 days after receiving linezolid 600 mg twice a day with symptoms of serotonin toxicity [19]. Reviewing the patient’s medication chart did not reveal any other serotonergic agents, and her symptoms resolved shortly after the discontinuation of linezolid.
Fourteen randomized controlled trials met criteria for inclusion with 3956 patients receiving linezolid and 3926 patients receiving comparator agents [20–33]. Five of these studies excluded patients that were receiving concomitant serotonergic agents, 2 studies excluded patients receiving monoamine oxidase inhibitors but no other serotonergic agents, and seven did not report any exclusion regarding serotonergic agents. Despite several studies not indicating the exclusion of serotonergic agents, none of these studies provided any further information about the co-administration of drugs. Therefore, we included all of the studies in the linezolid monotherapy category even though co-administration may have been allowed in some of the studies. The average duration of linezolid use ranged from 5 to 14 days; 1 study allowed durations beyond 14 days in particular circumstances; and 1 study had a duration of 14 or 24 days. There was no reported incidence of “serotonin syndrome” or “serotonin toxicity” reported in either the linezolid or comparator groups.
In 2012, Butterfield and colleagues conducted a patient-level analysis of 20 randomized controlled trials published prior to 2011 from a locked database with extensive patient information [6]. The database included patients receiving linezolid monotherapy and also patients receiving linezolid with a serotonergic agent. In patients receiving linezolid monotherapy, no patients were diagnosed with serotonin toxicity (0%, 0/3218 patients receiving linezolid). Utilizing an extensive patient-specific database search, no patients met the HSTC for serotonin toxicity. Although several patients in both the linezolid and comparator groups met the Sternbach criteria, it is possible that the locked database from which the information was pulled may have limited this outcome by potentially withholding patient information that could have made them eligible for the Sternbach criteria.
Combining the results of Butterfield and colleagues [6] with that of the 14 randomized controlled trials we reviewed (7174 patients receiving linezolid monotherapy), the 95% confidence interval of the incidence of serotonin toxicity in patients receiving short courses of linezolid (5–14 days) is 0.0 to 0.04% (Wilson interval performed in Stata version 14.2, Stata-Corp, College Station, TX, USA) and is no different than in controls.
Five retrospective/observational studies that evaluated the incidence of serotonin syndrome with linezolid monotherapy included 598 patients [13, 20, 34–36]. One patient in a retrospective case–control study was found to have clinical features consistent with serotonin syndrome using Sternbach and Hunter criteria [34]. Two patients in an observational cohort study were evaluated to have symptoms of serotonin toxicity based on a word search algorithm of electronic medical records [35]. No study found a significant difference in the incidence of serotonin syndrome with linezolid monotherapy to control; the incidence of serotonin toxicity on linezolid monotherapy through the retrospective/observational studies is 0.0050% (Table 1).
Linezolid co-administration with serotonergic agents
The studies that evaluated linezolid when co-administered with other serotonergic agents include 56 case reports, 11 retrospective cohort studies, and 1 analysis of previous randomized controlled trials.
We identified 56 case reports of serotonin toxicity in patients receiving linezolid and at least one other serotonergic agent from 2001 to March 30th, 2023. Of the 26 case reports that disclosed the patients’ complete comprehensive medication list, 18 patients were documented on > 1 serotoninergic agent. The mean age was 55 ± 21 years with a range of 4 months to 98 years. The average time to serotonin toxicity from linezolid administration was 46 ± 30 h with a range of 1 h to 20 days. Over 75% of cases reported serotonin toxicity achieved resolution within 24–48 h after discontinuation of the offending agent/s. Five of the 56 patients were administered cyproheptadine in addition to discontinuing the offending agent and shared similar serotonin syndrome (SS) resolution times with the cases that did not receive cyproheptadine.
Only 1 patient, an 81-year-old man with osteomyelitis due to MRSA, did not recover. This case report documented signs of altered mental status 1 week after oral linezolid therapy and drastic signs of SS at week 3. The patient died following 3 cardiac arrest episodes. The study, however, did not report if linezolid and/or the other serotonergic agent (citalopram) were discontinued. [37]. In addition to the medications list included in Table 1S, other medications were found in these case reports and might have contributed to serotonin toxicity when co-administered with linezolid. These medications are methadone, metoclopramide, lithium, L-tryptophan, and 5-hydroxytryptophan.
In the summary of a randomized controlled database by Butterfield and colleagues, none of the 2208 patients that received linezolid with another serotonergic agent were reported to experience serotonin toxicity. In searching patient records, those receiving a serotonergic agent with linezolid were similarly likely to meet the HSTC as comparator antibiotics (0.14% vs. 0.05% respectively, relative risk 2.79 (95% CI 0.29 to 26.85)). The same was true when evaluating those meeting the modified Sternbach criteria (relative risk 1.40 (95% CI 0.57 to 3.41)). In the 303 patients that received both linezolid and an SSRI, 0 met the HSTC, and 2 (0.66%) met the modified Sternbach criteria.
Eleven retrospective/observational studies evaluated the incidence of SS with linezolid combined with ≥ 1 serotonergic agent. As displayed in Table 1, 15 of the 1170 patients (0.0128%) had signs or symptoms consistent with serotonin toxicity. No reports of serious adverse events associated with serotonin toxicity were found in any study. One retrospective study highlighted the risk of SS with linezolid specifically in comparison with vancomycin. Eight (3.2%) linezolid patients and 22 (8.8%) vancomycin patients met HSTC (relative risk (RR) 0.36; 95% CI, 0.17 to 0.79; P 0.007) [35]. Among those who received at least one serotonin agonist, 4.2% of linezolid patients met the HSTC, while 12.3% of vancomycin patients met the HSTC by any word search algorithm. Although this study shows the highest number of reported serotonin toxicity cases when linezolid is combined with at least 1 other serotonergic agent (Table 1), the risk of meeting the HSTC was lower among linezolid patients than among vancomycin patients within the study. A detailed summary of the reported studies relevant to the incidence of serotonin syndrome with linezolid is illustrated in Table 2S with relevant information about the dosing regimen of linezolid and the other serotonergic agents, if available.
Discussion
The FDA warning in 2011 on a potential drug–drug interaction with linezolid and serotonergic psychiatric medications was based on the FDA’s Adverse Event Reporting System. The exact mechanism for the drug–drug interaction was declared unknown. Since the emergency and non-emergent recommendations by the FDA to consider the risk of serotonin toxicity with linezolid, clinical risk management of serotonin toxicity has limited the use of linezolid therapy. Clinical knowledgebase IBM Micromedex has linezolid concurrent use with other serotonergic agents as a “contraindicated” drug–drug interaction. In many clinical settings, a pharmacist will be alerted with a drug–drug interaction when a patient on serotonergic agents is prescribed linezolid.
The data we acquired reveals a low prevalence of serotonin toxicity in patients receiving linezolid monotherapy and linezolid in combination with other serotoninergic agents. In addition, analysis of the pharmacology and structure–activity relationship found linezolid to lack functional groups that cause potent serotonergic effect. Differences in methodology and diagnosis criteria separated the data we extracted based off of study design. Our collection of randomized controlled trials saw no incidence of serotonin toxicity in 8755 patients (95% CI: 0.00–0.003%). It is important to note that 27 of the 32 studies did not exclude concomitant serotonergic agents, implying patients may have been on > 1 serotoninergic agent. The included RCT study by Butterfield et al. did find a few patients that qualified for HSTC or Sternbach criteria [6]. However, no patient met both criteria, and the study noted most patients meeting criteria for serotonin toxicity had past or present comorbidities that may have contributed to the reported adverse events. This data concluded that linezolid-induced serotonin toxicity is not significantly different than comparators. The data collected from these RCTs generally administered only short-term use of linezolid (< 14 days) and the incidence of SS greater than this time period remains undetermined.
Though there was no linezolid-induced serotonin toxicity seen in the RCTs, toxicity was reported in smaller sample size retrospective and observational studies. The data we collected compared the incidence of serotonin toxicity with linezolid monotherapy (0.0050%) vs linezolid combination therapy (0.0134%%). The slight increase in serotonin toxicity with linezolid combination therapy remains at a very low incidence. There are however limitations within the studies. In retrospective studies, serotonin toxicity is generally not reported and is diagnosed retrospectively by collecting reported patient adverse effects and matching them to either HSTC or Sternbach criteria. The study by T.P. Lodise et al. noted that many of the patients that met the “rigidity” criteria in HSTC had longstanding conditions (e.g., Parkinson’s disease) independent of the drug assignment that resulted in a positive HSTC classification [35]; excluding rigidity from HSTC would cause only 4 patients to meet criteria instead of the reported 8. The ambiguous diagnosing criteria for serotonin toxicity may lead to false positives in retrospective studies due to different patient etiologies or medication changes. Retrospective study limitations included in this review may inflate the true incidence of serotonin toxicity.
The data collected from the 57 published case reports provide clear evidence that there is a potential for linezolid-induced serotonin toxicity, yet it also demonstrates the efficiency to reverse serotonin toxicity with recognition and discontinuation of the offending agent/s and supportive care. Every report had serotonin toxicity symptoms subside after discontinuation of offending agents within an average of 45.5 h (range; 2–120 h). One report of a patient who did not recover had no documentation of linezolid and other serotonergic agents being discontinued further highlighting the necessity of prompt discontinuation of serotoninergic agents [38]. The majority of symptoms were noted as mild, and patients recovered rapidly without residual effects. Serotonin toxicity as a disease itself has an overall mortality rate of 2 to 12% [39] which has led to the caution around linezolid’s drug–drug interactions. However, many of these reports of mortality are from illicit drug use. Our data shows that there is a lack of data supporting severe consequences of serotonin toxicity when including linezolid.
The FDA generally recommends against using linezolid in patients using serotonergic drugs. However, in case of life-threatening infections with VRE or MRSA, the benefit of linezolid therapy should be weighed against the risk of serotonin toxicity. The FDA recommends to consider the use of alternative interventions if the treatment benefit of linezolid does not exceed the risk of serotonin toxicity. Alternative intervention may include vancomycin and daptomycin, a lipopeptide antibiotic that can also treat MRSA and VRE.
However, using alternative therapy in emergent situations due to the concern of serotonin toxicity with linezolid may lead to patients not receiving optimal therapy for a rare and rapidly resolving adverse effect. For example, a cohort study found linezolid to be less commonly utilized due to a lack of effectiveness against VRE-BSI (40/167 versus D 24/60, p = 0.03) [40]. Nevertheless, linezolid may have greater efficacy when compared to other comparator agents. For example, a meta-analysis of 11 RCTs concluded that linezolid seems to be more effective than vancomycin for treating people with skin and soft tissue infections (SSTIs) [41].
The FDA additionally recommended that in non-emergent situations, serotonergic psychiatric drugs should be stopped at least 2 weeks in advance of linezolid treatment (5 weeks for fluoxetine). According to drug compendia UpToDate, the standard approach consistent with multiple treatment guidelines recommends discontinuation of antidepressants to progressively taper (reduce) the dose by a fixed amount or percent for at least two to four weeks. Patients that discontinue therapy abruptly, or through a briefer taper, may experience “discontinuation effects” that include a range of symptoms that are not limited to dizziness, insomnia, nausea, headache, and fatigue [42]. Additionally, re-initiation with several of the proposed serotonergic psychiatric drugs may take 4–6 weeks for full therapeutic effects. Patients may experience untreated mental illness during this time of medication withdrawal. Healthcare providers should carefully weigh the risk for a serotoninergic drug–drug interaction against the consequences of a timely untreated mental illness.
Although our review did not observe significant association between the risk of serotonin syndrome (SS) and concomitant administration of linezolid with other specific serotonergic agents, other studies have utilized the pharmacokinetics (PK) and pharmacodynamic (PD) properties of these agents to infer risk of SS. For example, a pharmacovigilance PK/PD analysis of the worldwide FDA Adverse Event Reporting System (FAERS) of linezolid-reported SS suggested that linezolid is more likely to cause SS when co-administered with citalopram, escitalopram, and methadone [43]. However, it should be mentioned that such suggestion is based on both the FAERS and PK/PD properties of the offending agents. It is known that pharmacovigilance analysis, including FAERS, has several limitations such as reporting bias, quality of reports, and inability to assess the onset of SS from the time of drug administration. Additionally, drug’s PK/PD properties are subject to wide inter-individual variations due to several physiological and pathological factors. Another pharmacovigilance study investigated the adverse events (AEs) associated with the use of tedizolid and linezolid, both of which are oxazolidinone antibiotics [44]. The data shows that while both drugs have been associated with AEs, linezolid has a higher incidence compared to tedizolid. Additionally, concomitant use of linezolid was reported in only a small number of cases with tedizolid. Of the AEs investigated, serotonin syndrome was reported with both drugs, but there was no significant difference in reporting between the two. The study found that the onset of AEs associated with tedizolid was longer than the approved time frame for most investigated AEs. Finally, the article notes that concomitant drugs potentially implicated in AEs were found in all cases of serotonin syndrome and in 66.7% of reports mentioning bone marrow failure.
The authors strongly encourage healthcare providers to continue to accurately report any adverse effect arising from linezolid when used as a monotherapy or in combination with other agents that have serotonergic properties. Effective reporting system is the key to understand the underlying mechanism of adverse events and therefore prepare effective preventive measures.
Conclusion(s)
The use of linezolid therapy when optimal should not be deferred due to the risk of serotonin toxicity. Drug–drug interactions with linezolid and serotonergic agents should be a clinical caution, rather than a contraindication. In an inpatient setting, patients on ≥ 1 serotonergic agent may be administered linezolid under added supervision for signs and symptoms of serotonin toxicity. If symptoms of serotonin toxicity do arise, prompt discontinuation of linezolid and corresponding serotonergic agents have shown to quickly resolve and mitigate any serious adverse effects of toxicity. The authors recommend close monitoring of patients who start linezolid concomitantly with known serotonergic agents (e.g., SSRIs) or have concurrent disorders associated with serotonin excess (e.g., neuroleptic malignant syndrome). If feasible, other serotonergic agents should be avoided.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Diekema DJ, Jones RN (2001) Oxazolidinone antibiotics. Lancet 358:1975–1982. https://doi.org/10.1016/S0140-6736(01)06964-1
Hashemian SMR, Farhadi T, Ganjparvar M (2018) Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther 12:1759–1767. https://doi.org/10.2147/DDDT.S164515
Vinh DC, Rubinstein E (2009) Linezolid: a review of safety and tolerability. J Infect 59(Suppl 1):S59-74. https://doi.org/10.1016/S0163-4453(09)60009-8
ZYVOX®Highlights (Linezolid) | Pfizer Medical Information - US. Available online: https://www.pfizermedicalinformation.com/en-us/zyvox/highlights. Retrieved 1 September 2022
Rubinstein E, Isturiz R, Standiford HC, Smith LG, Oliphant TH, Cammarata S, Hafkin B, Le V, Remington J (2003) Worldwide assessment of linezolid’s clinical safety and tolerability: comparator-controlled phase III studies. Antimicrob Agents Chemother 47:1824–1831. https://doi.org/10.1128/aac.47.6.1824-1831.2003
Butterfield JM, Lawrence KR, Reisman A, Huang DB, Thompson CA, Lodise TP (2012) Comparison of serotonin toxicity with concomitant use of either linezolid or comparators and serotonergic agents: an analysis of phase III and IV randomized clinical trial data. J Antimicrob Chemother 67:494–502. https://doi.org/10.1093/jac/dkr467
Discotto LF, Pucci MJ, Lawrence LE, Barrett JF (1998) 38th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), September 24–27, 1998, San Diego, CA, USA. Expert Opin Investig Drugs 7:2061–2077.https://doi.org/10.1517/13543784.7.12.2061
Zhanel GG, Shroeder C, Vercaigne L, Gin AS, Embil J, Hoban DJ (2001) A critical review of oxazolidinones: an alternative or replacement for glycopeptides and streptogramins? Can J Infect Dis 12:379–390. https://doi.org/10.1155/2001/260651
Fung HB, Kirschenbaum HL, Ojofeitimi BO (2001) Linezolid: an oxazolidinone antimicrobial agent. Clin Ther 23:356–391. https://doi.org/10.1016/s0149-2918(01)80043-6
Volpi-Abadie J, Kaye AM, Kaye AD (2013) Serotonin syndrome. Ochsner J 13:533–540
Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM (2003) The hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 96:635–642. https://doi.org/10.1093/qjmed/hcg109
Research C (2019) for D.E. and FDA Drug Safety Communication: serious CNS reactions possible when linezolid (Zyvox) is given to patients taking certain psychiatric medications. FDA
Woytowish MR, Maynor LM (2013) Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother 47:388–397. https://doi.org/10.1345/aph.1R386
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) PRISMA-P group preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1. https://doi.org/10.1186/2046-4053-4-1
Tokuyama R, Takahashi Y, Tomita Y, Suzuki T, Yoshida T, Iwasaki N, Kado N, Okezaki E, Nagata O (2001) Structure-activity relationship (SAR) studies on oxazolidinone antibacterial agents. 1. Conversion of 5-Substituent on Oxazolidinone. Chem Pharm Bull 49:347–352. https://doi.org/10.1248/cpb.49.347
Shi L, Yang Y, Li Z-L, Zhu Z-W, Liu C-H, Zhu H-L (2010) Design of novel nicotinamides as potent and selective monoamine oxidase a inhibitors. Bioorg Med Chem 18:1659–1664. https://doi.org/10.1016/j.bmc.2009.12.065
Kumar B, Sheetal S, Mantha AK, Kumar V (2016) Recent developments on the structure–activity relationship studies of MAO inhibitors and their role in different neurological disorders. RSC Adv 6:42660–42683. https://doi.org/10.1039/C6RA00302H
Mai A, Artico M, Valente S, Sbardella G, Turini P, Befani O, Vedova L, Agostinelli E (2005) 3-(1H-pyrrol-2-Yl)-2-oxazolidinones as novel monoamine oxidase type A inhibitors. MC 1:117–124. https://doi.org/10.2174/1573406053175247
Sutton J, Stroup J, Som M (2016) Linezolid-induced serotonin toxicity in a patient not taking monoamine oxidase inhibitors or serotonin receptor antagonists. Bayl Univ Med Cent Proc 29:214–215. https://doi.org/10.1080/08998280.2016.11929423
Lv X, Alder J, Li L, O’Riordan W, Rybak MJ, Ye H, Zhang R, Zhang Z, Zhu X, Wilcox MH (2019) Efficacy and safety of tedizolid phosphate versus linezolid in a randomized phase 3 trial in patients with acute bacterial skin and skin structure infection. Antimicrob Agents Chemother 63:e02252-e2318. https://doi.org/10.1128/AAC.02252-18
O’Riordan W, Green S, Overcash JS, Puljiz I, Metallidis S, Gardovskis J, Garrity-Ryan L, Das AF, Tzanis E, Eckburg PB et al (2019) Omadacycline for acute bacterial skin and skin-structure infections. N Engl J Med 380:528–538. https://doi.org/10.1056/NEJMoa1800170
Harbarth S, von Dach E, Pagani L, Macedo-Vinas M, Huttner B, Olearo F, Emonet S, Uckay I (2015) Randomized non-inferiority trial to compare trimethoprim/sulfamethoxazole plus rifampicin versus linezolid for the treatment of MRSA infection. J Antimicrob Chemother 70:264–272. https://doi.org/10.1093/jac/dku352
Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P (2014) Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 14:696–705. https://doi.org/10.1016/S1473-3099(14)70737-6
Prokocimer P, De Anda C, Fang E, Mehra P, Das A (2013) Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA 309:559. https://doi.org/10.1001/jama.2013.241
O’Riordan W, Cardenas C, Shin E, Sirbu A, Garrity-Ryan L, Das AF, Eckburg PB, Manley A, Steenbergen JN, Tzanis E et al (2019) Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): a phase 3, double-blind, multicentre, randomised, controlled, non-inferiority trial. Lancet Infect Dis 19:1080–1090. https://doi.org/10.1016/S1473-3099(19)30275-0
Lee J-K, Lee JY, Kim DK, Yoon HI, Jeong I, Heo EY, Park YS, Jo YS, Lee JH, Park SS et al (2019) Substitution of ethambutol with linezolid during the intensive phase of treatment of pulmonary tuberculosis: a prospective, multicentre, randomised, open-label, phase 2 trial. Lancet Infect Dis 19:46–55. https://doi.org/10.1016/S1473-3099(18)30480-8
Kingsley J, Mehra P, Lawrence LE, Henry E, Duffy E, Cammarata SK, Pullman J (2016) A Randomized, double-blind, phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother 71:821–829. https://doi.org/10.1093/jac/dkv411
Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW (2014) Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 370:2169–2179. https://doi.org/10.1056/NEJMoa1310480
Awad SS, Rodriguez AH, Chuang Y-C, Marjanek Z, Pareigis AJ, Reis G, Scheeren TWL, Sanchez AS, Zhou X, Saulay M et al (2014) A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia. Clin Infect Dis 59:51–61. https://doi.org/10.1093/cid/ciu219
Noel GJ, Draper MP, Hait H, Tanaka SK, Arbeit RD (2012) A randomized, evaluator-blind, phase 2 study comparing the safety and efficacy of omadacycline to those of linezolid for treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother 56:5650–5654. https://doi.org/10.1128/AAC.00948-12
Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J (2012) Linezolid in methicillin-resistant staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629. https://doi.org/10.1093/cid/cir895
Covington P, Davenport JM, Andrae D, O’Riordan W, Liverman L, McIntyre G, Almenoff J (2011) Randomized, double-blind, phase ii, multicenter study evaluating the safety/tolerability and efficacy of JNJ-Q2, a novel fluoroquinolone, compared with linezolid for treatment of acute bacterial skin and skin structure infection. Antimicrob Agents Chemother 55:5790–5797. https://doi.org/10.1128/AAC.05044-11
Craft JC, Moriarty SR, Clark K, Scott D, Degenhardt TP, Still JG, Corey GR, Das A, Fernandes P (2011) A randomized, double-blind phase 2 study comparing the efficacy and safety of an oral fusidic acid loading-dose regimen to oral linezolid for the treatment of acute bacterial skin and skin structure infections. Clin Infect Dis 52:S520–S526. https://doi.org/10.1093/cid/cir167
Karkow DC, Kauer JF, Ernst EJ (2017) Incidence of serotonin syndrome with combined use of linezolid and serotonin reuptake inhibitors compared with linezolid monotherapy. J Clin Psychopharmacol 37:518–523. https://doi.org/10.1097/JCP.0000000000000751
Lodise TP, Patel N, Rivera A, Tristani L, Lazariu V, Vandewall H, McNutt LA (2013) Comparative evaluation of serotonin toxicity among veterans affairs patients receiving linezolid and vancomycin. Antimicrob Agents Chemother 57:5901–5911. https://doi.org/10.1128/AAC.00921-13
Traver EC, Heil EL, Schmalzle SA (2022) A cross-sectional analysis of linezolid in combination with methadone or buprenorphine as a cause of serotonin toxicity. Open Forum Infect Dis 9:ofac331. https://doi.org/10.1093/ofid/ofac331
Bernard L, Stern R, Lew D, Hoffmeyer P (2003) Serotonin syndrome after concomitant treatment with linezolid and citalopram. Clin Infect Dis 36:1197–1197. https://doi.org/10.1086/374558
Hachem RY, Hicks K, Huen A, Raad I (2003) Myelosuppression and serotonin syndrome associated with concurrent use of linezolid and selective serotonin reuptake inhibitors in bone marrow transplant recipients. Clin Infect Dis 37:e8-11. https://doi.org/10.1086/375689
Frank C (2008) Recognition and treatment of serotonin syndrome. Can Fam Physician 54:988–992
Mckinnell JA, Eells SJ, Injean P, Whang D, Humphries R, Gregson A (2019) Linezolid versus daptomycin in the treatment of vancomycin-resistant enterococcal bloodstream infection (VRE-BSI) at a transplant center. Open Forum Infect Dis 2016:3. https://doi.org/10.1093/ofid/ofw172.1567
Li Y, Xu W (2018) Efficacy and safety of linezolid compared with other treatments for skin and soft tissue infections: a meta-analysis. Biosci Rep 38
Clarke C, Finnegan M, O’Dwyer A, Mc Donald C, O’Connell B, Cooney J (2014) Co-prescribing of linezolid and serotonergic agents in a general hospital setting. Ir J Psychol Med 31(3):191–193. https://doi.org/10.1017/ipm.2014.33
Radunz S, Juntermanns B, Kaiser GM, Treckmann J, Mathe Z, Paul A, Saner FH (2011) Efficacy and safety of linezolid in liver transplant patients. Transpl Infect Dis 13:353–358. All rights reserved
Lorenz RA, Vandenberg AM, Canepa EA (2008) Serotonergic Antidepressants and Linezolid: A Retrospective Chart Review and Presentation of Cases. Int J Psychiatry Med 38(1):81–90. https://doi.org/10.2190/PM.38.1.h
Taylor JJ, Wilson JW, Estes LL (2006) Linezolid and serotonergic drug interactions: a retrospective survey. Clin Infect Dis 43(2):180–7. https://doi.org/10.1086/504809
Bishop E, Melvani S, Howden B, Charles PG, Grayson ML (2006) Good clinical outcomes but high rates of adverse reactions during linezolid therapy for serious infections: a proposed protocol for monitoring therapy in complex patients. Antimicrob Agents Chemother 50(4):1599–602. https://doi.org/10.1128/AAC.50.4.1599-1602.2006
Go AC, Golightly LK, Barber GR, Barron MA (2010) Linezolid interaction with serotonin reuptake inhibitors: report of two cases and incidence assessment. 25(1–4):41–47. https://doi.org/10.1515/DMDI.2010.001
Thirot H, Briquet C, Frippiat F, Jacobs F, Holemans X, Henrard S, Tulkens PM, Spinewine A, Van Bambeke F (2021) Clinical Use and Adverse Drug Reactions of Linezolid: A Retrospective Study in Four Belgian Hospital Centers. Antibiotics 10:530. https://doi.org/10.3390/antibiotics10050530
Acknowledgements
The authors of this review would like to thank Brig Turner for their initial help with study design and abstract screening.
Author information
Authors and Affiliations
Contributions
Both authors have made equal and substantial contributions to all of the following: (1) the conception and design of the study, acquisition of data, analysis, and interpretation of data; (2) drafting the article and revising it critically for important intellectual content; and (3) final approval of the version to be submitted.
Corresponding author
Ethics declarations
Ethical approval
Due to the nature of the systematic review, no ethical approval was required.
Conflict of interest
The authors declare no competing interests.
Additional information
All authors meet the ICMJE authorship criteria
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elbarbry, F., Moshirian, N. Linezolid-associated serotonin toxicity: a systematic review. Eur J Clin Pharmacol 79, 875–883 (2023). https://doi.org/10.1007/s00228-023-03500-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03500-9