Abstract
Purpose
The purpose of this study is to investigate the association between polypharmacy with health-related quality of life (HRQoL) and medication regimen complexity with HRQoL in residential aged care facilities (RACFs).
Methods
A cross-sectional study of 383 residents from six Australian RACFs was conducted. The primary exposures were polypharmacy (≥9 regular medications) and the validated Medication Regimen Complexity Index (MRCI). The outcome measure was staff informant rated quality of life assessed using the Quality of Life Alzheimer’s disease (QoL-AD) scale. Covariates included age, sex, Charlson’s comorbidity index, activities of daily living, and dementia severity. Logistic quantile regression was used to characterize the association between polypharmacy and QoL-AD (model 1) and MRCI and QoL-AD (model 2).
Results
The median age of the 383 residents was 88 years and 297 (78 %) residents were female. In total, 63 % of residents were exposed to polypharmacy and the median MRCI score (range) was 43.5 (4–113). After adjusting for the covariates, polypharmacy was not associated with either higher or lower QoL-AD scores (estimate −0.02; 95 % confidence interval (CI) −0.165, 0.124; p = 0.78). Similarly, after adjusting for the covariates, MRCI was not associated with either higher or lower QoL-AD scores (estimate −0.0009, 95 % CI −0.005, 0.003; p = 0.63).
Conclusions
These findings suggest that polypharmacy and medication regimen complexity are not associated with staff informant rated HRQoL. Further research is needed to investigate how specific medication classes may impact change in quality of life over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polypharmacy and complex medication regimens are highly prevalent in residential aged care facilities (RACFs) [1–3]. The definition of polypharmacy is diverse in literature [1]. A recent systematic review revealed that the prevalence of polypharmacy is highly variable depending on the definition used for polypharmacy [1]. The polypharmacy definition of ≥5 medications is common in the community and outpatient settings [4, 5], whereas ≥9 medications is the most common polypharmacy definition in RACF settings [1]. Polypharmacy is currently a quality indicator in RACFs in some jurisdictions in Australia [6]. While polypharmacy is not always inappropriate, it is associated with a greater risk of adverse drug events, morbidity, mortality, and increased healthcare use [7–11]. Complex medication regimens have also been associated with increased potential adverse drug events, healthcare use and 30-day hospital readmission and inversely associated with adherence and hospital discharge directly home [12–14]. Addressing these areas has been an ongoing concern, in particular in the RACF setting because there is a higher prevalence of polypharmacy and more complex medication regimens than in the community setting [9, 15]. Investigating quality of life is also important in this setting because the palliative approach to provision of aged care services involves focusing on maintenance of quality of life as a goal of care [16]. People with dementia or cognitive impairment are a vulnerable group with high care needs that frequently require residential aged care.
The development of community-based models of care has led to people being admitted to RACFs later in life. Residents in RACFs aged ≥85 years accounted for 57 % and 43 % of residents in Australia and the USA, respectively, in 2011 [17, 18]. In residents of RACFs, health-related quality of life (HRQoL) measures have been identified as an important multidimensional outcome measure [19, 20]. Health-related quality of life measures encompass ratings of physical, psychological, and social variables in combination with recognition that HRQoL depends on the group being evaluated [21]. The purpose of such a measure is to quantify the degree to which the medical condition or its treatment impacts the individual’s life in a valid and reproducible way [22]. Currently, national voluntary quality indicators for aged care are being developed in Australia and are likely to include a measure of quality of life.
The association between certain comorbidities and HRQoL has been widely investigated. However, there has been limited research on the association between medications and HRQoL in RACFs in residents with or without dementia [23]. People with dementia may have different patterns of medication use and corresponding susceptibility to adverse drug events which may in turn affect quality of life [24]. People with dementia have limited eligibility for participation in clinical drug trials and, therefore, it is important to develop an evidence base for medication use in this population group [25]. To our knowledge, no previous studies have quantified the association between medication regimen complexity and resident HRQoL. Relatively few studies have investigated the association between polypharmacy and HRQoL [7, 26]. Two Western Australian studies investigated this association in the RACF resident population [7, 26]. The first study included 351 residents aged ≥65 years and found no association between number of medications (>10 medications) and resident self-reported HRQoL in adjusted cross-sectional analysis [26]. The second cross-sectional study used the same data source and reported an inverse association between polypharmacy (≥5 medications) and self-reported resident HRQoL [7]. To our knowledge, these are the only two published studies to quantify the association between polypharmacy and HRQoL in residents of RACFs.
The objective of this study was to investigate the association between polypharmacy with HRQoL and medication regimen complexity with HRQoL in residents of RACFs. We hypothesized that both medication regimen complexity and polypharmacy would be inversely associated with HRQoL.
Methods
Study design and setting
We conducted secondary analyses of data from a cross-sectional study of permanent residents in six RACFs in metropolitan Adelaide and regional Mt. Gambier in South Australia. Residential aged care facilities in Australia provide supported accommodation for people with care needs that can no longer be met in their own homes [27]. The term is synonymous with the terms “nursing home” and “long-term care facility” that are used internationally [28]. The overall study design, methodology, and data collection have been described in detail elsewhere [29]. The initial study investigated analgesic use, pain, and daytime sedation in people with and without dementia in RACFs [30].
Participants
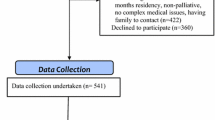
Permanent residents aged ≥65 years with and without dementia that had the ability to participate in structured assessments in English were included in the study sample. Residents deemed medically unstable (e.g., experiencing delirium) or estimated to have a life expectancy of less than 3 months were excluded from the study. There were 664 eligible residents in six residential aged care facilities and 603 residents were invited to participate due to exclusion by directors of care. Of these 603 residents, 220 were excluded for the following reasons: 106 declined; 34 were unwell, hospitalized, or palliative; 54 could not be recruited because third-party consent could not be arranged; and 26 residents were excluded for other reasons including being a new resident, behavior, or condition-precluded interview. The final study sample included 383 residents. There was no statistically significant differences between participants and all residents of RACFs in terms of age (mean 87.5 years [standard deviation SD 6.2] vs. 87.3 years [SD 6.4], t test, p = 0.66), sex (77.5 % female vs. 78.5 % female, chi square, p = 0.90), and dementia diagnosis (44.1 % vs. 46.8 %, chi square, p = 0.72).
Data source
A standardized data extraction form containing a range of validated and widely used scales (e.g., Katz Activities of Daily Living Scale (ADL), Dementia Severity Rating Scale (DSRS) was used for data collection. Residents’ medication administration charts were used to directly extract medication data. Clinical diagnoses of dementia and any other comorbidity were extracted from the electronic medical records. All data were collected by three experienced study nurses who undertook centralized training in the standard administration of the data collection tools. Data collection occurred from April to August 2014.
Medication assessment
To determine the prevalence of polypharmacy, the number of regular charted medications for each resident was counted. Polypharmacy was operationally defined as the “use of nine or more regular medications,” including prescription, non-prescription, dermatological products, and vitamin and herbal supplements. This definition was consistent with that recommended for use in residential aged care and is also the most frequent definition of polypharmacy in RACFs according to a recent systematic review [1]. Medication regimen complexity was computed using the 65-item validated Medication Regimen Complexity Index (MRCI) [31]. The MRCI incorporates a weighting corresponding to dosage form used, dosage frequency, and additional directions. The complexity index was the sum of scores for these three sections, with higher MRCI scores corresponding with more complex medication regimens. Regular and as-needed medications, including prescription, non-prescription, dermatological products, and vitamin and herbal supplements, were all included when computing the MRCI. The inclusion of non-prescription medications was consistent with other recent studies utilizing the MRCI [15, 32].
Primary outcome
The primary outcome measure was the Quality of Life in Alzheimer’s Disease (QoL-AD) scale. The staff informant version of the QoL-AD scale was completed by a registered nurse or care coordinator, who was required to have known the resident for a minimum of 2 weeks. The 15-item version for use in RACFs is an adaptation of the original 13-item version for use among community-dwelling people with dementia [33]. Previous research suggests that when used in people with dementia and mini-mental state examination scores greater than 10, the QoL-AD scale has good validity and reliability [21]. Possible scores range from 15 to 60, with higher scores indicating better HRQoL. Although some residents with dementia may have been able to self-report, the staff informant version of the QoL-AD scale was used for consistency and to maximize the number of completed scales.
Covariates
Covariates included age, sex comorbidity, ADL and dementia severity. Charlson’s Comorbidity Index (CCI) was calculated for each resident [23]. CCI consolidates individual comorbid conditions into a single, predictive variable. This is a widely used and validated method for use among older people in RACFs [34]. Activities of daily living were assessed using the 6-item Katz ADL scale [35]. The 12-item DSRS was completed by a staff informant as a valid assessment of dementia severity [36].
Statistical analysis
Demographic and clinical characteristics of residents were summarized numerically by MRCI tertile (≤37, >37 to 50 and >50) and polypharmacy (yes/no). Unadjusted and adjusted logistic quantile regression was used to investigate the association between polypharmacy as a dichotomous variable and HRQoL (analysis 1) and MRCI as a continuous variable and HRQoL (analysis 2) [37]. Logistic quantile regression is a regression technique that accommodates bounded continuous outcomes. The bounded continuous outcome was logit-transformed and regressed against the exposure variables using quantile regression. For the adjusted analyses, a full covariate model including age, sex, ADL, CCI, and DSRS was specified a priori based on previous research [1, 2]. Additionally, choice of covariates was informed by research suggesting polypharmacy is inversely associated with dementia in the RACF setting, and dementia may be associated with lower quality of life [1, 38–40]. Subgroup analyses were performed for analysis 1 and analysis 2 by limiting the sample to the 171 residents with a clinical diagnosis of dementia or who took antidementia medications (ATC code N06D). These subgroup analyses were conducted to compare to two previous studies on polypharmacy and HRQoL that were conducted in samples of residents with dementia only [7, 26]. All analyses were performed using R Version 3.0.1.
Results
Data were collected from 383 residents and 297 (78 %) residents were female (Table 1). At baseline, the median age of the participants was 88 years (range 66–106). The median number of regular charted medications per resident was 10 (range 1–22). In total, 243 (63 %) residents were exposed to polypharmacy (≥9 regular medications). The median score for QoL-AD in those exposed to polypharmacy was 37 (range 17–51) compared to 35 (range 15–49) among residents without polypharmacy. The median QoL-AD score for the total study sample was 36 (range 15–51).
Residents’ MRCI scores ranged from 4 to 113, with a median score of 43.5 and higher scores indicating a more complex medication regimen. Residents with a MRCI score ≤37 used a median of 6 (range 1–12) medications, residents with a MRCI score between 38 and 50 used a median of 10 (range 4–17) medications, while residents with a MRCI score >50 used a median of 14 (range 7–22) medications. Residents with a MRCI score >50 had a median QoL score of 36 (range 17–46).
The most prevalent dosing frequency for all medications was once daily (2807 medications, 54.6 %). The most prevalent dosage form was oral which included tablets or capsules (3718 medications, 70.4 %), followed by topical medication including creams, gels, paints, and patches (455 medications, 8.6 %). Other dosage forms (e.g., eye drops, inhalers, nasal sprays, injections) were used less commonly (1108 medication, 21 %). The five most prevalent medication classes used by residents with and without dementia were paracetamol (n = 362; 94.5 %), laxatives (n = 282; 73.6 %), antithrombotics (n = 238; 62.1 %), vitamins A and/or D (n = 212; 55.3 %), and medications for peptic ulcer and gastro-esophageal reflux disease (GORD) (n = 205; 53.5 %).
In the unadjusted model, there was no association between polypharmacy and either higher or lower QoL-AD scores (estimate 0.175; 95 % CI −0.093, 0.443; p = 0.20) (Table 2, Fig. 1). Likewise, MRCI was not associated with either higher or lower QoL-AD scores in the unadjusted model (estimate −0.002; 95 % CI: −0.008, 0.004; p = 0.49) (Table 2, Fig. 2). After adjusting for the covariates, polypharmacy was not associated with either higher or lower QoL-AD scores (estimate −0.02; 95 % CI −0.165, 0.124; p = 0.78) (Table 2). Similarly, after adjusting for covariates there was no association between MRCI and QoL-AD scores (estimate −0.0009; 95 % CI −0.005, 0.003; p = 0.63) (Table 2). Figures are available online as supplementary files.
There were 171 (45 %) residents with a clinical diagnosis of dementia or who took antidementia medications. Among these residents, 91 (53 %) were exposed to polypharmacy and the median MRCI score was 42 (range 6–113). In subgroup analyses for residents with dementia, there was no association between polypharmacy and OoL-AD scores in either the unadjusted (estimate −1.096E-09; 95 % CI −0.409, 0.409; p = 1.00) or adjusted (estimate 0.001; 95 % CI −0.332, 0.335; p = 0.99) analyses (Table 3). Similarly, there was no association between MRCI and QoL-AD scores in either the unadjusted (estimate −0.004; 95 % CI −0.016, 0.008; p = 0.52) or adjusted (estimate −0.001; 95 % CI −0.010, 0.009; p = 0.87) analyses (Table 3).
Discussion
The main findings of this study were that polypharmacy was not associated with staff informant rated HRQoL scores, and that medication regimen complexity was not associated with HRQoL scores even after adjusting for clinically important covariates. This finding is important because HRQoL is an important outcome measure for older people and it is crucial to determine the appropriateness of polypharmacy or regimen complexity as quality indicators in this resident population [7].
Previous studies on the association between medication use and HRQoL in the RACF setting have reported mixed findings. A Western Australian study by Bosboom et al. found that polypharmacy was inversely associated with self-reported HRQoL for residents with dementia when adjusting for a similar range of covariates as in our study [7]. In contrast, another Western Australian study by Beer et al. using the same data showed no association between the use of more than ten medications and self-rated HRQoL [26]. The Western Australian studies included residents with dementia only and Bosboom et al. defined polypharmacy as ≥5 medications, while Beer et al. investigated the use of >10 medications. These conflicting results highlight the importance of the definition of polypharmacy when investigating outcomes associated with polypharmacy in RACFs. In our sample, 63 % of residents were exposed to polypharmacy (≥9 medications) and these included residents with and without dementia. The definition of polypharmacy used in our study is the most commonly used definition in the RACF setting and is consistent with the polypharmacy indicator that has been used in RACFs in Australia and the USA [1]. Future studies should investigate the optimal definition of polypharmacy in this setting.
Residents with dementia may have different patterns of medication use than residents without dementia, and this may impact the association with quality of life [41]. In our study sample, polypharmacy was inversely associated with dementia severity. The subgroup analyses were limited to residents with dementia as prior studies by Bosboom et al. and Beer et al. limited their studies to residents with dementia. Similar to Beer et al., our subgroup analyses found no association between either polypharmacy or MRCI and HRQoL. Although Beer et al. used self-reported assessments for HRQoL, the comparison between our entire resident cohort and the subgroup limiting to dementia, demonstrates that regardless of dementia status there is no association between polypharmacy and MRCI with HRQoL [26]. Although there is no universal consensus on a superior instrument in assessing HRQoL in RACF residents, the QoL-AD is considered a measure of choice to assess the impact of interventions in dementia care [42]. While many residents of RACFs are able to self-report their HRQoL, studies suggest that there are discrepancies in HRQoL ratings made by people with dementia compared to those made by proxies (e.g., family carers and care staff) [26, 43, 44]. Notwithstanding the challenges associated with asking residents with dementia to self-report HRQoL, it may be important to include both self-reported and proxy measures in future research.
The complexity of an individual’s medication regimen not only takes into account the number of medications used but also the form of the medication, dosing frequency and additional directions required for correct administration. Medication regimen complexity was calculated using the MRCI. This is a widely used tool; however, it has not been specifically validated for use in the RACF setting [2]. Given most residents do not self-administer their medication, this may be a less useful indicator of HRQoL or other clinically important outcomes in this setting. Conversely, multiple daily dosing and the administration of multiple dose forms may be disruptive to the resident and adversely affect their HRQoL. Some residents may self-administer certain medications under supervision, e.g., metered dose inhalers. The MRCI was not scored differently for residents who do and do not self-administer their medications. A recent study conducted in Portugal of 415 residents aged ≥65 years showed that residents receiving from 1 to 5 different medications had a total MRCI score of 6.6 (±2.9), while those receiving from 6 to 20 medications had a total MRCI score of 20.2 (±8.9) (CI 99 % (p < 0.001)) [3]. Residents in our study had higher MRCI scores indicating they had more complex medication regimens than in the Portuguese study. Although we did not find an association with HRQoL, we did not assess the impact that complex regimens may have on staff. Additionally, complexity of medication regimens may highlight the opportunity for clinicians to consider simplifying regimens where appropriate and urge clinicians to consider regimen complexity when prescribing new medication.
There is a need for longitudinal studies into the association between medications and HRQoL. In a recent longitudinal study, it was suggested that the main predictors of HRQoL in residents were the baseline scores of the HRQoL measures and the number of chronic problems as assessed by CIRS-G comorbidity scale [45]. A Norwegian cluster randomized controlled study of residents of RACFs with dementia found that better baseline HRQoL (as measured by the QUALID scale) was associated with worsening HRQoL at 10 months [46]. The study found no association between baseline use of antipsychotics and change in HRQoL in adjusted analysis and approximately half of the residents QUALID scores did not deteriorate over a 10-month period [46]. Conversely, a study investigating potentially inappropriate medications (PIMs) with HRQoL found an association between PIMs and lower HRQoL [47]. Future research should investigate how medication review and use of specific medications is associated with maintenance or deterioration of quality of life changes over time [48]. With a sufficiently large sample size and multivariate analyses, it would be possible to consider whether specific medication classes are associated with changes in quality of life and other clinically relevant outcomes.
Strengths and limitations
Residents who participated in the study were similar in key demographics to all residents of the RACFs, indicating our sample was representative of residents in the RACFs from which they were recruited. However, the findings may not be generalizable to other settings. Diagnostic data were extracted from each resident’s current electronic medical record. However, we did not validate the clinical diagnoses listed in the medical records.
Informant ratings tend to underestimate self-reported HRQoL of people with dementia [26]. This is a limitation of our study; however, the registered nurse or care coordinator was required to have known the resident for at least 2 weeks. Although self-report is preferable where possible, staff informant rating scales are recommended as a substitute when people with dementia are not able to self-report. This principle is recognized by the Dementia Outcomes Measurement Suite of the Australian Dementia Collaborative Research Centres. Regardless, proxy bias cannot be excluded. Although, the QoL-AD instrument is suitable for use in people with dementia, the domains are applicable to people without dementia. Additionally, although only 44 % of participants had cognitive impairment that met the diagnostic criteria for dementia, many other participants had mild cognitive impairment or sub-threshold symptoms of dementia.
A limitation of our study is that we did not specifically consider the influence of depressive symptoms on HRQoL ratings. Depressive symptoms are recognized to be related to lower self-rated HRQoL, however the association between proxy-rated HRQoL and depressive symptoms is less clear [44]. Nevertheless, QoL-AD does include items related to residents’ mood and depressive symptoms.
Causality could not be determined given the cross-sectional nature of this study. It is not possible to determine whether residents with a more limited life expectancy and possibly lower HRQoL had medications stopped (deprescribed) which could lead them to belong to the non-polypharmacy group and therefore confound the results. However, we adjusted for multiple clinically relevant variables including comorbidity, dementia severity, and ADLs.
We considered the number of medication and not the clinical appropriateness of the medication, as polypharmacy alone is a current quality indicator in Australia and several other countries. Similar limitations also exist when applying explicit tools (e.g. Beers Criteria) to define medication appropriateness, most of which are not specific to the RACF setting nor among people with limited life expectancy (e.g. advanced dementia) [49]. More research on the clinical appropriateness of regimens in this setting will be of benefit as it may reduce inappropriate prescribing [49].
In conclusion, the use of polypharmacy and complex medication regimens is highly prevalent among residents of RACFs. Polypharmacy and medication regimen complexity were not associated with staff informant rated HRQoL in RACFs. Understanding whether or not medications contribute to lower quality of life is important for clinical practice because medications are a potentially modifiable risk factor for lower quality of life. Determining a quality indicator for medication use is progressively more important as the use of medications and associated regimens are increasing in this population group. Further research is needed to investigate how specific medication classes may impact on change in quality of life over time.
References
Jokanovic N, Tan E, Dooley M, Kirkpatrick C, Bell J (2015) Prevalence and factors associated with polypharmacy in long-term care facilities: a systematic review. J Am Med Dir Assoc 16(6):e531–512
Herson M, Bell J, Tan E, Robson L, Emery T, Wimmer B (2015) Factors associated with medication regimen complexity in residents of long-term care facilities. Eur Geriatr Med 6(6):561–564
Advinha A, de Oliveira-Martins S, Mateus V, Pajote S, Lopes M (2014) Medication regimen complexity in institutionalized elderly people in an aging society. Int J Clin Pharm 36(4):750–756
Gnjidic D, Hilmer S, Blyth F, Naganathan V, Waite L, Seibel M, McLachlan A, Cumming R, Handelsman D, Le Couteur D (2012) Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 65(9):989–995
Turner J, Jamsen K, Shakib S, Singhal N, Prowse R, Bell J (2016) Polypharmacy cut-points in older people with cancer: how many medications are too many? Support Care Cancer 24(4):1831–1840
Quality Indicators for Aged Care. (2015). https://www.dss.gov.au/ageing-and-aged-care/ensuring-quality/quality-indicators-for-aged-care. Accessed 21/10/2015
Bosboom P, Alfonso H, Almeida O, Beer C (2012) Use of potentially harmful medications and health-related quality of life among people with dementia living in residential aged care facilities. Dement Geriatr Cogn Dis Extra 2:361–371
Franic D, Jiang J (2006) Potentially inappropriate drug use and health-related quality of life in the elderly. Pharmacotherapy 26(6):768–778
Gurwitz J, Field T, Avorn J, McCormick D, Jain S, Eckler M, Benser M, Edmondson A, Bates D (2000) Incidence and preventability of adverse drug events in nursing homes. Am J Med 109(2):87–94
Lau D, Kasper J, Potter D, Lyles A, Bennett R (2005) Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Arch Intern Med 165(1):68–74
Onder G, Liperoti R, Foebel A, Fialova D, Topinkova E, van der Roest H, Gindin J, Cruz-Jentoft A, Fini M, Gambassi G, Bernabei R (2013) Polypharmacy and mortality among nursing home residents with advanced cognitive impairment: results from the SHELTER study. J Am Med Dir Assoc 14(6):450, e457-412
Schoonover H, Corbett C, Weeks D, Willson M, Setter S (2014) Predicting potential postdischarge adverse drug events and 30-day unplanned hospital readmissions from medication regimen complexity. J Patient Saf 10(4):186–191
Wimmer B, Dent E, Visvanathan R, Wiese M, Johnell K, Chapman I, Bell J (2014) Polypharmacy and medication regimen complexity as factors associated with hospital discharge destination among older people: a prospective cohort study. Drugs Aging 31(8):623–630
Mansur N, Weiss A, Beloosesky Y (2012) Looking beyond polypharmacy: quantification of medication regimen complexity in the elderly. Am J Geriatr Pharmacother 10(4):223–229
Wimmer B, Johnell K, Fastbom J, Wiese M, Bell J (2015) Factors associated with medication regimen complexity in older people: a cross-sectional population-based study. Eur J Clin Pharmacol 71(9):1099–1108
Beattie E, O’Reilly M, Moyle W, Chenoweth L, Fetherstonhaugh D, Horner B, Robinson A, Fielding E (2015) Multiple perspectives on quality of life for residents with dementia in long term care facilities: protocol for a comprehensive Australian study. Int Psychogeriatr 27(10):1739–1747
Department of Health and Human Services (2013) Nursing home data compendium. Center for Medicare and Medicaid Services. www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/downloads/nursinghomedatacompendium_508.pdf. Accessed 16/3/16
Residential aged care in Australia 2010–11: a statistical overview. (2012) Australian Institute of Health and Welfare. http://www.aihw.gov.au/publication-detail/?id=10737422821. Accessed 16/3/16
Hillen J, Vitry A, Caughey G (2015) Evaluating medication-related quality of care in residential aged care: a systematic review. Springer Plus 4:220
Naylor M, Hirschman K, Hanlon A, Abbott K, Bowles K, Foust J, Shah S, Zubritsky C (2015) Factors associated with changes in perceived quality of life among elderly recipients of long-term services and supports. J Am Med Dir Assoc
Logsdon R, Gibbons L, McCurry S, Teri L (2002) Assessing quality of life in older adults with cognitive impairment. Psychosom Med 64(3):510–519
Health-related quality of life research. (2016) International Society for Quality of Life Research. http://www.isoqol.org/about-isoqol/what-is-health-related-quality-of-life-research. Accessed 11/3/16
Frenkel W, Jongerius E, Mandjes-van Uitert M, van Munster B, de Rooij S (2014) Validation of the Charlson Comorbidity Index in acutely hospitalized elderly adults: a prospective cohort study. J Am Geriatr Soc 62(2):342–346
Bell J, Taipale H, Soini H, Pitkala K (2010) Sedative load among long-term care facility residents with and without dementia: a cross-sectional study. Clin Drug Investig 30(1):63–70
Cooper C, Ketley D, Livingston G (2014) Systematic review and meta-analysis to estimate potential recruitment to dementia intervention studies. Int J Geriatr Psychiatry 29(5):515–525
Beer C, Flicker L, Horner B, Bretland N, Scherer S, Lautenschlager N, Schaper F, Almeida O (2010) Factors associated with self and informant ratings of the quality of life of people with dementia living in care facilities: a cross sectional study. PLoS ONE 5(12):e15621
Aged care. (2016) Australian Institute of Health and Wellfare. http://www.aihw.gov.au/aged-care/. Accessed 16/3/16
Sanford A, Orrell M, Tolson D, Abbatecola A, Arai H, Bauer J, Cruz-Jentoft A, Dong B, Ga H, Goel A, Hajjar R, Holmerova I, Katz P, Koopmans R, Rolland Y, Visvanathan R, Woo J, Morley J, Vellas B (2015) An international definition for “nursing home”. J Am Med Dir Assoc 16(3):181–184
Tan E, Visvanathan R, Hilmer S, Vitry A, Quirke T, Emery T, Robson L, Shortt T, Sheldrick S, Lee S, Clothier R, Reeve E, Gnjidic D, Ilomaki J, Bell J (2014) Analgesic use, pain and daytime sedation in people with and without dementia in aged care facilities: a cross-sectional, multisite, epidemiological study protocol. BMJ Open 4(6):e005757
Tan E, Visvanathan R, Hilmer S, Emery T, Robson L, Vitry A, Hughes J, Jones M, Moawad S, Ilomaki J, Quirke T, Bell J (2015) Analgesic use and daytime sleepiness in residents with and without dementia in residential aged care facilities. Drugs Aging 32(12):1045–1053
George J, Phun Y, Bailey M, Kong D, Stewart K (2004) Development and validation of the medication regimen complexity index. Ann Pharmacother 38(9):1369–1376
Hirsch J, Metz K, Hosokawa P, Libby A (2014) Validation of a patient-level medication regimen complexity index as a possible tool to identify patients for medication therapy management intervention. Pharmacotherapy 34(8):826–835
Edelman P, Fulton B, Kuhn D, Chang C (2005) A comparison of three methods of measuring dementia-specific quality of life: perspectives of residents, staff, and observers. Gerontologist 45 Spec No 1 (1):27–36
Buntinx F, Niclaes L, Suetens C, Jans B, Mertens R, Van den Akker M (2002) Evaluation of Charlson’s comorbidity index in elderly living in nursing homes. J Clin Epidemiol 55(11):1144–1147
Katz S, Ford A, Moskowitz R, Jackson B, Jaffe M (1963) Studies of illness in the aged. The index of ADL: A standardized measure of biological and pscychosocial function JAMA 185:914–919
Clark C, Ewbank D (1996) Performance of the dementia severity rating scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord 10(1):31–39
Bottai M, Cai B, McKeown R (2010) Logistic quantile regression for bounded outcomes. Stat Med 29:309–317
Lai C, Leung D, Kwong E, Lee R (2015) Factors associated with the quality of life of nursing home residents in Hong Kong. Int Nurs Rev 62(1):120–129
Wetzels R, Zuidema S, de Jonghe J, Verhey F, Koopmans R (2010) Determinants of quality of life in nursing home residents with dementia. Dement Geriatr Cogn Disord 29(3):189–197
Hurt C, Banerjee S, Tunnard C, Whitehead D, Tsolaki M, Mecocci P, Kloszewska I, Soininen H, Vellas B, Lovestone S (2010) Insight, cognition and quality of life in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 81(3):331–336
Tan E, Jokanovic N, Koponen M, Thomas D, Hilmer S, Bell J (2015) Prevalence of analgesic use and pain in people with and without dementia or cognitive impairment in aged care facilities: a systematic review and meta-analysis. Curr Clin Pharmacol 10(3):194–203
Moniz-Cook E, Vernooij-Dassen M, Woods R, Verhey F, Chattat R, De Vugt M, Mountain G, O’Connell M, Harrison J, Vasse E, Droes R, Orrell M (2008) A European consensus on outcome measures for psychosocial intervention research in dementia care. Aging Ment Health 12(1):14–29
Bosboom P, Alfonso H, Almeida O (2013) Determining the predictors of change in quality of life self-ratings and carer-ratings for community-dwelling people with Alzheimer disease. Alzheimer Dis Assoc Disord 27(4):363–371
Beerens H, Zwakhalen S, Verbeek H, Ruwaard D, Hamers J (2013) Factors associated with quality of life of people with dementia in long-term care facilities: a systematic review. Int J Nurs Stud 50(9):1259–1270
Castro-Monteiro E, Forjaz M, Ayala A, Rodriguez-Blazquez C, Fernandez-Mayoralas G, Diaz-Redondo A, Martinez-Martin P (2014) Change and predictors of quality of life in institutionalized older adults with dementia. Qual Life Res 23(9):2595–2601
Mjorud M, Rosvik J, Rokstad A, Kirkevold M, Engedal K (2014) Variables associated with change in quality of life among persons with dementia in nursing homes: a 10 months follow-up study. PLoS ONE 9(12):e115248
Juola A, Pylkkanen S, Kautiainen H, Bell J, Bjorkman M, Finne-Soveri H, Soini H, Pitkala K (2016) Burden of potentially harmful medications and the association with quality of life and mortality among institutionalized older people. J Am Med Dir Assoc 17(3):276, e279-276 e214
Pitkala K, Juola A, Kautiainen H, Soini H, Finne-Soveri U, Bell J, Bjorkman M (2014) Education to reduce potentially harmful medication use among residents of assisted living facilities: a randomized controlled trial. J Am Med Dir Assoc 15(12):892–898. doi:10.1016/j.jamda.2014.04.002
Kaufmann C, Tremp R, Hersberger K, Lampert M (2014) Inappropriate prescribing: a systematic overview of published assessment tools. Eur J Clin Pharmacol 70(1):1–11
Acknowledgments
The authors thank Resthaven staff and residents for their participation in this study. The work was supported by the Alzheimer’s Australia Dementia Research Foundation via the Resthaven Incorporated Dementia Research Award, with additional funding provided by Resthaven Incorporated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Verbal and written information about the study were provided to all participants. The resident’s written consent was obtained directly. If the residents did not have the capacity to consent, written informed consent was obtained from their guardian, next of kin or significant other. The study was approved by the Royal Australian College of General Practitioners (RACGP) National Research and Evaluation Ethics Committee and the Monash University Human Research Ethics Committee.
Conflict of interest
No conflicts of interest. The authors alone are responsible for the content and writing of the manuscript.
Rights and permissions
About this article
Cite this article
Lalic, S., Jamsen, K.M., Wimmer, B.C. et al. Polypharmacy and medication regimen complexity as factors associated with staff informant rated quality of life in residents of aged care facilities: a cross-sectional study. Eur J Clin Pharmacol 72, 1117–1124 (2016). https://doi.org/10.1007/s00228-016-2075-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2075-4