Abstract
Purpose
Potentially inappropriate medication (PIM) is suggested to give rise to adverse drug events. To study this suggestion for elderly psychiatric patients, an observational analysis related prescription of PRISCUS PIMs and drug-induced side effects in old aged (≥65 years) psychiatric inpatients and outpatients under conditions of everyday pharmacotherapy.
Methods
Request forms from a therapeutic drug monitoring (TDM) survey and medical files were screened for medication to identify PIMs of the PRISCUS list and assessed using the Udvalg for Kliniske Undersøgelser (UKU) side effect rating scale.
Results
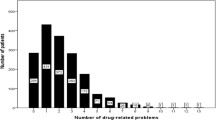
From 914 TDM request forms, data were available for 168 patients (64.3 % female). Patients (mean ± SD age 73.0 ± 5.5 years) received by mean 6.4 ± 3.9 drugs per day. More than half of them (53.0 %, n = 89) had at least one PIM, inpatients 0.9 ± 0.8 and outpatients 0.5 ± 0.7. Predominant PIMs were hypnotic drugs (69 %) in inpatients and antipsychotic drugs (35.6 %) in outpatients. The number of PIMs correlated with the total number of drugs administered per day (Spearman correlation coefficient 0.225, p < 0.01, CI 95 %). Side effects were documented for 106 patients (63 %). Severity of side effects did not correlate significantly (p > 0.05) with number of PIMs. However, only 6 of 77 patients who took no PRISCUS PIMs but 2 of 3 patients who took 3 PRISCUS PIMs exhibited severe side effects.
Conclusions
Though the prevalence for PIMs and side effects was high in old aged psychiatric inpatients and outpatients, PIMs could not be identified as major determinants of overall unwanted side effects. Nevertheless, prescription of PIMs should be minimized, especially of hypnotic drugs, to improve safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Healthcare is confronted with a growing number of elderly patients suffering from numerous disorders including psychiatric diseases. These patients require multiple drugs. Because of polypharmacy [1], multimorbidity, and age-related changes in pharmacokinetics and pharmacodynamics [2–4], they are high-risk patients for the development of drug-induced adverse drug reactions (ADRs) [5]. Especially in gerontopsychiatry, physicians have to be aware about an increased sensitivity to neuroleptic drugs [6, 7]. In particular, patients with diagnosed frailty-syndrome are highly sensitive to ADRs [8–10]. Polypharmacy increases the ADR risk due to pharmacodynamic and pharmacokinetic drug–drug interactions [11]. Therefore, ADRs are often the results of cumulative effects of multiple drugs, especially by summation of delirogenic properties [12, 13]. In addition, prescribing potentially inappropriate medication (PIM) is suggested to be an important risk factor for the development of ADRs in older patients [14–17]. Many commonly prescribed psychotropic drugs are categorized as potentially inappropriate in the elderly [16, 18]. This gives rise to the suggestion that avoidance or reduction of PIM prescription will improve safety and tolerability of drugs [17].
Lists of PIMs for elderly patients have been developed by different expert teams [18–21, 16] aiming to improve safety and tolerability of pharmacotherapy. The PRISCUS list [16] was established and published in 2010 for the German pharmaceutical market [22, 23]. It contains 83 drugs from 18 classes.
Before integrating the use of the PRISCUS list in every day practice, it has to be shown that intake of PIMs is really associated with the occurrence of adverse events. A review of medical records from emergency cases aged 65 years or older revealed a high rate of adverse events. Prescription of PRISCUS PIMs, however, accounted for only 6.1 % of this adverse events [15]. Since many PRISUS PIMs are psychoactive drugs and factors such as depression or polypharmacy have been identified as risk factors for PIM intake [24–27], it may be suggested that elderly psychiatric patients have a high prevalence for prescription of PIMs and that the impact of inducing unwanted side effects is more pronounced in elderly psychiatric patients than in emergency patients. Data on old aged psychiatric patients, however, relating prescription of PIMs and the occurrence of drug-induced side effects are so far lacking.
We analyzed prescription of PRISCUS PIMs in old aged psychiatric patients for whom monitoring of drug concentrations in blood had been requested. It was hypothesized that there is a high rate of PIM prescriptions and that this is related to side effects. Moreover, it was hypothesized that inpatients receive more PIMs than outpatients since they are sicker than outpatients and that there is a gender difference with more prescription of drugs in female than in male patients [24, 27].
Methods
Patients
The present study was a retrospective analysis, conducted on psychiatric patients who were treated in academic and nonacademic psychiatric hospitals with inclusion of three outpatient care units. Due to the retrospective nature of the study, real-life pharmacotherapy could be studied, since included patients were treated under everyday clinical conditions. Data were obtained from inpatients and outpatients for whom therapeutic drug monitoring (TDM) had been requested. Request forms included information on patients’ characteristics, medication, and side effects [28]. For inpatients, data from TDM request forms were supplemented with information from medical files.
Patients were excluded when evaluation of side effects in relation to prescribed medication was not possible, for example, in the case of acute detoxification or moderate to severe dementia at the time of blood withdrawal. Elderly patients who were identified by TDM as noncompliant at the time of blood withdrawal were also excluded. Each patient was included for analysis only once. When multiple serum level measurements were requested for the same patient, only the latest information was considered. No restriction was made according to diagnosis and severity of illness, length of treatment, or comedication.
The total number of drugs prescribed per day was calculated, excluding topical, ophthalmic, inhaled, and otologic medications. Dietary supplements and medical devices were also excluded from calculation.
Identification of PIM
Potentially inappropriate medication (PIM) was identified using the PRISCUS list [16], considering the criteria dosage or formulations that are not recommended.
Evaluation of side effects
Side effects and their severity were assessed by the treating physician using the Udvalg for Kliniske Undersøgelser (UKU) rating scale for side effects [29] as documented on the TDM request form (0 = no, 1 = mild, 2 = moderate, 3 = severe side effect). In the case of incomplete information on the request form, a clinical pharmacist checked the medical records if side effects had been reported around the time of blood withdrawal.
Statistical analysis
Descriptive statistics of patients’ data were presented as mean values ± standard deviations (mean ± SD). Referring to the hypothesis of the study, multiple comparisons between patient groups were conducted using one-way analysis of variance (ANOVA), as normal distribution was assumed and two independent groups were compared concerning one metric criterion. For post hoc comparison, Tukey’s HSD was used. If heterogeneity in data was detected, the Welch’s test was applied. A significant association between the occurrence of side effects and the intake or number of PRISCUS PIMs was analyzed using the chi-square (χ 2) test for independence. Using Spearman’s correlation analysis, a possible correlation between various factors in the two patient groups was determined. Statistical analysis was carried out by using IBM® SPSS® Statistics version 21.0 (IBM GmbH, Ehningen, Germany). A p value of <0.05 was considered as statistically significant.
Results
Patients
Overall, 914 request forms for determination of drug concentrations in blood were available from 206 old aged patients (117 inpatients and 89 outpatients). Using the last observation per patient and excluding patients with incomplete information, 168 patients (64.3 % female) underwent our analyses. Their mean ± SD age was 73.0 ± 5.5 years (Table 1). The most frequent diagnoses (ICD-10) were recurrent depressive disorder (F33, 47.9 %), schizophrenia (F20, 16.8 %), and depressive episode (F32, 10.8 %). For outpatients, the most frequent diagnoses (ICD-10) were recurrent depressive disorder (F33, 38.6 %), schizophrenia (F20, 26.1 %), and bipolar affective disorder (F31, 12.5 %). With regard to hospitalized patients, recurrent depressive disorder (F33, 58.2 %) and depressive episode (F32, 16.5 %) were predominant. Schizophrenia (F20) was diagnosed for only 6.3 % of the patients.
Medication and prescription of PRISCUS PIMs
The 168 patients included for retrospective analysis of prescribed PIMs and side effects received by mean ± SD of 6.4 ± 3.9 drugs per patient and day, inpatients twofold more than outpatients, i.e., 8.8 ± 3.9 and 4.2 ± 4.3, respectively (p < 0.01). In total, 53.0 % (n = 89) of all patients received at least one PIM, by mean 0.7 ± 0.8 (range 0–3, Table 1). Considering the mean number of 6.4 drugs and 0.7 PIMs per patient, the relative number indicated that 10.9 % of prescribed drugs were PIMs. This percentage was similar in inpatients (11.9 %) and outpatients (10.2 %). With regard to gender, there were no significant differences (p > 0.05) concerning the number of prescribed PIMs. The mean number of administered drugs was similar in females (6.3 ± 3.9) and males (6.4 ± 3.9). The amount of prescribed PIMs correlated significantly with the total number of drugs administered per day, Spearman’s correlation coefficient was 0.225 (p < 0.01, CI 95 %). Outpatients received significantly (p < 0.01) fewer PIMs than inpatients (0.5 ± 0.7 and 0.9 ± 0.8, respectively). Altogether, 40.4 % of the outpatients (n = 36) received at least one PIM compared to 67.1 % of the inpatients (n = 53, Table 1).
Pharmacological properties of PRISCUS PIMs
For the 89 patients who received at least one PIM, the most frequently prescribed PIMs were lorazepam >2 mg/day (n = 31, 26.1 %), zopiclone >3.75 mg/day (n = 11, 9.2 %), diazepam (n = 10, 8.4 %), haloperidol >2 mg/day (n = 8, 6.7 %), amitriptyline (n = 7, 5.9 %), clozapine (n = 7, 5.9 %), and zolpidem >5 mg/day (n = 7, 5.9 %) as shown in Table 2. Hypnotic/anxiolytic drugs were thus by far the most frequently prescribed PIMs (n = 63, 52.9 %), most of them benzodiazepines (n = 45). Other frequently prescribed PIMs were antipsychotic (n = 23, 19.3 %), antidepressant (n = 20, 16.8 %), and antihypertensive drugs (n = 6, 5.0 %). With respect to pharmacological properties of PIMs, GABAA receptors (n = 63, 23.5 %) were the most frequent target structures, followed by alpha 1-adrenoceptors (n = 43, 16.0 %), muscarinic (mAch) receptors (n = 37, 13.8 %), histamine (H) receptors (n = 36, 13.4 %), serotonin (5-HT) receptors (n = 33, 12.3 %), and dopamine (D) receptors (n = 25, 9.3 %).
In inpatients, more than two thirds (n = 51, 68.9 %) of PIMs were hypnotics/anxiolytics (Fig. 1), which consisted of benzodiazepines (n = 35) and the Z-drugs zopiclone and zolpidem (n = 16). Other PIMs were antidepressants (n = 8, 10.8 %) and antipsychotics (n = 7, 9.5 %).
In outpatients, the predominant PIMs (Fig. 1) were antipsychotics (n = 16, 35.6 %), mostly haloperidol >2 mg/day (n = 7, 15.6 %) and clozapine (n = 5, 11.1 %), antidepressants (n = 12, 26.7 %), and hypnotics/anxiolytics (n = 12, 26.6 %; lorazepam >2 mg/day, n = 6, 13.3 %).
PRISCUS PIM intake and related side effects
Information on the occurrence or absence of side effects was available for all patients except two. Side effects were explicitly documented as absent in 60 (35.7 %) and present in 106 patients (63.1 %). For patients without side effects, no (n = 31, 51.7 %), one (n = 22, 36.7 %), or two (n = 7, 11.7 %) PIMs had been prescribed. For 49 patients (29.2 %) with mild side effects, an intake of no (n = 23, 46.9 %), one (n = 17, 34.7 %), and two (n = 9, 18.4 %) PIMs was found. Moderate side effects were observed in 45 patients (26.8 %) and associated with an intake of no (n = 17, 37.8 %), one (n = 19, 42.2 %), two (n = 8, 17.8 %), and three (n = 1, 2.2 %) PIMs. Severe side effects were found in 12 patients (7.1 %). They received no (n = 6, 50.0 %), one (n = 4, 33.3 %), or three (n = 2, 16.7 %) PIMs.
Most side effects reported for the 106 patients were cardiovascular disturbances (n = 32, 30.2 %), agitation (n = 25, 23.6 %), extrapyramidal symptoms (n = 22, 20.8 %), drowsiness/sedation (n = 22, 20.8 %), gastrointestinal disturbances (n = 20, 18.9 %), and cognitive decline (n = 16, 15.1 %).
A statistically significant correlation between the severity of side effects and number of PRISCUS PIMs or between PIM intake and the overall occurrence of side effects was not found in this study (p > 0.05). However, only 6 of 77 patients who took no PRISCUS PIMs but 2 of 3 patients who took 3 PRISCUS PIMs exhibited severe side effects.
Overall, these 12 patients who exhibited severe side effects received by mean 0.83 ± 1.1 PIMs, and the most frequent side effects were falls (n = 5) and cardiovascular symptoms (n = 5), followed by extrapyramidal symptoms (n = 4), agitation (n = 3), cognitive decline (n = 3), drowsiness/sedation (n = 2), hyposalivation (n = 2), and sleep disturbances (n = 2). For single cases, hypersalivation, gastrointestinal disturbances, urogenital disturbances, tremor, sweating, constipation, paresthesia, photosensibility, dysarthria, and diminished appetite were considered as severe. Five of these patients received inappropriate hypnotics/anxiolytics, two had received antiemetics and antidepressants as PIMs, and the remaining six patients with severe side effects took no PIMs.
Discussion
This study analyzed medication and related prescription of PIMs with side effects of elderly psychiatric inpatients and outpatients. As this study was conducted in Germany, the German PRISCUS list, and not the Beers criteria, was used as screening tool for PIM use. The PRISCUS list is based on a selective literature search, a qualitative analysis of international PIM lists, amongst others, the Beers criteria, and a structured survey of expert opinions. The PRISCUS list was considered as useful, since Bauer and colleagues [30] detected that drugs from the PRISCUS list correlated with the incidence of injuries, and Dormann and coworkers [15] detected in older patients admitted to an emergency unit that PRISCUS PIMS were a risk factor for the development of an adverse drug reaction.
Our expectation that elderly patients receive frequently PRISCUS PIMs was confirmed. More than half of the patients had at least one PRISCUS PIM. The absolute number of PIMs was higher in inpatients than in outpatients, the relative number of about 11 % PIMs of prescribed drugs, however, was similar in inpatients and outpatients. In inpatients, most PIMs (69 %) were hypnotic/anxiolytic drugs and antipsychotic drugs in outpatients. Drug-related side effects were reported rather frequently, by mean 63 % for inpatients and outpatients. However, we found no significant association between PIM intake and overall side effects. There was just a trend between severe side effects and the number of PRISCUS PIMs, as only 6 of 77 patients who took no PRISCUS PIMs but 2 from 3 patients who took 3 PRISCUS PIMs exhibited severe side effects.
The prevalence rate of PIM intake of 53.0 % (40.4 % in outpatients and 67.1 % in inpatients) was about twofold higher in our old aged psychiatric patient sample than in other types of patients that had been analyzed from data of health insurances (22.0 %–25.0 % [24, 27]). In a study conducted in a geriatric hospital for rehabilitation [31], 35 % of the patients received a PIM at admission, 43 % during hospitalization, and 29 % at discharge. A high prevalence rate of PRISCUS PIM use in psychiatric patients was also observed by Berger and coworkers [32] who reported that 40 % of old aged patients (≥65 years) with the diagnosis of generalized anxiety disorder received potentially inappropriate agents. For these patients, however, it was not analyzed if PIM intake is associated with poor drug tolerability. Our finding of a relation of drug consumption and PRISCUS PIM use in the hospitalized setting was consistent with the findings of Siebert and coworkers [31] for geriatric inpatients.
In inpatients, most inappropriate medications were hypnotic/anxiolytic drugs. A similar high prevalence of prescription of 33 % of hypnotic drugs was reported by Berger and coworkers [32]. Siebert and colleagues [31] analyzed retrospectively the prevalence and type of PIMs in a clinic for geriatric rehabilitation. The main drug groups with 64 % PIMs at admission, 88 % while hospitalized and 86 % at discharge, were sedatives/hypnotics, antidepressants, and antiarrhythmics. Thus, 17 % received a potentially inadequate hypnotic/anxiolytic drug at admission, 35 % while hospitalized, and 21 % at discharge.
In our patients, the number of PIMs correlated significantly (p < 0.05) with the number of administered drugs per day. This was in accordance with other studies that detected number of drugs as risk factor for PIM intake [26, 24, 25].
No significant gender differences concerning number of prescribed PIMs could be detected. Other studies that investigated claims data from statutory health insurances reported that female patients have a higher risk for receiving a PIM than male patients [24, 27]. Since the mean number of administered drugs was similar in females (6.3 ± 3.9) and males (6.4 ± 3.9) in our patient sample but higher for females in other investigations, it is concluded that the increased risk for receiving PIMs was primarily due to number of drugs and not due to gender-specific differences.
Side effects were reported rather frequently, for 63 % of all patients. Most reported side effects were in accordance with the pharmacological properties of prescribed drugs. Our primary hypothesis, however, that overall side effects may be explained substantially by inappropriate medication could not be confirmed. There was just a trend between the number of PRISCUS PIMs and severe side effects that occurred in 12 patients. For these few patients, the most frequent drug-related severe side effects were falls (n = 5), cardiovascular disturbances (n = 5), and extrapyramidal motor symptoms (n = 4). The overall occurrence of severe side effects was 67 % with three PIMs and only 8 % without PIM prescription. However, one must also be aware that six of the 12 patients with severe side effects had not received any PIM. A number of comorbidities, renal or hepatic insufficiency, or heart failure are further risk factors for adverse drug reactions [15, 33]. Moreover, appropriately prescribed antipsychotic and antidepressant drugs may also give rise to adverse drug reactions [34, 14]. PRISCUS PIMs should thus be regarded as one of multiple risk factors that influence the tolerability of drugs in elderly psychiatric patients. On the other hand, Bauer and colleagues [30] found in routine data of frail elderly persons aged ≥65 years a positive correlation between appropriate medication with antidepressant, anxiolytic, hypnotic, sedative, and antiarrhythmic drugs as well as drugs from the PRISCUS list with falls and injuries. Dormann and coworkers [15] reported that elderly patients who were treated in the emergency department of a hospital and who took PIMs had an increased risk of adverse drug reactions and medication errors. However, most drug-related adverse reactions were due to non-PIMs.
Regarding medications of the PRISCUS list, one should also be aware that PRISCUS drugs are not absolutely contraindicated for elderly psychiatric patients. Prescription of drugs of the PRISCUS list should therefore always include an individual risk-benefit analysis, and it must be critically evaluated if safer alternatives are available which are not listed as PIMs [16].
Limitations
Since included patients were selected from a therapeutic drug monitoring (TDM) database, a selection bias may be assumed. According to the AGNP guidelines for TDM in psychiatry [28], TDM is recommended for elderly patients. Moreover, a number of indications such as suspected noncompliance, lack of clinical improvement, combination treatment with a drug known for its interaction potential, or pharmacokinetic comorbidities are specific indications to use TDM. The participating hospitals are regular users of TDM, especially for their elderly patients. Inpatients and outpatients of our study were therefore regarded as representative for elderly psychiatrically ill patients. This view was supported by the finding that the medication of the selected patient sample was similar to that of other studies on geriatric psychiatric patients, especially the frequent use of benzodiazepines [32, 35, 36].
The interpretation of our results is restricted by a relatively small sample size of only 168 patients. Multivariate analysis and adjustment for number of prescriptions, comorbidities, or other variables were not possible. On the other hand, it should be stressed that this study related prescription of PIMs and drug side effects in elderly psychiatric patients. This approach so far has not been applied by other investigators.
Finally, this study relies on retrospective data which has the limitation that information on side effects may be assumed less reliable than in the case of a prospective study. To overcome the problem of missing data, a trained clinical pharmacist checked the medical records for documented side effects. Under conditions of a prospective observational study, however, it must be considered that a prospective study may affect treatment. When patients and treating physicians are aware that the appropriateness of the medication is surveyed, treating psychiatrists will be cautious and try to avoid inappropriate medication. Therefore, it was suggested that a retrospective study design as ours reflects everyday practice of pharmacotherapy possibly better than a prospective study.
Conclusions
Our study is the first analysis that related prescription of PRISCUS PIMs and reported side effects in elderly psychiatric patients. It was revealed that prevalence of PIMs is considerably high as expected. However, a significant association between PIM intake and the occurrence of side effects was not observed. There was just a trend in a minority of patients that severe side effects might be associated with PRISCUS PIMs. Based on our observations and in accordance with another study that used the STOPP and Beers criteria [37], PIMs of the PRISCUS list can be considered as one of multiple factors that influence the tolerability of drugs in elderly psychiatric patients.
Conflict of interest
Christoph Hiemke has received speaker’s or consultancy fees from Bristol-Myers Squibb, Janssen-Cilag, Pfizer, Eli Lilly, and Servier. He is the Managing Director of psiac GmbH, which provides an Internet-based drug–drug interaction program for psychopharmacotherapy. He and all other authors report no conflicts of interest.
References
Fulton MM, Allen ER (2005) Polypharmacy in the elderly: a literature review. J Am Acad Nurse Pract 17(4):123–132. doi:10.111/j.1041-2972.2005.0020.×
McLean AJ, Le Couteur DG (2004) Aging biology and geriatric clinical pharmacology. Pharmacol Rev 56(2):163–184. doi:10.1124/pr.56.2.4
Turnheim K (2003) When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp Gerontol 38(8):843–853
Aymanns C, Keller F, Maus S, Hartmann B, Czock D (2010) Review on pharmacokinetics and pharmacodynamics and the aging kidney. Clin J Am Soc Nephrol 5(2):314–327. doi:10.2215/cjn.03960609
Goldberg RM, Mabee J, Chan L, Wong S (1996) Drug-drug and drug-disease interactions in the ED: analysis of a high-risk population. Am J Emerg Med 14(5):447–450. doi:10.1016/s0735-6757(96)90147-3
Leon C, Gerretsen P, Uchida H, Suzuki T, Rajji T, Mamo DC (2010) Sensitivity to antipsychotic drugs in older adults. Curr Psychiatry Rep 12(1):28–33. doi:10.1007/s11920-009-0080-3
Trifiro G, Spina E (2011) Age-related changes in pharmacodynamics: focus on drugs acting on central nervous and cardiovascular systems. Curr Drug Metab 12(7):611–620
Ahmed N, Mandel R, Fain MJ (2007) Frailty: an emerging geriatric syndrome. Am J Med 120(9):748–753. doi:10.1016/j.amjmed.2006.10.018
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research G (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56(3):M146–M156
Onder G, Pedone C, Landi F, Cesari M, Della Vedova C, Bernabei R, Gambassi G (2002) Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). J Am Geriatr Soc 50(12):1962–1968
Mallet L, Spinewine A, Huang A (2007) The challenge of managing drug interactions in elderly people. Lancet 370(9582):185–191. doi:10.1016/s0140-6736(07)61092-7
Mittal V, Muralee S, Williamson D, McEnerney N, Thomas J, Cash M, Tampi RR (2011) Review: delirium in the elderly: a comprehensive review. Am J Alzheimers Dis Other Demen 26(2):97–109. doi:10.1177/1533317510397331
Mintzer J, Burns A (2000) Anticholinergic side-effects of drugs in elderly people. J R Soc Med 93(9):457–462
Field TS, Gurwitz JH, Avorn J, McCormick D, Jain S, Eckler M, Benser M, Bates DW (2001) Risk factors for adverse drug events among nursing home residents. Arch Intern Med 161(13):1629–1634
Dormann H, Sonst A, Muller F, Vogler R, Patapovas A, Pfistermeister B, Plank-Kiegele B, Kirchner M, Hartmann N, Burkle T, Maas R (2013) Adverse drug events in older patients admitted as an emergency: the role of potentially inappropriate medication in elderly people (PRISCUS). Dtsch Arztebl Int 110(13):213–219. doi:10.3238/arztebl.2013.0213
Holt S, Schmiedl S, Thurmann PA (2010) Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int 107(31–32):543–551. doi:10.3238/arztebl.2010.0543
Hamilton HJ, Gallagher PF, O’Mahony D (2009) Inappropriate prescribing and adverse drug events in older people. BMC Geriatr 9:5. doi:10.1186/1471-2318-9-5
American Geriatrics Society Beers Criteria Update Expert P (2012) American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 60(4):616–631. doi:10.1111/j.1532-5415.2012.03923.×
Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH (2003) Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 163(22):2716–2724. doi:10.1001/archinte.163.22.2716
Gallagher P, O’Mahony D (2008) STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Ageing 37(6):673–679. doi:10.1093/ageing/afn197
Laroche ML, Charmes JP, Merle L (2007) Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol 63(8):725–731. doi:10.1007/s00228-007-0324-2
Thiem U (2012) Potentially inappropriate medication: the quality of pharmacotherapy in the elderly. Internist Berl 53(9):1125–1130. doi:10.1007/s00108-012-3087-5
Thiem U, Theile G, Junius-Walker U, Holt S, Thurmann P, Hinrichs T, Platen P, Diederichs C, Berger K, Hodek JM, Greiner W, Berkemeyer S, Pientka L, Trampisch HJ (2011) Prerequisites for a new health care model for elderly people with multimorbidity: the PRISCUS research consortium. Z Gerontol Geriatr 44(2):115–120. doi:10.1007/s00391-010-0156-z
Schubert I, Kupper-Nybelen J, Ihle P, Thurmann P (2013) Prescribing potentially inappropriate medication (PIM) in Germany’s elderly as indicated by the PRISCUS list. An analysis based on regional claims data. Pharmacoepidemiol Drug Saf 22(7):719–727. doi:10.1002/pds.3429
Zimmermann T, Kaduszkiewicz H, van den Bussche H, Schon G, Brettschneider C, Konig HH, Wiese B, Bickel H, Mosch E, Luppa M, Riedel-Heller S, Werle J, Weyerer S, Fuchs A, Pentzek M, Hanisch B, Maier W, Scherer M, Jessen F, AgeCoDe-Study G (2013) Potentially inappropriate medication in elderly primary care patients: a retrospective, longitudinal analysis. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 56(7):941–949. doi:10.1007/s00103-013-1767-5
Fiss T, Thyrian JR, Fendrich K, van den Berg N, Hoffmann W (2013) Cognitive impairment in primary ambulatory health care: pharmacotherapy and the use of potentially inappropriate medicine. Int J Geriatr Psychiatry 28(2):173–181. doi:10.1002/gps.3806
Amann U, Schmedt N, Garbe E (2012) Prescribing of potentially inappropriate medications for the elderly: an analysis based on the PRISCUS list. Dtsch Arztebl Int 109(5):69–75. doi:10.3238/arztebl.2012.0069
Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, Fric M, Gerlach M, Greiner C, Grunder G, Haen E, Havemann-Reinecke U, Jaquenoud Sirot E, Kirchherr H, Laux G, Lutz UC, Messer T, Muller MJ, Pfuhlmann B, Rambeck B, Riederer P, Schoppek B, Stingl J, Uhr M, Ulrich S, Waschgler R, Zernig G (2011) AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry 44(6):195–235
Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K (1987) The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 334:1–100
Bauer TK, Lindenbaum K, Stroka MA, Engel S, Linder R, Verheyen F (2012) Fall risk increasing drugs and injuries of the frail elderly—evidence from administrative data. Pharmacoepidemiol Drug Saf 21(12):1321–1327. doi:10.1002/pds.3357
Siebert S, Elkeles B, Hempel G, Kruse J, Smollich M (2013) The PRISCUS list in clinical routine. Practicability and comparison to international PIM lists. Z Gerontol Geriatr 46(1):35–47. doi:10.1007/s00391-012-0324-4
Berger A, Mychaskiw M, Dukes E, Edelsberg J, Oster G (2009) Magnitude of potentially inappropriate prescribing in Germany among older patients with generalized anxiety disorder. BMC Geriatr 9:31. doi:10.1186/1471-2318-9-31
Onder G, Petrovic M, Tangiisuran B, Meinardi MC, Markito-Notenboom WP, Somers A, Rajkumar C, Bernabei R, van der Cammen TJ (2010) Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: the GerontoNet ADR risk score. Arch Intern Med 170(13):1142–1148. doi:10.1001/archinternmed.2010.153
Bates DW, Miller EB, Cullen DJ, Burdick L, Williams L, Laird N, Petersen LA, Small SD, Sweitzer BJ, Vander Vliet M, Leape LL (1999) Patient risk factors for adverse drug events in hospitalized patients. ADE Prevention Study Group. Arch Intern Med 159(21):2553–2560
Dolder CR, McKinsey J (2011) Antipsychotic polypharmacy among patients admitted to a geriatric psychiatry unit. J Psychiatr Pract 17(5):368–374. doi:10.1097/01.pra.0000405368.20538.cd
Lesen E, Petzold M, Andersson K, Carlsten A (2009) To what extent does the indicator “concurrent use of three or more psychotropic drugs” capture use of potentially inappropriate psychotropics among the elderly? Eur J Clin Pharmacol 65(6):635–642. doi:10.1007/s00228-009-0623-×
Hamilton H, Gallagher P, Ryan C, Byrne S, O’Mahony D (2011) Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med 171(11):1013–1019. doi:10.1001/archinternmed.2011.215
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hefner, G., Stieffenhofer, V., Gabriel, S. et al. Side effects related to potentially inappropriate medications in elderly psychiatric patients under everyday pharmacotherapy. Eur J Clin Pharmacol 71, 165–172 (2015). https://doi.org/10.1007/s00228-014-1796-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1796-5