Abstract
Objectives
To develop population pharmacokinetic (PK) models for piperacillin/tazobactam in neonates and infants of less than 2 months of age in order to determine the appropriate dosing regimen and provide a rational basis for the development of preliminary dosing guidelines suitable for this population.
Methods
A two-stage, open-label study was conducted in neonates and infants less than 2 months of age in the neonatal intensive care unit (NICU). A total of 207 piperacillin and 204 tazobactam concentration–time data sets from 71 patients were analyzed using a nonlinear mixed-effect modeling approach (NONMEM VII). PK models were developed for piperacillin and tazobactam. The final models were evaluated using both bootstrap and visual predictive checks. External model evaluations were made in 20 additional patients.
Results
For neonates and young infants less than 2 months of age, the median central clearance was 0.133 and 0.149 L/h/kg for piperacillin and tazobactam, respectively. Postmenstrual age (PMA) was identified as the most significant covariate on central clearance of piperacillin and tazobactam. However, the combination of current bodyweight (BW) and postnatal age proved to be superior to PMA alone. BW was the most important covariate for apparent central volume of distribution. Both internal and external evaluations supported the prediction of the final piperacillin and tazobactam PK models. The dosing strategy 44.44/5.56 mg/kg/dose piperacillin/tazobactam every 8 or 12 h evaluated in this study achieved the pharmacodynamic target (free piperacillin concentrations >4 mg/L for more than 50 % of the dosing interval) in about 67 % of infants.
Conclusions
Population PK models accurately described the PK profiles of piperacillin/tazobactam in infants less than 2 months of age. The results indicated that higher doses or more frequent dosing regimens may be required for controlling infection in this population in NICU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Piperacillin/tazobactam, an injectable antibacterial combination consisting of the semisynthetic piperacillin sodium and the β-lactamase inhibitor tazobactam sodium, has been indicated for the treatment of moderate to severe infections, including Pseudomonas aeruginosa infections [1], complicated urinary tract infections [2], complicated skin and soft tissue infections [3], complicated intra-abdominal infection [4], and severe sepsis and septic shock [5]. Piperacillin/tazobactam has been approved for the treatment of pediatric patients (age 2 months to 17 years) in the USA [6]. Tornøe et al. conducted a pharmacometric analysis to evaluate appropriate dosing regimens for pediatric patients as young as 2 months [7], but to date there are no data on which to base additional recommendations for pediatric patients younger than 2 months. In addition, there is no published description of piperacillin/tazobactam pharmacokinetics (PK) in neonates and young infants less than 2 months of age using population-based techniques. Previous data suggest that the protein-binding capacities of piperacillin and tazobactam are approximately 20 to 30 % and 20 to 23 %, respectively. Both piperacillin and tazobactam are eliminated via the kidney by glomerular filtration and tubular secretion [8]. The antibacterial activity of piperacillin is related to the time that the free drug concentration exceeds the minimal inhibitory concentration (MIC) of the infecting pathogen (f%T >MIC%, where f is the the free drug fraction and T is time) during a dosing interval. Data on the T >MIC% required for optimal activity for β-lactams has been obtained from dynamic in vitro and in vivo animal models and suggest that an f%T >MIC% of approximately 50 % of the dosing interval for Gram-negative bacteria is necessary [9–11]. Piperacillin MIC50 values of extended-spectrum beta-lactamase (ESBL) Escherichia coli and Pseudomonas aeruginosa in the Asia-Pacific region are 4 and 8 mg/L, respectively [12]; the MIC50 value of ESBL-producing Klebsiella pneumoniae is 32 mg/L global [13].
Children differ significantly from adults in the way they dispose of and respond to drugs. Pharmacodynamic and PK parameters also vary as children grow from neonates through to adolescence. The importance of medication efficacy and safety in neonates and young infants has been gaining increasing attention in the past decades with the realization that neonates and young infants are not “little adults.” Postnatal anatomical, physiological, and psychological developments make it hard to these patients by just scaling adult doses on the basis of their age, weight, or body surface area [14]. Atypical or indirect responses may occur in newborns [15], and premature birth can complicate treatment even more. A knowledge of PK and PD parameters in neonates and young infants is significantly important to maximize the clinical utility and reduce antimicrobial resistance.
We therefore designed and conducted a two-stage, open-label clinical trial in the neonatal intensive care unit (NICU) with the objective of developing population PK models for piperacillin/tazobactam to provide the appropriate dosing regimen and a rational basis for the development of preliminary dosing guidelines suitable for use in neonates and infants younger than 2 months.
Methods
Study design
At stage 1, a total of 71 patients receiving antimicrobial therapy for suspected or confirmed bacterial infection were enrolled in the study, including 46 newborns and 25 young infants. Newborns were stratified into 4-week periods based on gestational age at birth (GA) between 24 and 43 weeks, and the target recruitment was approximately 8–12 patients per 4-week period. Each patient was enrolled to receive piperacillin/tazobactam (Bangda®; Qilu Pharmaceutical, Jinan, China; each glass vial contains 1.0 g of piperacillin sodium and 0.125 g of tazobactam sodium) 44.44/5.56 mg/kg/dose, every 12 h. For neonates and infants with severe infection, the dosing interval could be decreased to 8 h based on the decision of the attending physician. A small number of samples (n = 3) were drawn for each patient on day 1 (first dose) or the day when steady state concentrations were reached. A population PK model was established to investigate piperacillin/tazobactam PK parameters and their variability estimates in this population.
At stage 2, according to the preliminary population PK model, a simulation was conducted to determine the optimal dosing regimen in this population with similar characteristics. The simulated optimal dosing regimen was evaluated in an additional 20 neonates and young infants.
Patients and data collection
Data were collected from neonates and young infants in the NICU at Children’s Hospital of Fudan University. Inclusion criteria included an age of <61 days, sufficient intravascular access (either peripheral or central) to receive the study drug, suspected or confirmed bacterial infection that necessitated treatment with piperacillin/tazobactam as part of the standard of care following the decision of the attending physician. Participants were excluded for the following conditions: (1) so severely ill that they were not likely to survive the duration of the trial (at least 8 days), (2) history of allergic reactions to any penicillin, cephalosporin, or beta-lactamase inhibitor, (3) hepatic dysfunction, as evidenced by aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels of ≥90th percentile for corrected postmenstrual age (PMA) [16], (4) renal dysfunction, as evidenced by a serum creatinine (Scr) level of ≥90th percentile for infants with PMA of <28 weeks [17] and ≥28 weeks [mean + 3 standard deviations (SD)] for other gestational ages [18, 19], (5) having received a systemic investigational drug or (6) having any condition which in the opinion of the investigator made the subject unsuitable for enrollment. The study protocol was approved by the Ethics Committee of Children's Hospital of Fudan University. All study participants were enrolled after obtaining written permission (informed consent) from each child’s parents or legal guardians.
The following demographic factors were collected for all patients on enrollment: gender (SEX), gestational age (GA), postnatal age (PNA),PMA, birth body weight (BBW), body weight (BW), height (HT), body surface area [BSA: BW(kg)0.5378 × HT(m)0.3964 × 0.024265)] [20], serum creatinine (Scr), creatinine clearance rate [CLcr: Schwartz CLcr (mL/min/1.73 m2) = k × HT (cm)/Scr (mg/dL), where k = 0.45 for term infants throughout the first year of life] [21], and concomitant drug therapy.
Patients who met inclusion/exclusion criteria were assigned to receive piperacillin/tazobactam. The drug was reconstituted with 0.9 % sodium chloride for injection and was further diluted to a total volume of 2 ml. The study drug was infused intravenously over 5 min by a nurse by using a calibrated syringe. Immediately following infusion of the study drug, the intravenous infusion tubing was flushed with 2 ml of 0.9 % sodium chloride to ensure administration of the total dose. Patients were divided into four groups according to the dosing scheme, and sampling times are shown in Table 1. Blood (0.5 ml) was collected in disposable syringes and was transferred immediately into heparinized tubes. After gentle infusion, blood samples were centrifuged, separated, and frozen at −70°C until the time of analysis.
HPLC/MS/MS analysis
Piperacillin and tazobactam concentrations were simultaneously determined by a liquid chromatography/tandem mass spectrometry (LC/MS/MS) system consisting of an API 3000 mass spectrometer (Applied Biosystems, Foster City, CA) equipped with a turbo ion spray source in the electro-spray positive ion mode (ESI+) and a chromatographic Shimadzu 20A system (Shimadzu, Kyoto, Japan) consisting of two LC-20 AD pumps, a vacuum degasser, a SIL-HTC autosampler, and a controller module. The concentration ranges of standard curves were approximately 0.1–10 mg/L for piperacillin and 0.03–3 mg/L for tazobactam. There were duplicates of four quality controls, including for the LLOQ (lower limit of quantification) for each standard curve to ensure assay accuracy and precision. The intra- and inter-day accuracy of piperacillin was approximately 95.8–103.7 % and 94.9–104.4 %, respectively. The intra- and inter-day accuracy of tazobactam was approximately 93.7–97.5 % and 88.9–99.8 %, respectively. The coefficient variations of the intra- and inter-assays for total piperacilln were less than 10 and 7.3 %, respectively; for tazobactam, these were less than 5.3 and 5 %, respectively. The concentrations of the unknown samples above the qualification limits were prediluted with tested drug-free human serum [22].
PK analysis
Data analysis was performed with the NONMEM program (ver. VII; Icon Development Solutions, Ellicott City, MD) in conjunction with Wings for NONMEM. The first order conditional estimation (FOCE) method with the interaction option was used to estimate PK parameters and their variability. One- and two-compartment models were employed as the PK base model, respectively. The one-compartment PK parameters were clearance (CL) and apparent volume of distribution (V). The two-compartment PK parameters were central clearance (CL), apparent central volume of distribution (V1), inter-compartmental clearance (Q), and apparent peripheral volume of distribution (V2). The initial parameters were obtained by the $ESTIMATION of NONMEM according to the literature.
The demographic factors obtained for PK parameters were used to perform an initial selection of covariates. The selection was carried out by plotting the parameter estimates against demographic factors and then retaining those with statistical significance (P <0.05) as initial covariates. In the case of continuous covariates (GA, PNA, PMA, BBW, BW, HT, BSA, Scr, CLcr), the statistical significance of the PK parameters was evaluated by means of the correlation coefficient, whereas in the case of categorical covariates (SEX, concomitant drug therapy), Student’s t-test was applied. Continuous covariates were separately implemented in the model, using an allometric equation:
where P i represents the individual parameter estimate of the ith subject, P p represents the population parameter estimate, Cov is the covariate, and k is the exponent. If weight was included in the PK base model, parameter values were standardized for a body weight of 70 kg using an allometric model:
where P i represents the individual parameter estimate of the ith subject, P std represents the individual parameter estimate with a body weight of 70 kg, and W i represents the body weight of the ith subject. Allometric scaling has a strong theoretical and empirical basis with a PWR exponent of 0.75 for clearance parameters (CL and Q) and 1 for volume parameters (V1 and V2). Categorical covariates were included using a category variable equation. The selection of covariates was determined using a forward selection process and a backward elimination process. During forward selection, any covariate that reduced the objective function value by >3.84 (P <0.05, χ2 distribution with 1 df) was considered to be significant and added to the model. A full model was constructed with all statistically significant covariates included. The importance of each covariate was then re-evaluated by backward elimination. Each covariate was independently removed from the model one at a time to identify its relevance. An increase in the objective-function value (OFV) of >10.83 (P <0.001, χ2 distribution) was required for confirmation. When two or more covariates were found to significantly improve the model, the covariate causing the largest reduction in the OFV was left in the model. The resulting model was called the final model and included all significant covariates that cannot be eliminated from the full model.
The residual variability was evaluated with additive, proportional, and combined residual error models, respectively.
Model evaluation
The goodness of fit was evaluated by using several diagnostic scatter plots as follows: (1) observed versus population predicted concentrations (DV vs. PRED); (2) observed versus individual predicted concentrations (DV vs. IPRED); (3) conditional weighted residuals versus time (CWRES vs. TIME); (4) conditional weighted residuals versus population predicted concentrations (CWRES vs. PRED).
The stability of the final model was assessed by means of an internal validation method involving a non-parametric bootstrap. Re-sampling of the dataset was repeated 1,000 times using NONMEM in the final model. The values of estimated parameters, such as the median and standard error (SE) from the bootstrap procedure were compared with those estimated from the original dataset. It could be proved that the model was stable if the values of parameters were not significantly different. The 95 % confidence intervals (CIs) were obtained as the point estimate ± 1.96 × SE of the estimate.
A visual predictive check (VPC) was performed to evaluate the predictive performance of the model. One thousand datasets were simulated based on the final model. The observed concentration versus time data was graphically overlaid with the median values along with the 5th and 95th percentiles from the simulated data profiles. The model was deemed precise if the observed concentration data were appropriately approximately distributed within the 5th to 95th prediction interval.
To perform an external evaluation for the final piperacillin/tazobactam two-compartment model, we enrolled an additional 20 neonates and infants in the study. Each patient was simulated 1,000 times according to the final piperacillin model to determine the optimal dosing regimen by NONMEM. The population-predicted piperacillin/tazobactam concentrations were compared to the observed piperacillin/tazobactam concentrations, and the predictive performance of the final models was evaluated by means of precision and bias. The median prediction error (MDPE), median absolute prediction error (MDAPE), and BIAS were used as measures of precision and bias. These are calculated by using the following equations:
where Obsi represents the observed concentration of the ith subject, and Predi represents the population-predicted concentration of the ith subject. MDPE and MDAPE are expressed in the results as a percentage by multiplying by 100.
Assessment of PD target
The final model was used to simulate concentrations to evaluate whether the optimal piperacillin (the determinate component of this combination) f%T >MIC% had been obtained when the original dosing regimen was applied. One hundred serum piperacillin concentration datasets were generated after half of the dosing interval on day 1 and one-half on the day when steady state concentrations were reached for virtual neonates and young infants with characteristics similar to those observed for the study patients. A piperacillin MIC50 value of ESBL E. coli in the Asia-Pacific region of 4 mg/L [10] was chosen as a representative value. The level of unbound piperacillin was set at 70 % [6].
To explore the appropriate dosing regimen, we performed additional simulations using NONMEM based on 1,000 patients to evaluate 10, 20, 40, 60, 80, and 100 mg/kg doses administered every 6, 8, or 12 h in neonates and young infants with a BW of approximately 0.5–5 kg and a PNA of approximately 1–60 days. As the safety of piperacillin in the treatment of infections of neonates and young infants of less than 2 months of age was unknown, 100 mg/kg piperacillin was restricted as the maximum dosage in our trial. For purposes of the simulations, the dosing regimens of a PD target of 50 % f%T >MIC was established in >95 % of neonates and young infants younger than 2 months.
Results
Data analysis
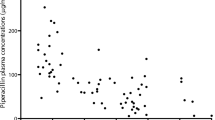
The characteristics of the 71 patients enrolled in the study are presented in Table 2. The final PK database consisted of 207 piperacillin concentrations and 204 tazobactam concentrations. Individual piperacillin and tazobactam concentration plots (concentration versus time) are shown in Fig. 1.
Population PK modeling
Preliminary analysis for the base model showed that the OFV of one- and two-compartment models were 1316.620 and 1282.748 for piperacillin, and 452.264 and 412.968 for tazobactam, respectively. Thus, a two-compartment model resulted in a better fit to describe piperacillin and tazobactam concentrations. Residual variability was best described by a proportional model.
During the procedure for determining the covariates for the model, each covariate that may affect the inter-individual variation was analyzed. As many weight-related covariates were highly correlated in this population, weight was a priori determined to be descriptor included into the model before confirming other weight-related covariates. The inclusion of BW, PNA to CL of piperacillin/tazobactam, and BW to V1 of piperacillin/tazobactam produced the most significant decrease in the OFV and inter-individual variances of the PK parameters. For piperacillin, the OFV was 1116.897, and the between-subject variance (BSVS) for CL and V1, and ηCL and ηV1 decreased from 0.83 to 0.34 and from 0.41 to 0.21, respectively, with the inclusion of BW in the base model. For tazobactam, the OFV was 234.835, and ηCL and ηV1 decreased from 0.85 to 0.35 and from 0.34 to 0.20, respectively. Given the strong physiological basis, allometric scaling was explored as potential covariates for the model. However, no improvement was observed in model fit when exponents for weight were fixed at 0.75 and 1 for clearance parameters (CL and Q, respectively) and at 1 for volume parameters (V1 and V2, respectively). The OFV were 1201.593 and 312.297 for piperacillin and tazobactam, respectively. For piperacillin, ηCL and ηV1 decreased from 0.83 to 0.63 and from 0.41 to 0.33, respectively; for tazobactam, ηCL and ηV1 decreased from 0.85 to 0.67, and from 0.34 to 0.21, respectively. Therefore, we insisted on the previous covariates in the model. Further investigation of PNA on CL showed a similar decrease of CL as a function of PNA; the .ηCL of piperacillin and tazobactam decreased from 0.34 to 0.16, and from 0.35 to 0.16, respectively.
The final population PK models for the disposition of both piperacillin and tazobactam were therefore the base model that included BW and PNA as covariates. The OFV of the final models were 1048.767 and 144.293 for piperacillin and tazobactam, respectively. The final population PK parameter estimates for piperacillin and tazobactam are given in Tables 3 and 4.
Model evaluation
Diagnostic plots for the final models of piperacillin and tazobactam showed a good model fit (Fig. 2). The results of 1,000 bootstrap replicates for piperacillin/tazobactam are summarized in Tables 3 and 4. The number of runs successfully converged was 993 for piperacillin and 994 for tazobactam. The median parameter estimates from the bootstrap procedure were very close to the values of the final population model. In addition, the parameters from the bootstrap procedure followed a normal distribution and contained all of the parameter estimates from the final population model. The results indicate that the estimates for the population PK parameters in the final model were precise and that the model was stable. The VPC of piperacillin/tazobactam is given in Fig. 3. During the procedure, 1,000 datasets on day 1 were simulated to assess the predictive performance of the model. This visual internal validation of the model showed that approximately 90 % of data fit well within the 5th to 95th percentiles of simulation (Exact Binomial Test,1.68 % out of limits observed, 95 % CI 0.204–5.94) and were symmetrically distributed (Pearson’s chi-square test, χ2 = 9.67, df = 3, Pvalue = 0.02) for piperacillin. For tazobactam, 90 % of data fit well within approximately the 5th to 95th percentiles of simulation (Exact Binomial Test, 2.5 % out of limits observed, 95 % CI 0.527–7.25) and were symmetrically distributed (Pearson’s chi-square test, χ2 = 11.84, df = 3, P value = 0.008).
Diagnostic scatter plots for piperacillin (left)/tazobactam (right) final model. a Population-predicted versus the observed concentration. b Individual-predicted versus the observed concentration. c Conditional weighted residual versus time. d Conditional weighted residual versus the predicted concentration
The optimal dosing regimen was determined by simulations according to the final piperacillin model and infections caused by pathogens for each of the additional 20 neonates and infants less than 2 months of age. In the simulation, the most significant covariates of individual BW and PNA were used to calculate the dosing recommendation. The external evaluation suggested that the final models accurately characterized the PK profiles of piperacillin and tazobactam in the population. Plots of population-predicted piperacillin/tazobactam concentrations versus observed concentrations are shown in Fig. 4. The validation of the final model is given in Table 5. As can be seen, MDPE, MDAPE, and BIAS were low for both piperacillin and tazobactam, indicating a satisfactory prediction error and good precision.
Assessment of PD target
The dosing strategy evaluated in this study achieved the PD target (free piperacillin concentrations of >4 mg/L for more than 50 % of the dosing interval) in about 67 % of infants. Furthermore, a more frequent dosing regimen may be more successful in patients administered the same dose, i.e., about 76 % of these infants achieved the target concentration if they received piperacillin/tazobactam every 8 h, while only 63 % of them achieved the target concentration when the dosing interval was increased to 12 h. Piperacillin dosage simulations against ESBL E. coli in the Asia-Pacific region were performed for this population (BW range approx. 0.5– 5 kg; PNA range approx. 1–40 days). The results of simulations for developing the appropriate dosing regimen are illustrated in Table 6.
Discussion
In a previous PK study conducted in patients aged 2 months to 12 years receiving a single dose of 50/6.25 mg/kg or 100/12.5 mg/kg of piperacillin/tazobactam, the estimated non-compartmental PK parameters (mean ± SD) in young infants approximately 2–5 months were a mean clearance of 0.198 ± 0.048 L/h/kg for piperacillin and 0.198 ± 0.042 L/h/kg for tazobactam [23]. Tornøe et al. developed equations to calculate dosing recommendations for paediatric patients as young as 2 months of age [7]. In our study, piperacillin and tazobactam were well described by two-compartment models. For neonates and young infants (PNA <2 months), the median clearance was 0.133 L/h/kg for piperacillin and 0.149 L/h/kg for tazobactam. The central clearance values for piperacillin and tazobactam decreased to approximately two thirds and three quarters of the respectively values in patients less than 2 months of age compared to young infants approximately 2–5 months of age. Although the precise mechanism underlying the decrease in piperacillin and tazobactam clearance has not been clarified, it might be explained by the immature development of renal function in the younger study group.
Tornøe et al. investigated the influence of demographic covariates of body weight and age on CL in patients aged 2 months to 12 years by non-linear regression [7]. In our study, additional demographic covariates that may influence the PK behavior of the drug were investigated by NONMEM. To our knowledge, no previous studies have assessed the influence of patient covariates on the PK profiles of piperacillin/tazobactam in neonates and infants younger than than 2 months, and no covariate models have been proposed for these patient groups and drugs. During the selection of covariates, most of the demographic covariates showed a statistically significant effect on the CL of piperacillin/tazobactam when they were individually tested, and the greatest decrease in the OFV was obtained with PMA. This result was in accordance with the initial stratification based on the GA; however, with the incorporation of other covariates, piperacillin/tazobactam clearance was best described by a combination of BW and PNA. This may be partly explained by the fact that size parameters, such as BW, were frequently highly correlated with other development- or maturation-related parameters, such as PMA in pediatric PK data sets. Furthermore, allometric size adjustments using the fixed allometric coefficient of 0.75 for clearance terms have repeatedly been used in pediatric PK analyses and have especially been reported in more recent publications [24, 25]. However, careful consideration had to be given to the fact that allometric scaling may not hold for all of the studied populations, and concerns have been raised recently about the values used for allometric coefficients [26]. Hu et al. suggested an exponent of 0.75 for the clearance of drugs that were eliminated mainly by metabolism or by metabolism and excretion combined [27]. In our study, after the identification of the appropriate base model, BW with an allometric exponent of 0.75 for CL or Q and an exponent of 1 for V1 or V2 were used as covariate models, but no greater decrease in the objective function was observed. The disposition characteristics defined for tazobactam were very similar to those for the behavior of piperacillin, indicating a compatible match for the combination.
In terms of concomitant therapy, it has been reported that the administration of probenecid prolongs the half-life of piperacillin by 21 % and that of tazobactam by 71 % [6]. In our study, the majority of patients were treated with a single antibiotic (the study drug piperacillin/tazobactam). Furthermore, during the selection of covariates, no other drug was retained as a significant covariate in the final model.
Integrating the concept of time above the MIC with the piperacillin/tazobactam population PK model generated in our study provided a rational basis for the development of preliminary dosing guidelines to neonates and infants less than 2 months of age. The results indicate that a dose of 44.44/5.56 mg/kg every 8 or 12 h may be not enough for controlling infection in neonates and infants less than 2 months of age in the NICU. They also revealed that higher doses or more frequent regimens may be required for this population and are likely to achieve the target concentration.
Simulations using NONMEM for several subgroups were performed in order to obtain the doses required to achieve the presumed most favorable outcome related to f%T >MIC% greater than 50 %. In a typical infant (BW 1 kg and PNA 3 days), the administration of 10 mg/kg every 8 h as the initial piperacillin dose afforded a 95 % probability of achieving an antibiotic exposure against ESBL E. coli in the Asia-Pacific region. In order to attain the same result, the piperacillin dose needed to be increased to 100 mg/kg given every 6 h in an infant with a BW of 4.5 kg BW and a PNA of 7 days. This simulated evaluation highlighted how dosage individualization can affect clinical outcomes in this critical population and might explain some clinical failures.
In conclusion, two-compartment PK models were developed for piperacillin/tazobactam in children less than 2 months of age. BW and PNA were identified as significant covariates influencing the PK of piperacillin/tazobactam. The final models were evaluated using bootstrap and a visual predictive check. The final models were also evaluated on an additional 20 neonates and infants enrolled at stage 2 and showed accurate predictive performances. Our results may be used to recommend the initial dosage of piperacillin/tazobactam in hospitals in patient populations with similar characteristics.
References
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD et al (2007) Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(Suppl 2):S27–S72
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P et al (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of America. Clin Infect Dis 49:1–45
Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P et al (2005) Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 41:1373–1406
Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ et al (2010) Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the surgical infection society and the infectious diseases society of America. Surg Infect (Larchmt) 11:79–109
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM et al (2008) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Federal Drug Administration (2007) Zosyn® (Piperacillin and Tazobactam for Injection). Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050684s051s052lbl.pdf. Accessed Feb 2007
Tornøe CW, Tworzyanski JJ, Imoisili MA, Alexander JJ, Korth-Bradley JM et al (2007) Optimising piperacillin/tazobactam dosing in paediatrics. Int J Antimicrob Agents 30:320–324
Sörgel F, Kinzig M (1993) The chemistry, pharmacokinetics and tissue distribution of piperacillin/tazobactam. J Antimicrob Chemother 31[Suppl A]:39–60
Craig WA (1998) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10
Lodise TP, Lomaestro BM, Drusano GL; Society of Infectious Diseases Pharmacists (2006) Application of antimicrobial pharmacodynamic concepts into clinical practice: focus on beta-lactam antibiotics: insights from the society of infectious diseases pharmacists. Pharmacotherapy 26:1320–1332
Frei CR, Wiederhold NP, Burgess DS (2008) Antimicrobial breakpoints for gram-negative aerobic bacteria based on pharmacokinetic-pharmacodynamic models with Monte Carlo simulation. J Antimicrob Chemother 61:621–628
Farrell DJ, Turnidge JD, Bell J, Sader HS, Jones RN (2010) The in vitro evaluation of tigecycline tested against pathogens isolated in eight countries in the Asia-Western Pacific region (2008). J Infect 60:440–451
Garrison MW, Mutters R, Dowzicky MJ (2009) In vitro activity of tigecycline and comparator agents against a global collection of Gram-negative and Gram-positive organisms: tigecycline evaluation and surveillance trial 2004 to 2007. Diagn Microbiol Infect Dis 65:288–299
Bartelink IH, Rademaker CM, Schobben AF, van den Anker JN (2006) Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet 45:1077–1097
McIntyre J, Choonara I (2004) Drug toxicity in the neonate. Biol Neonate 86:218–221
Victor S, Helen D, Mark T (2011) Plasma aminotransferase concentrations in preterm infants. Arch Dis Child Fetal Neonatal Ed 96:F144–F145
Thayyil S, Sheik S, Kempley ST, Sinha A (2008) A gestation- and postnatal age-based reference chart for assessing renal function in extremely premature infants. J Perinatol 28:226–229
van den Anker JN, de Groot R, Broerse HM, Sauer PJ, van der Heijden BJ et al (1995) Assessment of glomerular filtration rate in preterm infants by serum creatinine: comparison with inulin clearance. Pediatrics 96:1156–1158
Chantler C (1973) The measurement of renal function in children: a review. Guy's Hosp Rep 122:25–41
Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93:62–66
Schwartz GJ, Feld LG, Langford DJ (1984) A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr 104:849–854
Li Z, Li Q, Wang Y, Cao D, Chen C (2012) Determination of free and total Piperacillin-Tazobactam in Plasma by HPLC–MS–MS: an adapted method for neonates. Chromatographia 75:533–539
Reed MD, Goldfarb J, Yamashita TS, Lemon E, Blumer JL (1994) Single-dose pharmacokinetics of piperacillin and tazobactam in infants and children. Antimicrob Agents Chemother 38:2817–2826
Rajagopalan P, Gastonguay MR (2003) Population pharmacokinetics of ciprofloxacin in pediatric patients. J Clin Pharmacol 43:698–710
Zuppa AF, Nicolson SC, Barrett JS, Gastonguay MR (2011) Population pharmacokinetics of pentobarbital in neonates, infants, and children after open heart surgery. J Pediatr 159:414–419
Agutter PS, Wheatley DN (2004) Metabolic scaling: consensus or controversy? Theor Biol Med Model 1:13
Hu TM, Hayton WL (2001) Allometric scaling of xenobiotic clearance: uncertainty versus universality. AAPS PharmSci 3:E29
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Chen, Y., Li, Q. et al. Population pharmacokinetics of piperacillin/tazobactam in neonates and young infants. Eur J Clin Pharmacol 69, 1223–1233 (2013). https://doi.org/10.1007/s00228-012-1413-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1413-4