Abstract
Purpose
Cisplatin during hyperthermic intraperitoneal chemotherapy (HIPEC) has not previously been measured with a selective technique. The primary aims were to examine the pharmacokinetics of active cisplatin and its monohydrated complex (MHC) during HIPEC using a specific measuring technique, to compare cisplatin’s systemic absorption with oxaliplatin, and to compare active cisplatin levels to that of total platinum.
Methods
Ten patients treated with cytoreductive surgery and HIPEC (cisplatin 50 mg/m2,doxorubicin 15 mg/m2) were recruited. Blood and perfusate samples were drawn during and after HIPEC. Cisplatin analysis was conducted using liquid chromatography (LC) with post-column derivatization with diethyldithiocarbamate and compared with inductively coupled plasma-mass spectrometry (ICP-MS).
Results
The mean half-life (t1/2) of perfusate cisplatin was 18.4 min, with area under the time-concentration curve (AUC) 0–90 min of 2.87 mM·min and estimated 0–60 min of 2.45 mM·min. The absorption t1/2 was 9.0 min for cisplatin and 18.2 min for oxaliplatin. The ratio of total platinum to active cisplatin increased in a linear manner by time of perfusion.
Conclusions
Cisplatin is absorbed quicker than oxaliplatin. Lowering the perfusion time to 60 min does not significantly change the pharmacokinetics of cisplatin, and is therefore to be considered. As the HIPEC perfusion progresses, the ICP-MS technique does not adequately reflect active cisplatin levels in the perfusate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperthermic intraperitoneal chemotherapy (HIPEC) treatment is a growing field. It is being used intra-operatively during cytoreductive surgery (CRS). cis-Diamminedichloroplatinum, also known as cisplatinum or cisplatin, is a commonly used drug in this setting, particularly for ovarian and gastric tumors [1, 2]. The drug’s target molecule is DNA producing intra-strand and inter-strand adducts. The main intracellular form of the drug thought to react in this way is the monohydrated complex (MHC) [3–5].
In 2002, Elias et al. conducted a study using oxaliplatin instead of cisplatin in HIPEC for colorectal peritoneal carcinomatosis (PCs) [6]. They chose a 30 min perfusion time, using the maximum intraperitoneal concentration and the concentration in tumor nodules as the end point of the chemo-perfusion. The area under the time-concentration curve (AUC) of oxaliplatin in the perfusate was not an endpoint in the study. This is in contrast to earlier cisplatin studies with 90 min perfusion, where comparisons have been made primarily using the perfusate AUC as a measure of efficacy. As oxaliplatin is a derivative of cisplatin, a third generation platinum compound, the question arises whether the same rationale used in oxaliplatin can be used in cisplatin. One justification given for the two different perfusion times between the platinum compounds is that oxaliplatin has been proposed to have a quicker systemic uptake, which would warrant a shorter perfusion time using a higher concentration [7].
The terminal half-life (t1/2) in the perfusate of oxaliplatin is around 30 min, which has been demonstrated both by a general measuring technique, flameless atomic absorption spectrophotometry (AAS) or inductively coupled plasma mass spectrometry (ICP-MS); and a specific technique, liquid chromatography with post-column derivatization using diethyldithiocarbamate as the reagent [6, 8]. Using the AAS or ICP-MS technique, which will codetermine low-molecular-weight complexes of platinum with endogenous compounds, cisplatin has a terminal t1/2 in the perfusate of between 25.8 and 99.6 min [9–12]. No studies have investigated the pharmacokinetic profile of cisplatin using a specific measuring technique, and considering the great range in t1/2, there is a need of determining the pharmacokinetic profile using a specific measuring technique (measuring only active cisplatin). Moreover, this may have important implications for the future of cisplatin in HIPEC, if the perfusion time can be decreased.
The monohydrated form of cisplatin (MHC), which has been implicated in the nephrotoxicity [13] of cisplatin in intravenous administration [14], has never previously been measured during HIPEC. As such, the primary aim of this study was to examine the pharmacokinetics of active cisplatin and MHC during HIPEC using a specific measuring technique, and to compare the systemic absorption of cisplatin with that of oxaliplatin. The secondary aim was to compare the results of the specific technique (liquid chromatography with post-column derivatization using diethyldithiocarbamate as the reagent) with measurements of total platinum using ICP-MS.
Methods and patients
Patients
A recent study on sample size has shown that a reasonable estimate on pharmacokinetics can be achieved at a minimal sample size of five [15]. Thus, a sample size of ten was chosen, in order to compensate for possible technical difficulties. Ten consecutive patients treated at Uppsala University Hospital for peritoneal carcinomatosis (PC) where the drug of choice was cisplatin were invited into the study. The eligibility requirements for treatment were the following: histologically confirmed diagnosis of PC; no distant metastases; adequate renal, hematopoietic and liver functions; and World Health Organization (WHO) performance status less than or equal to 2. Exclusion citeria were the following: pregnancy, disease preventing chemotherapy administration (such as immunological deficiencies), other cancer disease still under follow-up. There were six patients with pseudomyxoma peritonei (PMP), two with mucinous colorectal tumors, one with ovarian cancer, and one with small bowel adenocarcinoma. The following clinical data was collected: age, gender, body surface area (BSA), body mass index (BMI), tumor histopathology, cisplatin dose given, volume of perfusate, erythrocyte fraction volume preoperatively and postoperatively, postoperative plasma albumin, and surgical parameters (such as blood loss and operating time). The regional ethics committee approved the study and informed consent was obtained from each patient.
Surgery and HIPEC
The patients were operated on with peritonectomy and visceral resections, as described by Sugerbaker [16]. The extent of tumor load and result of the surgical procedures were recorded at surgery as peritoneal cancer index (PCI) and completeness of cytoreduction score (CC) respectively. The PCI lesion score divides the abdomen into 13 sections according to size: 0 = no visible tumor, 1 = tumor up to 0.5 cm, 2 = tumor up to 5 cm, and 3 = tumor >5 cm. Maximum PCI is 39 (13 × 3) with lesions > 5 cm in all 13 sections. The CC score is based upon the remaining tumor nodules after cytoreduction: 0 = no peritoneal seeding visible, 1 = nodules up to 2.5 mm, 2 = nodules up to 2.5 cm, and 3 = nodules > 2.5 cm.
After cytoreduction, the patients received HIPEC with cisplatin and doxorubicin. It was administered using the coliseum technique, as described earlier [17]. Briefly, a Tenchoff inflow catheter was centrally placed in the abdomen and four outflow catheters were inserted through separate stab incisions through the abdominal wall. Both the inflow and outflow catheters were connected to a perfusion pump and a heat exchanger. The skin of the abdomen was attached to a retractor ring and covered with a plastic film. Prior to the start of the treatment, the patient’s core temperature was reduced to 35 °C with a cooling blanket. A dose of 50 mg/m2 cisplatin and 15 mg/m2 doxorubicin was injected into the circulating perfusate (dianeal peritoneal dialysis fluid) that was kept at a temperature of 41.1–43 °C. This treatment was given during 90 min. Afterward, the abdomen was rinsed and closed up. Four intra abdominal drains were left in place after surgery in all the patients as part of the postoperative care.
Sampling and pharmacokinetic analysis
Perfusate and arterial blood samples were drawn immediately before start of HIPEC (time 0) and then at seven different intervals—2, 5, 10, 15, 30, 60, and 90 min during the HIPEC perfusion. Additional samples (arterial blood only) were drawn at 1, 15, 45, 75, and 105 min after completing the HIPEC perfusion.
The samples were collected in pre-chilled vacutainer tubes and stored on ice. The blood and a portion of the perfusate samples were ultrafiltrated centripetally at 4 °C (4000×g, 20 min) within 30 min. After centrifugation, the resulting filtrates were promptly put on dry ice, stored at −80 °C, and analyzed within 3 weeks. This is in accordance with the known stability of cisplatin and MHC [18]. The analysis was done by liquid chromatography using a post-column derivatization technique, as described earlier, to determine the concentrations of cisplatin[19]. MHC was analyzed using the same conditions, but with a pH 8.2 HEPES buffer, and this method has been previously shown to clearly distinguish between mono and dihydrated forms of platinum [20, 21]. While these methods have been internally validated; as of yet, they lack a full Food and Drug Administration (FDA) standardised bioanalytical method validation. The area under the concentration time curve (AUC), peak concentration, and terminal t1/2 for cisplatin in blood ultrafiltrate (UF) were calculated using the WIN NONLIN 1.5 SCI software in a compartmental model. The AUC for MHC in both perfusate and blood was determined using the trapezoidal rule, as it provided a better model of MHC levels than the NONLIN compartmental model. Pharmacokinetic calculations for the perfusate samples were performed by Graph Pad Prism (version 3.02, Graph Pad Software, San Diego, CA, USA). The AUC ratio was calculated by dividing the perfusate AUC of the 90 min cisplatin perfusion by the resulting systemic blood UF AUC. The perfusate AUC between 0 and 60 min was also estimated.
Cisplatin versus oxaliplatin
The comparison between the absorption of cisplatin and oxaliplatin was conducted by calculating the absorption constant (ka) during the HIPEC phase. Unpublished data on oxaliplatin’s absorption constant (ka) was retrieved from an earlier study on oxaliplatin pharmacokinetics in eight patients, performed at our institution using the same HIPEC coliseum method (only the carrier solutions differed: oxaliplatin with electrolyte-free glucose and cisplatin with dianeal peritoneal dialysis fluid) [8]. Further characteristics of these patients are detailed in the previous study [8]. The mean value was calculated with the standard deviation and 95 % confidence interval. The absorption constant as calculated from the systemic uptake was expressed in terms of a t1/2 in min. The Mann–Whitney U test was used to evaluate the statistical difference between cisplatin and oxaliplatin. A p value <0.05 was considered statistically significant.
Inductively coupled plasma mass spectrometry versus liquid chromatography in perfusate samples
Two samples from every patient were sent for total platinum analysis using ICP-MS. These samples were taken at 10 min and 90 min during the HIPEC perfusion. For patient 2, samples were taken from all seven sampling time points during the HIPEC perfusion and sent for total platinum analysis. The ICP-MS results were then compared with the liquid chromatography results by a ratio (ICP-MS/liquid chromatography).
Results
Clinical results
Basic patient descriptive data are displayed in Table 1. The tumor load, as estimated by PCI, had a mean value of 24.4; and out of the ten patients, seven reached a CC score of 0, two had a CC score of 0–1, and one had CC score of 2. The mean operating time was 10.75 h (range: 6.5–12.67) with a mean blood loss of 675 ml (range: 200–2,000). The mean loss of EVF (erythrocyte volume fraction) during surgery was 30 % (absolute value 12.4 % from 40.8 % to 28.4 %). The mean postoperative value of albumin was 29.8 g/L. No grade III–IV hematological or renal toxicity was observed. However, in four patients, there was a transient grade I increase in creatinine an average of 7 days after treatment.
Pharmacokinetics
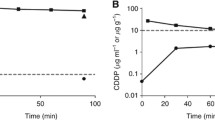
The results of the pharmacokinetic analysis are displayed in Tables 2, 3, and 4. The mean t1/2 of cisplatin in the perfusate UF was 18.4 min with an AUC of 2.87 mM × min. The mean t1/2 of cisplatin in the blood UF was 36.6 min with an AUC of 0.46 mM × min. The AUC perfusate UF/blood UF ratio was 6.28 for cisplatin and the mean perfusate and blood concentrations are displayed in Fig. 1. The AUC of the MHC in the perfusate UF was 0.66 mM × min, whereas the blood UF was 0.09 mM × min (Table 4). The mean perfusate UF AUC from 0 to 60 min was 2.45 mM × min ± 0.62 (SD).
Cisplatin versus oxaliplatin
The absorption constant (ka) ± standard deviation for cisplatin was 0.077 ± 0.026 (95 % CI: 0.059–0.096) as compared with 0.038 ± 0.007 for oxaliplatin (95 % CI: 0.033–0.043), p = 0.005. The corresponding t1/2 was 9.0 min for cisplatin and 18.2 min for oxaliplatin.
Inductively coupled plasma mass spectrometry versus liquid chromatography in perfusate samples
The ratio of total cisplatin (as measured by ICP-MS) to active cisplatin (as measured by liquid chromatography) over time in the perfusate is demonstrated in Fig. 2. The ratio of total platinum to cisplatin increased in a linear manner by time of perfusion.
Discussion
This is the first pharmacokinetic analysis of cisplatin and MHC in HIPEC using a selective technique. The t1/2 of active cisplatin in the perfusate UF was 18.4 min. This differs from earlier studies where t1/2 was reported to range between 25.8 and 99.6 min [9–12], or between 43.8 and 48 min with the same dosing (50 mg/m2) as the current study [9, 11]. This would indicate that after 75 min there is little active cisplatin left in the perfusate, having been either absorbed locally in the abdomen, systemically, or been bound to compounds (low-molecular weight compounds such as thiols) in the perfusate.
There are several findings in this study that would support a change in the current protocol from a 90-min to a 60-min perfusion time for cisplatin in HIPEC. Firstly, considering the short t1/2, reducing from 90 to 60 min would not significantly change the AUC in the perfusate (from 2.87 to 2.45 mM x min). Secondly, the cytotoxic effect of cisplatin is both time and concentration dependent, meaning that its cytotoxic effect can be enhanced by either increasing exposure time or the concentration [22, 23]. One in vitro study, combining both hyperthermia (at different temperatures) and cisplatin (at increasing concentrations), demonstrated only a few percent cell survival after 1 h cisplatin exposure at 7 mg/L (23.3 μM) with 42 °C hyperthermia [24]. Our study has an average concentration of 40 μM (2.4 mM x min/60 min) during the first 60 min with a hyperthermic temperature between 41 and 43 °C, which is consistent with a good cell kill rate according to Barlogie and colleagues. Thirdly, our comparison with oxaliplatin systemic absorption clearly shows that cisplatin is absorbed twice as fast as oxaliplatin (t1/2: 9.0 min vs. 18.2 min). This refutes the earlier rationale that cisplatin should have a longer perfusion time based on longer systemic absorption [7]. One weakness in this study is the low number of patients, which may limit the generalizability of the results. However, the results are quite congruent as shown in Tables 2 and 3, where the standard deviation of the AUC of the perfusate is only 18 % of the mean value and only 11 %of the blood UF. Therefore, with such consistency, it was deemed unnecessary to increase the sample size. Using the current protocol with 50 mg/m2 dosing, it appears feasible to reduce the perfusion time to 60 min.
Could the perfusion time be reduced even more, to 30 min, as used for oxaliplatin? Considering the more rapid systemic uptake and the shorter perfusate t1/2 in this study compared to oxaliplatin, it seems relevant to perform a dose escalating study on cisplatin within the framework of 30 min. However, there is some contention as to what should be the pharmacological endpoint. Some stress the exposure time as important, arguing in favour of repeated dosing [25], while others stress the local uptake in the tumor nodule as the endpoint, which is much more concentration dependant [6, 26]. In either case, it appears important to confirm pharmacokinetic models with in vitro studies in order to verify that there indeed is a similar or improved rate of tumor cell death.
Figure 2 displays the ratio between total platinum and cisplatin, and it appears that the longer the perfusion continues, the less reliable total platinum measurements of cisplatin are, with a median difference in the concentration of cisplatin of more than 40 % at the end of the perfusion. This difference is important, as it affects the pharmacokinetic modelling. The AAS or ICP-MS techniques measure all the platinum in a sample, but cisplatin can bind to various other low-molecular-weight endogenous compounds in the ultrafiltrate, such as thiols, leading to a lowering of bioactive platinum [27, 28]. There is a time-dependent increase of protein and albumin levels in the perfusate during HIPEC, probably due to raw peritoneal surfaces [9]. This could explain the skewed difference over time, as cisplatin can continue to react with new endogenous compounds that continuously leak into the peritoneal cavity during the perfusion. This also explains why this study’s pharmacokinetics differs from earlier studies, as they have not taken into account the continuous inactivation of cisplatin in the perfusate by new compounds leaking into the abdominal cavity from raw dissected peritoneal surfaces. These studies only measure total platinum, which cannot differentiate between active and inactive cisplatin (even after ultrafiltration). This supports the use of more specific measurements than total platinum as a basis for pharmacokinetic modeling of cisplatin in the perfusate during HIPEC.
When comparing results with intravenously (IV) administered cisplatin using the same liquid chromatography technique, the AUC of plasma UF (0.46 mM × min—Table 3) in HIPEC was 46 % of the AUC of plasma UF (1 mM × min) in IV administration [14]. This is interesting as the dose given during HIPEC is exactly half that given during IV administration (50 mg/m2 vs. 100 mg/m2). This is in contrast to earlier findings, where the AUC of plasma UF was similar for HIPEC at 50 mgm2 (0.8 mM × min) and IV at 100 mg/m2 (0.7 mM × min) measured as total platinum [9, 29]. The locoregional treatment of peritoneal carcinomatosis is not aimed at reaching therapeutic systemic levels. However, this may mean there is a margin on which to increase the cisplatin dose, particularly if the perfusion time were to decrease. There is one dose escalation study with a cisplatin perfusion of 90 min that increased the dose from 100 to 400 mg/m2. However, this study simultaneously administered sodium thiosulfate intravenously as a protective agent against nephrotoxicity, but how much that leaks into the peritoneal cavity inactivating cisplatin is yet unknown [30]. Furthermore, Cotte observed that they could not increase the dose (1–1.5 mg/kg which is a similar dose as 50 mg/m2) without sodium thiosulfate, as they were already observing temporary renal failures [31]. As such, one needs to either add sodium thiosulfate IV (maybe also determine its presence in the perfusate) or decrease the perfusion time if the dose is to be increased.
MHC presence in the perfusate UF and blood UF, as measured by the AUC, was 18 % in both. This metabolite is a very toxic form of cisplatin, both in terms of tumor cytotoxicity and nephrotoxicity [13, 14]. Its production appears to be similar in both the perfusate and blood ultrafiltrate. It should be important to quantify the concentration of MHC when evaluating the influence of the composition of the perfusion solution, since both chloride concentration and pH will affect the formation and thus, the cytotoxicity of cisplatin [32].
In conclusion, the pharmacokinetics of cisplatin demonstrates that the absorption of active cisplatin is more rapid than oxaliplatin and quicker than previously known. Lowering the perfusion time from 90 to 60 min does not significantly change the pharmacokinetic profile of active cisplatin during HIPEC and may, therefore, be considered. Further studies are needed to discern if a high-dose cisplatin and 30 min perfusion is possible. As the HIPEC perfusion progresses, the ICP-MS technique does not adequately reflect active cisplatin levels. Thus, pharmacokinetic modelling of cisplatin in HIPEC is improved by using a selective measuring technique, such as the one used in this study.
Abbreviations
- AAS:
-
Flameless atomic absorption spectrophotometry
- AUC:
-
Area under the time-concentration curve
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- CC:
-
Completeness of cytoreduction
- CRS:
-
Cytoreductive surgery
- HIPEC:
-
Hypthermic intraperitoneal chemotherapy
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- IV:
-
Intravenous(ly)
- MHC:
-
Monohydrated complex of cisplatin
- PCI:
-
Peritoneal cancer index
- PMP:
-
Pseudomyxoma peritonei
- UF:
-
Ultrafiltrate
References
Yang X-J, Huang C-Q, Suo T et al (2011) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 18:1575–1581
Deraco M, Kusamura S, Virzì S et al (2011) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as upfront therapy for advanced epithelial ovarian cancer: Multi-institutional phase-II trial. Gynecol Oncol 122:215–220
Bancroft D, Lepre C, Lippard S (1990) (195)PT NMR kinetic and mechanistic studies of cis-and trans-diamminedichloroplatinum (II) binding to DNA. J Am Chem Soc 112:6860–6871
Segal E, Le Pecq JB (1985) Role of ligand exchange processes in the reaction kinetics of the antitumor drug cis-diamminedichloroplatinum(II) with its targets. Cancer Res 45:492–498
Sherman S, Lippard S (1987) Structural aspects of platinum anticancer drug interactions with DNA. Chem Rev 87:1153–1181
Elias D, Bonnay M, Puizillou JM et al (2002) Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: Pharmacokinetics and tissue distribution. Ann Oncol 13:267–272
Ceelen WP, Van Nieuwenhove Y, Van Belle S, Denys H, Pattyn P (2009) Cytoreduction and hyperthermic intraperitoneal chemoperfusion in women with heavily pretreated recurrent ovarian cancer. Ann Surg Oncol. doi:10.1245/s10434-009-0878-6
Mahteme H, Wallin I, Glimelius B, Påhlman L, Ehrsson H (2008) Systemic exposure of the parent drug oxaliplatin during hyperthermic intraperitoneal perfusion. Eur J Clin Pharmacol 64:907–911
Royer B, Guardiola E, Polycarpe E et al (2005) Serum and intraperitoneal pharmacokinetics of cisplatin within intraoperative intraperitoneal chemotherapy: Influence of protein binding. Anticancer Drugs 16:1009–1016
Kern W, Braess J, Kotschofsky M et al (2002) Application of cisplatin as intraoperative hyperthermic peritoneal lavage (IHPL) in patients with locally advanced gastric cancer: Analysis of pharmacokinetics and of nephrotoxicity. Anticancer Res 22:3099–3102
Stephens AD, Belliveau JF, Sugarbaker PH (1996) Intraoperative hyperthermic lavage with cisplatin for peritoneal carcinomatosis and sarcomatosis. Cancer Treat Res 81:15–30
Chatelut E, de Forni M, Canal P et al (1991) Teniposide and cisplatin given by intraperitoneal administration: preclinical and phase I/pharmacokinetic studies. Ann Oncol 2:217–221
Jones MM, Basinger MA, Beaty JA, Holscher MA (1991) The relative nephrotoxicity of cisplatin, cis-[Pt(NH3)2(guanosine)2]2+, and the hydrolysis product of cisplatin in the rat. Cancer Chemother Pharmacol 29:29–32
Andersson A, Fagerberg J, Lewensohn R, Ehrsson H (1996) Pharmacokinetics of cisplatin and its monohydrated complex in humans. J Pharm Sci 85:824–827
Mahmood I, Duan J (2009) Population pharmacokinetics with a very small sample size. Drug Metabol Drug Interact 24:259–74
Sugarbaker PH (1995) Peritonectomy procedures. Ann Surg 221:29–42
Sugarbaker P (1998) Management of peritoneal surface malignancy using intraperitoneal chemotherapy and cytoreductive surgery. A manual for physicians and nurses, 3rd edn. The Ludann Company, Grand Rapids, MI
Andersson A, Ehrsson H (1995) Stability of cisplatin and its monohydrated complex in blood, plasma and ultrafiltrate–implications for quantitative analysis. J Pharm Biomed Anal 13:639–644
Pierre PV, Wallin I, Eksborg S, Ehrsson H (2011) Quantitative liquid chromatographic determination of intact cisplatin in blood with microwave-assisted post-column derivatization and UV detection. J Pharm Biomed Anal 56:126–130
Andersson A, Ehrsson H (1994) Determination of cisplatin and cis-diammineaquachloroplatinum(II) ion by liquid chromatography using post-column derivatization with diethyldithiocarbamate. J Chromatogr B 652:203–210
Ehrsson H, Wallin I, Andersson A, Edlund P (1995) Cisplatin, transplatin, and their hydrated complexes: Separation and identification using porous graphitic carbon and electrospray mass spectrometry. Anal Chem 67:3608–3611
Bergerat JP, Barlogie B, Göhde W, Johnston DA, Drewinko B (1979) In vitro cytokinetic response of human colon cancer cells to cis-dichlorodiammineplatinum(II). Cancer Res 39:4356–4363
Niell HB, Wood CA, Mickey DD, Soloway MS (1982) Time- and concentration-dependent inhibition of the clonogenic growth of N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide-induced murine bladder tumor cell lines by cis-diamminedichloroplatinum(II). Cancer Res 42:807–811
Barlogie B, Corry PM, Drewinko B (1980) In vitro thermochemotherapy of human colon cancer cells with cis-dichlorodiammineplatinum(II) and mitomycin C. Cancer Res 40:1165–1168
Royer B, Delroeux D, Guardiola E et al (2008) Improvement in intraperitoneal intraoperative cisplatin exposure based on pharmacokinetic analysis in patients with ovarian cancer. Cancer Chemother Pharmacol 61:415–421
Van der Speeten K, Stuart OA, Sugarbaker PH (2009) Pharmacokinetics and pharmacodynamics of perioperative cancer chemotherapy in peritoneal surface malignancy. Cancer J 15:216–224
De Waal WA, Maessen FJ, Kraak JC (1990) Analytical methodologies for the quantitation of platinum anti-cancer drugs and related compounds in biological media. J Pharm Biomed Anal 8:1–30
Cole WC, Wolf W (1980) Preparation and metabolism of a cisplatin/serum protein complex. Chem Biol Interact 30:223–235
Fournier C, Vennin P, Hecquet B (1988) Correlation between free platinum AUC and total platinum measurement 24 h after i.v. bolus injection of cisplatin in humans. Cancer Chemother Pharmacol 21:75–77
Cho HK, Lush RM, Bartlett DL et al (1999) Pharmacokinetics of cisplatin administered by continuous hyperthermic peritoneal perfusion (CHPP) to patients with peritoneal carcinomatosis. J Clin Pharmacol 39:394–401
Cotte E, Colomban O, Guitton J, Tranchand B, Bakrin N, Gilly FN, Glehen O, Tod M (2011) Population pharmacokinetics and pharmacodynamics of cisplatinum during hyperthermic intraperitoneal chemotherapy using a closed abdominal procedure. J Clin Pharmacol 51:9–18
Yachnin JR, Wallin I, Lewensohn R, Sirzén F, Ehrsson H (1998) The kinetics and cytotoxicity of cisplatin and its monohydrated complex. Cancer Lett 132:175–180
Acknowledgments
ALF funding through the Uppsala University hospital was used during this study, as well as funding from Apoteket AB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cashin, P.H., Ehrsson, H., Wallin, I. et al. Pharmacokinetics of cisplatin during hyperthermic intraperitoneal treatment of peritoneal carcinomatosis. Eur J Clin Pharmacol 69, 533–540 (2013). https://doi.org/10.1007/s00228-012-1405-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1405-4