Abstract
Purpose
To investigate the association of ethnicity with objectively, electronically measured adherence to inhaled corticosteroids (ICS) in a multicultural population of children with asthma in the city of Amsterdam.
Methods
The study was designed as a prospective, observational multicenter study in which adherence to ICS and potential risk factors for adherence to ICS were measured in a cohort of Moroccan and native Dutch children with asthma. Electronic adherence measurements were performed for 3 months per patient using a Real Time Medication Monitoring (RTMM) system. Ethnicity and other potential risk factors, such as socio-economic status, asthma control and parental medication beliefs, were extracted from medical records or parent interviews. The association between adherence and ethnicity was analysed using multivariate linear regression analysis.
Results
A total of 90 children (aged 1–11 years) were included in the study and data of 87 children were used for analysis. Average adherence to ICS was 49.3 %. Native Dutch children showed higher adherence to ICS than Moroccan children (55.9 vs. 42.5 %, respectively; p = 0.044, univariate analysis). After correction for confounders (>3 annual visits to the paediatric outpatient clinic, regular use of a spacer during inhalation), the final regression model showed that ethnicity was independently associated with adherence (p = 0.028).
Conclusions
In our Western European population of inner city children with asthma, poor adherence to ICS was a serious problem, and even somewhat more so in ethnic minorities. Paediatricians involved in asthma treatment should be aware of these cultural differences in medication-taking behaviour, but further studies are needed to elucidate the causal mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma is the most common chronic disease in children, with a prevalence of almost 10 % [1]. Almost all children with asthma use asthma medication, of which inhaled corticosteroids (ICS) are a main category [2]. Regular use of ICS can improve asthma control [3, 4] and reduce hospital admissions and mortality [5, 6]. Still, non-adherence is an important problem in healthcare in general and is of specific concern in asthma. A World Health Organisation report from 2003 [7] stated that 6–44 % of (all) asthma patients do not fill their first prescriptions of ICS. Amongst those who do, adherence rates range from 40 to 70 % [7–12]. After 1 year only 8–13 % of patients with first prescriptions of ICS still use these inhalers [13, 14].
Poor asthma control seems to be a particular problem amongst ethnic minority patients [15, 16]. Some evidence exists that ethnic disparities in asthma may be caused by poor adherence to ICS in ethnic minority patients [8, 9, 17]. Van Dellen et al. explored differences in adherence to ICS between children from different ethnic backgrounds in The Netherlands and did not find any difference in adherence between ethnic groups [18]. However, adherence in their study was measured with pharmacy record data and patient self-reports, two methods which are known to result in an over-reporting of adherence and therefore potentially unreliable [19, 20]. A more reliable method is the use of electronic measurements [10, 21, 22].
Another limitation of the data currently available in the literature is that the majority of studies on the relation between ethnicity and asthma originate from the USA. Extrapolation of the results to other countries may be complicated by considerable differences in national social security systems and public healthcare insurance. Also, large differences exist between the cultural identity of ethnic minority populations in the USA (e.g. Afro-Americans, Latin-Americans) and those in Western Europe (e.g. North-Africans, Turkish people). Therefore, additional studies focussing on the role of ethnicity in adherence to ICS are needed. This need is emphasised by the fact that approximately 10 % of the population in The Netherlands is of non-Western origin and even over 50 % in some of The Netherlands’ larger cities [23], as is the case in many other large urban communities in Western Europe.
The aim of our study was to determine the association of ethnicity with objectively measured adherence to ICS in a multicultural population of children with asthma in The Netherlands.
Subjects and methods
Study design
The study was designed as a prospective, observational, multicenter study in which adherence to ICS was electronically measured for 3 months in a cohort of Moroccan and native Dutch children with asthma. Patients were included from the St. Lucas Andreas Hospital, the BovenIJ Hospital and the Academic Medical Centre/Emma Children’s Hospital in Amsterdam. The study design was approved by the medical ethics committee of the VU Medical Centre in Amsterdam and by the institutional review boards of the participating hospitals.
Study population
Patient records were selected from the hospital administration for cases involving children aged 11 years or younger and diagnosed with asthma or wheezing (children under 6 years of age are usually diagnosed based on a symptom score; this is registered as wheezing [2]). Children with Moroccan and native Dutch ethnicity were identified using a name recognition technique. In this procedure, the names of potential participants were initially screened by an investigator with a Moroccan/Dutch ethnicity. Identification of Moroccan and Dutch ethnicity based on names is considered to be highly distinctive if carried out by a native speaker [15]. Parents of potential participants were contacted by telephone to verify whether the children had used an ICS with a pressurized metered dose inhaler (pMDI) for at least the past 3 months and to verify ethnicity. If the child or (at least one of) his/her parents were born in Morocco, the child was considered to have Moroccan ethnicity. Following the definition of Statistics Netherlands, children are considered to have Dutch ethnicity if they and both of their parents were born in the Netherlands. Only patients using fluticasone alone or fluticasone combined with the long-acting beta agonist salmeterol could enter the study because of compatibility with the Real Time Medication Monitoring (RTMM) devices. Spacers also had to be compatible with the RTMM-device. Eligible patients were invited to visit the paediatric outpatient department. An introduction letter and informed consent form were sent to their home address. Children and their parents who declined to participate in the study were excluded from further analysis.
Data collection
Patient contacts
During the initial visit to the paediatric outpatient clinic, the study design, including the data collection method by the RTMM-device, was further explained, and the parents were requested to sign the informed consent form. In a subsequent interview with one of the parents a number of potential risk factors were registered (section “potential risk factors”). Moroccan patients were interviewed by bilingual research assistants. Each patient received an RTMM-device (section Outcome measures) which was attached to their own pMDI. After receiving instructions, patients used the RTMM-device for 3 months for inhalation of their normal ICS dose. The patients and their parents knew they were being monitored during the study. After 3 months of using the RTMM-device, patients were invited for a second visit to the paediatric outpatient department in which the RTMM-device was collected and an exit interview took place. During the entire study period an independent paediatrician was available for consultation by parents or children participating in the study. However, no parents consulted this independent paediatrician.
Outcome measures
The number of ICS inhalations was registered by the RTMM-devices, which operate as follows. Each time the pMDI was fired a data message containing patient-ID and time and date of administration was sent to the study database by way of the mobile telephone network. In order to prevent incomplete registration caused by an insufficient network connection, the RTMM-device was so designed as to be able to use two different networks: GPRS and GSM (dual band). If both networks were unavailable at the time of inhalation, a data message was prepared for sending at a later time.
For each administration, data were compared to the prescribed ICS dosing schedule. Adherent administrations were defined as inhalations registered within a 6-h timeframe around the prescribed time of inhalation (from 3 h before until 3 h after), which is a common measure for twice-daily dosing regimens [24–26]. For each patient the proportion of adherent administrations of the number of prescribed administrations was calculated and used as the outcome measure.
Potential risk factors
Children and their parents were interviewed by healthcare workers specialised in ethnic diversity for collection of relevant characteristics that could not be extracted from the medical records. Potential risk factors (i.e. secondary determinants, collected as potential confounders for the association between ethnicity and adherence) registered during this study included age, gender, type of ICS (fluticasone or fluticasone/salmeterol), fluticasone dosage and dosing frequency, Dutch language skills of the parents (assessed by investigators), parental level of education (highest education that was successfully finished by the parents), family income, quality of housing (e.g. degree of insulation, problems with indoor humidity; assessed by parents), parental smoking habits (at home), use of a spacer during inhalation, identity of paediatrician and hospital. The frequency of hospitalisation and of visits to the paediatric outpatient department in the 12 months preceding the study period were collected as an indicator of the level of asthma control. Finally, parental medication beliefs were measured using the Beliefs about Medicines Questionnaire (BMQ) Specific, which contains a scale for beliefs in the necessity (nec) of ICS and one for concerns (conc) about long-term toxicity and disruptive effects of ICS [27, 28]. Both scales range from 5 to 25, with higher scores indicating stronger beliefs. Subtraction gives a necessity:concerns differential (range −20 to +20), indicating the balance between the patients’ trust in the efficacy and concerns about side effects. Combining the separate necessity and concern scales results in the creation of four attitudinal groups: skeptical (nec <15, conc >15), indifferent (nec <15, conc <15), ambivalent (nec >15, conc >15) and accepting (nec >15, conc <15) [29].
Data analysis
The sample size calculation was based on the assumption that in the final linear regression model four independent variables would be included. With a type-1 error of 0.05, a power of 80 % and an estimated effect size of 0.15, a sample size of in total 84 children was determined. In anticipation of patient drop-out, a few additional patients were included.
Patients for whom <5 inhalations were registered during the study period were excluded from the data analysis since they had apparently stopped using ICS and therefore did not meet the inclusion criteria.
Data were processed and analysed using SPSS ver. 18.0 (SPSS, Chicago, IL). Categorical determinants were first converted into dummy variables.
For both the primary determinant and the secondary determinants we started with a univariate analysis [independent samples t test or one-way analysis of variance (ANOVA)] on the outcome measure (percentage of adherent inhalations). Secondary determinants that showed a borderline association (p ≤ 0.2) with adherence in the univariate analysis were subsequently added to a multivariate linear regression model on the association between ethnicity and adherence, and were only left in the final model if they had a significant contribution (p < 0.05).
Results
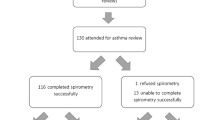
Of all 1,026 patients with the correct age and diagnosed with asthma or wheezing, 939 were excluded for several reasons (Fig. 1).
All 90 patients included in the study completed the follow-up. At the end of data collection period three patients were excluded from the analysis: two (one with Dutch and one with Moroccan ethnicity) had taken fewer than five ICS inhalations during the entire study period and were therefore assumed to have stopped ICS therapy before entering the study; one patient had used a RTMM-device that suffered from technical failure. The final study population thus included 87 patients of whom 44 were Dutch and 43 Moroccan. Baseline characteristics of the 87 children included in the analyses are presented in Table 1. Several baseline characteristics were unevenly distributed between children with Dutch and Moroccan ethnicity, including sex, hospital, parental level of education, quality of housing, parental Dutch language skills, family income and medication beliefs (BMQ) (Table 1).
The mean percentage of adherent inhalations was 49.3 % [standard deviation (SD) 31.2 %]. Only 18 % of patients (16/87) showed an adherence rate of >80 %. More than one-half of the study population (49/87) had <50 % adherent ICS inhalations and almost a quarter of the participants (21/87) consistently took <20 % of inhalations. On average, patients did not use any ICS on 36.9 % of the study days. On 63.1 % of the study days at least one ICS inhalation was taken and at least two inhalations on 40.7 % of the days.
Overall, native Dutch children showed a higher percentage of adherent ICS inhalations than children with Moroccan ethnicity (55.9 vs. 42.5 %, respectively;p = 0.044, univariate analysis). Determinants that did not show any association with adherence in the univariate analysis were age, sex, hospital, paediatrician, dosing frequency, daily dose, smoking habits parents, parental Dutch language skills, hospital admissions for asthma, BMQ necessity and BMQ necessity minus concerns.
The remaining secondary determinants showed an association with adherence (p ≤ 0.2) in the univariate analyses and are presented in Table 2.
These determinants were subsequently added to the model in a multivariate linear regression analysis estimating the association of ethnicity with adherence. The final model contained ‘>3 annual visits to the paediatric outpatient clinic’ and ‘regular use of a spacer during inhalation’ as confounders and resulted in an independent, statistically significant association of ethnicity with adherence (p = 0.028; Table 3)
Discussion
In this study, an average of 49 % of prescribed ICS inhalations was taken within the predefined 6-h timeframe around the planned time of inhalation. This result corresponds with adherence rates found in earlier studies in asthma patients (range 40–50 %) in which adherence was objectively assessed by measuring canister weight, counting doses or electronic measurement [10, 12, 30, 31].
In our study, a significantly higher adherence rate was found in native Dutch children (55.9 %) than in Moroccan children (42.5 %), even after adjustment for confounders known to be associated with adherence (p = 0.028). This result is in agreement with those from several studies in the USA looking at the association of ethnic disparities with ICS adherence [9, 10, 17, 30]. In spite of the different ethnic background of minority patients in these American studies (Afro-American, Asian and Latin-American) and other socio-economic differences (i.e. insurance status, income/social security system), we found similar adherence rates and ethnic/racial differences in our population. This difference in adherence rate between Dutch and Moroccan children was not (fully) explained by the determinants we collected, including those that showed a significant association with ethnicity (e.g. parental level of education, quality of housing, parental language skills, family income, medications beliefs; Table 1) or with adherence (use of a spacer during inhalation; Table 2). This is in contrast with other studies, in which socio-economic status and negative patient beliefs are found to mediate the ethnicity–adherence relationship [8, 30]. Other cultural issues may contribute to the observed ethnicity-related difference in adherence.
Several limitations of this study need to be discussed. First, this study was designed to investigate ethnicity as an independent risk factor for non-adherence to ICS. This association was adjusted for covariates that were unevenly distributed between ethnicities or that had an association with adherence. However, larger studies looking into specific risk factors within ethnic subgroups need to be designed to identify factors that may explain the association between ethnicity and adherence to ICS. Furthermore, an investigation of culture-specific determinants of adherence to asthma medication is needed for all major ethnic minority populations in large Western European cities. Although electronic monitoring, such as RTMM is considered to be more sensitive for measuring non-adherence than other, subjective tools for adherence measurement [10, 19, 32] and although we found a mean adherence rate of 49 %, we believe that we may still have overestimated the actual adherence to ICS. The participating patients were aware that they were being observed, so they may have acted more adherent than usual. Also, the RTMM-devices can only detect that the pMDI is being fired; consequently, deliberate faking of the adherence measurement remains undetected. A common criticism of electronic medication monitoring based on the time and date the inhaler is fired is the inability to confirm whether the medication is actually taken or that no more or no less than the prescribed dose is taken. Only drug assays can confirm ingestion. However, studies comparing the sequence of medication events with projected and periodically measured concentrations of the drug in plasma have confirmed the validity of medication event monitors. In these studies, mismatches between medication events and actual dosing were too rare to create substantial differences between projected and actual plasma concentrations of the drug [33–36].
Patients who had decided to quit taking ICS (without consulting a physician) were not been included into this study. At patient selection, the parents of 203 patients claimed that their child not longer used a fluticasone-containing pMDI. Only two patients were excluded for this reason after completing the study (<5 inhalations were registered in 3 months). If some of the excluded patients still had an indication for taking ICS, they would have had a 0 % adherence rate. Taking this into account, the mean adherence rate would be even considerably lower than 49 %.
Although a considerable number of potential risk factors for poor adherence were identified in our study, we may have missed one or more. For example, we did not collect data on the number and type of drugs concomitantly used with ICS, asthma control, asthma severity, patient self efficacy and parental asthma knowledge. This omission may have resulted in insufficient adjustment of the association between ethnicity and adherence. Healthcare insurance status was deliberately not collected in this study. According to Dutch law, every Dutch citizen is required to have a basic healthcare insurance (which covers medication costs). Children receive free healthcare insurance. Therefore, we believe that non-adherence caused by lack of healthcare insurance is not a relevant risk factor in The Netherlands.
Finally, this study was carried out in a mixed population of children with a (spirometry-confirmed) diagnosis of asthma and in children with wheezing. The latter had not been officially diagnosed with asthma and therefore may have benefitted less from ICS treatment than the others, which in turn may have influenced adherence behavior and medication beliefs.
A strength of this study is the use of RTMM as an objective and reliable method for adherence measurement. This method provides less room for bias than other measurement methods, such as patient self-reporting (socially acceptable patient response), adherence questionnaires (misjudgement of patient behaviour) and pharmacy dispensing data based on, for example, refill rate and persistence (possible overestimation of adherence). The results of this study also provide a better understanding of medication behaviour of a multicultural population of European inner-city children with asthma. This is especially relevant since ethnic minority children are present in abundant in large European cities and have not previously been intensively studied [23].
In conclusion, our multicultural population of children with asthma showed an average adherence to ICS of 49 %. We found a significantly higher adherence rate in native Dutch children than in Moroccan children. Therefore, we conclude that poor adherence to ICS is a major healthcare concern in our Western European population of inner city children with asthma, and somewhat more so in ethnic minority children. Paediatricians involved in asthma treatment should be aware of these cultural differences in medication-taking behaviour, but further studies are needed to elucidate the causal mechanism.
Statements
What is known about this topic?
-
Non-adherence to inhaled corticosteroids is a major healthcare problem in children with asthma.
-
Ethnicity may be a risk factor for non-adherence.
-
The exact role of ethnicity is unknown due to the use of subjective measurement methods for adherence in earlier studies.
-
Previous adherence studies have been primarily conducted in the USA, with a focus on Afro-American, Asian and Latin-American populations.
What this study adds
-
Adherence to inhaled corticosteroids was objectively measured by RTMM.
-
Non-adherence to inhaled corticosteroids in children with asthma is common.
-
Ethnicity is an independent risk factor for non-adherence to ICS.
-
This study adds insight into medication taking behaviour of Arabic immigrants in Western Europe.
References
Hirasing RA, Aardoom HA, Van den Heuvel EW et al (1995) Prevalentie van door ouders gerapporteerde chronische aandoeningen bij schoolkinderen (Prevalence of chronic diseases in school going children as reported by their parents; in Dutch). Ned Tijdschr Geneesk 139:934–938
Zuidgeest MG, Van Dijk L, Smit HA et al (2008) Prescription of respiratory medication without an asthma diagnosis in children: a population based study. BMC Health Serv Res 8:16. doi:10.1186/1472-6963-8-16
Johnston NW, Sears MR (2006) Asthma exacerbations. 1: epidemiology. Thorax 61:722–728. doi:10.1136/thx.2005.045161
O’Connell EJ (2004) Optimizing inhaled corticosteroid therapy in children with chronic asthma. Pediatr Pulmonol 39:74–83
Suissa S, Ernst P, Kezouh A (2002) Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax 57:880–884
Suissa S, Ernst P, Benayoun S et al (2000) Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 343:332–336. doi:10.1056/NEJM200008033430504
Sabete E (2003) WHO report ‘Adherence to long-term therapies – evidence for action’. p 7,48,49
Apter AJ, Boston RC, George M et al (2003) Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol 111:1219–1226
Williams LK, Joseph CL, Peterson EL et al (2007) Race-ethnicity, crime, and other factors associated with adherence to inhaled corticosteroids. J Allergy Clin Immunol 119:168–175. doi:10.1016/j.jaci.2006.09.029
Bender B, Wamboldt FS, O’Connor SL et al (2000) Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol 85:416–421
Bosley CM, Fosbury JA, Cochrane GM (1995) The psychological factors associated with poor compliance with treatment in asthma. Eur Respir J 8:899–904
Celano M, Geller RJ, Keith Phillips KM et al (1998) Treatment adherence among low-income children with asthma. J Pediatr Psychol 23:345–349
Herings RMC, Leufkens HGM, Heerdink ER et al (2002) Pharmo report ‘Chronic pharmacotherapy forever’. CIP Data Royal Library, The Hague
Schirm E, de Vries TW, Tobi H et al (2006) Prescribed doses of inhaled steroids in Dutch children: too little or too much, for too short a time. Br J Clin Pharmacol 62:383–390. doi:10.1111/j.1365-2125.2006.02699.x
Van Dellen QM, Stronks K, Bindels PJ et al (2007) Predictors of asthma control in children from different ethnic origins living in Amsterdam. Respir Med 101:779–785. doi:10.1016/j.rmed.2006.08.002
Akinbami LJ, Moorman JE, Garbe PL et al (2009) Status of childhood asthma in the United States, 1980–2007. Pediatrics 123:S131–S145. doi:10.1542/peds.2008-2233C
Apter AJ, Reisine ST, Affleck G et al (1998) Adherence with twice-daily dosing of inhaled steroids - socioeconomic and health-belief differences. Am J Respir Crit Care Med 157:1810–1817
Van Dellen QM, Stronks K, Bindels PJ, PEACE Study Group et al (2008) Adherence to inhaled corticosteroids in children with asthma and their parents. Respir Med 102:755–763. doi:10.1016/j.rmed.2007.12.005
Milgrom H, Bender B, Ackerson L et al (1996) Non-compliance in and treatment failure in children with asthma. J Allergy Clin Immunol 98:1051–1057
Horne R, Weinman J (2002) Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatments beliefs in explaining non-adherence to preventer medication. Psychol Health 17:17–32
Apter AJ, Tor M, Feldman HI (2001) Testing the reliability of old and new features of a new electronic monitor for metered dose inhalers. Ann Allergy Immunol 86:421–424. doi:10.1016/S1081-1206(10)62488-X
O’Connor SL, Bender BG, Gavin-Devitt LA et al (2004) Measuring adherence with the doser CT in children with asthma. J Asthma 41:663–670
Van Dellen QM, Van Aalderen WMC, Bindels PJE, PEACE study Group et al (2008) Asthma beliefs among mothers and children from different ethnic origins living in Amsterdam, the Netherlands. BMC Publ Health 8:380–390. doi:10.1186/1471-2458-8-380
De Klerk E, Van der Heijde D, Landewé R et al (2003) Patient compliance in rheumatoid arthritis, polymyalgia rheumatica, and gout. J Rheumatol 30:44–54
Schroeder K, Fahey T, Hay AD et al (2006) Adherence to antihypertensive medication assessed by self-report was associated with electronic monitoring compliance. J Clin Epidemiol 59:650–651. doi:10.1016/j.jclinepi.2005.10.013
Maitland D, Jackson A, Osorio J et al (2008) Switching from twice-daily abacavir and lamivudine to the once-daily fixed-dose combination tablet of abacavir and lamivudine improves patient adherence and satisfaction with therapy. HIV Medicine 9:667–672. doi:10.1111/j.1468-1293.2008.00618.x
Horne R, Weinman J, Hankins M (1999) The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 14:1–24
Horne R, Weinman J (1999) Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 47:555–567
Menckeberg TT, Bouvy ML, Bracke M et al (2008) Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res 64:47–54. doi:10.1016/j.jpsychores.2007.07.016
Tao TL, Bilderback A, Bender B et al (2008) Do asthma medication beliefs mediate the relationship between minority status and adherence to therapy? J Asthma 45:33–37
Jónasson G, Carlsen KH, Mowinckler P (2000) Asthma drug adherence in a long term clinical trial. Arch Dis Child 83(4):330–333
Burgess SW, Sly PD, Morawska A, Devadason SG (2008) Assessing adherence and factors associated with adherence in young children with asthma. Respirology 13:559–563. doi:10.1111/j.1440-1843.2008.01292.x
Rubio A, Cox C, Weintraub M (1992) Prediction of diltiazem plasma concentration curves from limited measurements using compliance data. Clin Pharmacokin 22:238–246
Girard P, Sheiner LB, Kastrissios H et al (1996) Do we need full compliance data for population pharmacokinetic analysis? J Pharmacokin Biopharm 24:265–282
Vrijens B, Goetghebeur E (1999) The impact of compliance in pharmacokinetic studies. Stat Methods Med Res 8:247–262
Vrijens B, Tousset E, Rode R et al (2005) Successful projection of the time course of drug concentration in plasma during a 1-year period from electronically compiled dosing-time data used as input to individually parameterized pharmacokinetic models. J Clin Pharmacol 45:461–467. doi:10.1177/0091270004274433
Acknowledgements
We are indebted to Loes Thomaes, Chaled Abdel Gawad, Marloes van Hest, Sander Borgsteede, Fatma Karapinar, Marjo Janssen, Svetlana Belitser, Hein van Onzenoort, Inge Berger, Caroline Dijkstra, Karin Geutjes, Fatima Antar, Sufian Alariachi, Saidan Karaman and Mahmut Yilmaz for their comments on the study protocol and for collection of the data, and to the colleagues of the paediatric outpatient clinics of the participating hospitals for their contribution to this study.
Financial disclosure
The study was supported by a non-conditional grant from the health insurance company Agis and partially sponsored by the manufacturer of the RTMM-devices, Evalan BV.
Conflict of interest
The manufacturer of the RTMM-devices, Evalan BV, partially sponsored the study by providing devices at cost price. Both AGIS and Evalan BV did not have any role in the study design, nor in the analysis and interpretation of the data, nor in the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Netherlands Trial Registry (NTR)
Identification code: NTR1373 (www.trialregister.nl)
Rights and permissions
About this article
Cite this article
Vasbinder, E., Dahhan, N., Wolf, B. et al. The association of ethnicity with electronically measured adherence to inhaled corticosteroids in children. Eur J Clin Pharmacol 69, 683–690 (2013). https://doi.org/10.1007/s00228-012-1380-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1380-9