Abstract
Purpose
The aim of this study was to investigate the genetic polymorphisms of UGT1A3, UGT1A6, and UGT2B7 in Chinese epilepsy patients and their potential influence on the pharmacokinetics of valproic acid (VPA).
Methods
The genetic architectures of UGT1A3, UGT1A6, and UGT2B7 in 242 epilepsy patients were detected by DNA sequencing and PCR-restriction fragment length polymorphism. Steady-state plasma concentrations of VPA in 225 patients who had received VPA (approx. 250–1,000 mg/day) for at least 2 weeks were determined and associated with UGT polymorphisms.
Results
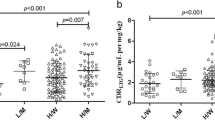
The allelic distribution of UGT1A3 in our Chinese epilepsy patients was significantly different from that in healthy subjects based on reference data. The standardized trough plasma concentration (CS) of VPA was much lower in our patients with the UGT1A3*5 variant than in the wild type carriers (3.24 ± 1.05 vs. 4.68 ± 1.24 μg·kg·mL-1·mg-1, P < 0.01). UGT polymorphisms had no influence on the pharmacokinetic interactions between carbamazepine and VPA.
Conclusion
Our results suggest that UGT1A3*5 may be an important determinant of individual variability in the pharmacokinetics of VPA and that it may be necessary to increase VPA dose for UGT1A3*5 carriers to ensure its therapeutic range of 50–100 μg/mL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Valproic acid (2-propylpentanoic acid, VPA) is an antiepileptic drug (AED) that has been in long use as an anticonvulsant medication in epilepsy and also used in the treatment of other brain diseases [1]. Recent studies have revealed that VPA is also a potential anticancer agent because of its inhibitory activity on histone deacetylase [2]. VPA has a narrow therapeutic range (50–100 μg/mL) in the treatment of epilepsy and shows considerable individual variability in both its pharmacokinetics and pharmacodynamics. Although the exact mechanisms of VPA individual variability remain elusive, drug co-medications and gene polymorphisms have been found to be two major causes [3–7].
VPA is extensively metabolized and dozens of metabolites are produced from oxidation catalyzed by various cytochrome P450s, mitochondrion-mediated β-oxidation, and glucuronidation catalyzed by uridine diphosphate glucuronosyltransferases (UGTs) [8, 9]. Glucuronide metabolites account for an up to 50% of the VPA dose [10, 11], suggesting a pivotal role of UGTs in VPA metabolism. UGT2B7, UGT1A3, and UGT1A6 are the major UGT isozymes involved in the production of VPA glucuronides [14, 15]. UGT isozymes were found to be highly polymorphic, and some of the polymorphisms can lead to both transcriptional and functional changes of the enzymes [12, 13]. The isozyme UGT2B7 shows the highest activity for VPA glucuronidation; however, its variant (UGT2B7*2, H286Y) has been found to have little influence on the pharmacokinetics of VPA in healthy volunteers [6]. UGT1A6*2 (T181A/R184S) possesses a twofold higher activity for catalyzing VPA glucuronidation in vitro than the wild type [16]. A total of 17 single nucleotide polymorphisms in UGT1A3 have been identified, and some of these showed functional significance [17–19]. However, currently available information on the influence of UGT polymorphisms on VPA pharmacokinetics is extremely limited, and no studies have been performed with epilepsy patients.
Given the pivotal role of UGTs in VPA metabolism, we hypothesized that genetic polymorphisms of the major UGT isozymes responsible for its metabolism, including UGT1A3, UGT1A6, and UGT2B7, are possibly important determinants of its pharmacokinetic inter-individual variability. We have therefore investigated the genetic polymorphisms of UGT1A3, UGT1A6, and UGT2B7 in a population of Chinese epilepsy patients, as well as their association with VPA pharmacokinetics. We also determined the potential influence of genetic polymorphisms on the pharmacokinetic interactions of co-administered carbamazepine with VPA.
Methods
Patients and blood sampling
A total of 242 epilepsy outpatients were enrolled between March 2008 and October 2009 at the department of Neurology at Jinling Hospital (Nanjing, China). All patients had been diagnosed with epilepsy, with normal liver and kidney function, based on their seizure history and the results of the electroencephalogram and biochemical laboratory tests. The protocol of this study was approved by the Ethics Committee of Nanjing Jinling Hospital, and written consent was obtained from all patients prior to enrollment.
Patients received sodium valproate (Deparkin; Sanofi-Synthelabo Minsheng Pharmaceutical, Hangzhou, China) (250–1,000 mg/day) or other AEDs to control epilepsy. The dosing regimen was maintained stably for at least 2 weeks (>5 half-lives) to ensure that the blood sampling was performed at the steady-state of VPA pharmacokinetics. Venous blood samples (5 mL) for analysis were collected immediately before the morning dose. The blood samples were separated into two tubes, one of which was centrifuged immediately to obtain plasma and then stored at −70°C until used for drug analysis, and the other was immediately stored at −20°C until used for DNA isolation.
Quantification of VPA plasma concentrations
Steady-state trough plasma concentrations of VPA were determined by a fluorescence polarization immunoassay (FPIA) performed using TDx equipment (Abbott Laboratories, Abbott Park, IL); this is a standard method applied in routine therapeutic drug monitoring (TDM) [20]. The method developed in this study was validated for biosample analysis in a linear range of 0.7–150 μg/mL; inter- and intra-batch variations were <10% and the lower limit of quantification (LOQ) was 0.7 μg/mL.
Genetic analysis of UGTs
Genomic DNA was extracted from peripheral blood lymphocytes using the blood DNA extraction kit according to the manufacturer’s recommendations (BioMed, China). DNA concentrations were determined by spectrophotometry at 260 nm, and all samples were stored at −20°C until analysis. The presence of UGT1A6 (A541Gand A552C) and UGT2B7 C802T variants was identified by PCR-restriction fragment length polymorphism (RFLP) [21, 22]. UGT1A3 was genotyped directly by gene sequencing according to the manufacturer’s instructions on an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA), and analyzed with Gene Scan software (Applied Biosystems). Sequencing data were checked for the presence of six frequently identified single nucleotide polymorphisms (SNPs) including A17G, T31C, A81G, C133T, T140C, and A477G. The primers of UGT1A3 were designed using Primer Premier 5.0 software (Premier Biosoft, Palo Alto, CA). All primers were synthesized by integrated DNA technologies (Invitrogen, Shanghai, China); primers sequences, and specific annealing temperatures are listed in Electronic Supplementary Material (ESM) Table 1. Restriction enzymes (Fermentas, Ontario, Canada) for UGT1A6 and UGT2B7 are listed in ESM Table 2. In total, 5 % of the samples were randomly selected for validation by gene sequencing.
Statistical and computational genetic analysis
Hardy–Weinberg equilibrium analysis of UGT1A3, UGT1A6, and UGT2B7 SNPs was performed using Haploview 4.2 software (available at: www.broad.mit.edu/mpg/haploview). Allelic and genotypic frequencies of UGTs in our population of Chinese epilepsy patients were compared with those previously reported in Chinese healthy volunteers and Japanese and German–Caucasian populations by the Fisher’s Exact chi-square test.
Trough plasma concentrations of VPA were standardized by adjusting with patients’ weight and dose and expressed as CS [CS = trough plasma concentration/(dairy dose/weight )] [23]. Data were expressed as mean ± standard deviation (SD). Statistical differences in VPA CS among various groups classified by genotypes and/or drug combinations were analyzed preferentially by nonparametric methods (Kruskal–Wallis and Mann–Whitney test for multiple comparisons); if the analysis was significant (P < 0.05), a one-way analysis of variance (ANOVA) test with Dunnett’s post hoc test was then applied to compare the difference between groups. A p value of <0.05 was considered to be significant. Statistical analysis was performed using SPSS ver. 16.0 software (SPSS, Chicago, IL).
Results
Clinical characteristics of epileptic patients
Genotyping was performed in a total of 242 epilepsy patients; 136 of these were treated with VPA mono-therapy, while 38 were concomitantly treated with carbamazepine, 19 with topiramate, three with phenytoin, six with piracetam, and 23 with vitamins and cardiovascular agents. The remaining 17 patients received no VPA but other ADEs. All participants are from the Chinese Han population. Of these patients, 163 were male with an average [± standard deviation (SD)] age of 31.1 (±16.1) years, and 79 female, with an average age of 27.5 (±14.0) years. The average body weight was 64.7 (±17.0) and 55.0 (±12.4) kg for male and female patients, respectively.
SNPs, alleles, and haplotype analysis of UGT1A3, UGT1A6, and UGT2B7 in Chinese epilepsy patients
DNA samples from the whole blood of 242 patients were screened by RFLP-PCR and gene sequencing to determine the prevelance of UGT1A3, UGT1A6, and UGT2B7 polymorphisms in the Chinese epilepsy patients (Table 1). The allelic distributions of all SNPs detected in this study were consistent with Hardy–Weinberg equilibrium. As our study was the first to determine the distribution of UGT polymorphisms in epilepsy patients, we compared our findings with those previously identified in Chinese, Japanese, and Caucasian healthy volunteers [18, 24–30]. Previous studies found that UGT1A3 SNPs comprise four UGT1A3 allele variants, including UGT1A3*2b (T31C-G81A-T140C-A477G), UGT1A3*3b (T31C-G81A-A447G), UGT1A3*4(C133T), and UGT1A3*5 (A17G-T31C-G81A-A477G) [18, 22, 31]. We identified significant differences between our Chinese epilepsy patients and healthy subjects for the gene frequencies of some of UGT1A3 alleles, including UGT1A3*1 (42 vs. 68 %, P < 0.05), UGT1A3*2b (21 vs. 9 %, P < 0.05), and UGT1A3*3b (18 vs. 7 %, P < 0.05) (Table 2). In contrast, there was little difference in the genotype frequencies of UGT1A6 and UGT2B7 in our Chinese epilepsy patients and the healthy subjects (Table 3).
Haplotype analysis was performed among the eight SNPs of the UGT1 locus (UGT1A3 and 1A6) to determine their potential cooperative contributions in alternating the enzyme activity. A total of 20 haplotypes were identified (ESM Table 3). Dominant haplotypes include II (9.5%, UGT1A3*1–UGT 1A6 541 A > G-552 A > C), III (9.5%, all reference alleles except UGT1A3*3b), IV (8.68%, all reference alleles except UGT1A3*2b), V (6.20%, all reference alleles except UGT1A6 552 A > C), VI (5.79%, UGT1A3*2b–UGT1A6 541 A > G-552 A > C), and VII (5.79%, all reference alleles except UGT1A3*5); the frequencies of other haplotypes were less than 5% (ESM Table 3).
Association of UGT polymorphisms with VPA plasma trough concentrations
To determine the influence of polymorphisms of the identified UGTs on VPA pharmacokinetics, we conducted an association analysis of UGT SNPs, genotypes, and haplotypes with VPA CS. Among all of the UGT SNPs detected, only the UGT1A3 A17G polymorphism showed a significant influence, with carriers of UGT1A3 A17G polymorphism characterized by having a much lower VPA CS than the wild-type carriers (ESM Table 4). Patients who were carriers of UGT1A3*5 alleles harboring the A17G mutation were consistently characterized with a significant lower VPA CS (Table 4), whereas polymorphisms of UGT1A6 and UGT2B7 had no significant effect on VPA CS (Table 5). The same result was obtained from the association analysis of UGT1A haplotypes and VPA CS, with only those patients with haplotype VII and XV, both harboring the UGT1A3*5 mutation, characterized with a significant lower VPA CS (ESM Table 5).
Association of UGT polymorphisms with drug interactions
The patients receiving concomitant therapy with carbamazepine and VPA were characterized with a much lower VPA CS than those receiving VPA monotherapy (3.53 ± 1.67 vs. 4.57 ± 1.48, P < 0.01) (ESM Table 6). To determine whether UGT genotypes influence VPA–carbamazepine interactions, we sub-classified the patients receiving concomitent carbamazepine and VPA according to UGT genotypes and found no significant difference among the different patients (Table 6).
Discussion
Uridine diphosphate glucuronosyltransferases have been previously proven to be highly polymorphic, and their genetic polymorphisms have been associated with altered metabolism of many endogenous and exogenous compounds and with the susceptibility to various diseases [32–35]. Although there have been several previous reports of an association between UGT polymorphisms and VPA pharmacokinetics, none of these studies involved epilepsy patients. In one study, genetic polymorphisms of UGT2B7, the UGT isozyme with the highest activity towards VPA glucuronidation, were found to have little influence on VPA pharmacokinetics in healthy subjects [6]. However, because of the small sample size (14 subjects), the authors themselves were somewhat uncertain about the exact influence of UGT2B7 polymorphisms on VPA pharmacokinetics. Our results, based on the analysis of 136 epilepsy patients receiving VPA monotherapy, may lead to a more definitive conclusion that UGT2B7 polymorphism exerts little influence on the steady-state plasma concentration of VPA. A previous study using recombinant UGT1A6 variant enzymes found that the UGT1A6*2 allele possessed a twofold higher activity than the wild type towards VPA glucuronidation [14]. However, we found that the genetic polymorphism of UGT1A6 had no influence on the VPA pharmacokinetics in Chinese epilepsy patients. Such an inconsistency may be explained by poor in vitro to in vivo correlations that have been observed in multiple genotype–phenotype association studies on UGTs.
Allelic distributions of UGT1A3 vary to a significant extent in different racial populations, especially between Asians and Caucasians [15, 18, 24, 25]. Our study further verified the differential racial distribution of various UGT1A3 polymorphisms. It was of interest to find that the UGT1A3 allelic distribution in our Chinese epilepy patients varies significantly from that in Chinese healthy subjects (Table 2); the functional significance of this difference may warrant further research to explore whether or not the UGT1A3 polymorphism is a genetic determinant of epileptic susceptibility.
Our genotype and phenotype association analysis showed that our Chinese epilepsy patients with the UGT1A3 A17G polymorphism and UGT1A3*5 allele were characterized with a significant lower VPA CS. This result seems to be inconsistent with those from previous reports where the UGT1A3*5 variant was found to be associated with a slightly lower activity (86%) than the wild type towards the metabolism of its typical substrate estrogen [24]. However, previous reports on the enzyme activities of different UGT1A3 variants were largely controversial. For example, in one study the UGT1A3*2 allele was identified with a 3.7-fold higher intrinsic clearance than the wild type towards the metabolism of estrone, whereas in another study the authors reported a moderate lower activity of this allele. There is still no information available on the activity of UGT1A3 variants in metabolizing VPA; thus, it is difficult to conclude that the lower plasma concentration of VPA in the UGT1A3*5 carriers is caused by the increased enzyme activity of this variant. In addition, previous reports on the influence of the UGT1A3 polymorphism on its transcription and protein expression were also quite controversial. In one study, UGT1A3*2 was found to be closely associated with a 7.3-fold increase in the protein expression of UGT1A3 in the human liver [19]; in contrast, an analysis using reporter gene transfection in Hepg2 cells revealed a 60% lower transcriptional activity of UGT1A3*2[15]. Therefore, much work remains to be done to determine the detailed enzymatic properties of various UGT1A3 variants, including UGT1A3*5, a potential genetic determinant of VPA pharmacokinetics.
The co-medication of drugs, such as carbamazepine, is another important determinant of the inter-individual pharmacokinetic variability of VPA [36]. Taking into account that the genetic polymorphisms of UGTs may influence drug–drug interactions [37, 38], we sub-grouped the patients co-medicated with carbamazepine based on their UGT genotypes to determine the possible influence of UGT polymorphisms on carbamazepine–VPA interactions. Our results showed no significant difference among the differently genotyped subgroups, suggesting that the carbamazepine–VPA pharmacokinetic interaction might not be influenced by UGT genotype.
In conclusion, our results demonstrate that the genotype distribution of UGT1A3 in a population of Chinese epilepsy patients was significant different from that in Chinese healthy subjects. Based on a detailed genotype-to-phenotype assay of all three major UGT isozymes involved in VPA metabolism, including UGT1A3, UGT1A6, and UGT2B7, we found that only UGT1A3*5 is a potential genetic determinant leading to the reduced plasma levels of VPA in our Chinese epilepsy patients. The results obtained from this study suggest that it may be necessary to increase VPA dose for UGT1A3*5 carriers to maintain its therapeutic range of 50–100 μg/mL.
References
Rogawski MA, Loscher W (2004) The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med 10:685–692
Kuendgen A, Gattermann N (2007) Valproic acid for the treatment of myeloid malignancies. Cancer 110:943–954
Patsalos PN, Perucca E (2003) Clinically important drug interactions in epilepsy: interactions between antiepileptic drugs and other drugs. Lancet Neurol 2:473–481
Nakajima Y, Mizobuchi M, Nakamura M et al (2004) Mechanism of the drug interaction between valproic acid and carbapenem antibiotics in monkeys and rats. Drug Metab Dispos 32:1383–1391
Perucca E (2006) Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 61:246–255
Chung JY, Cho JY, Yu KS et al (2008) Pharmacokinetic and pharmacodynamic interaction of lorazepam and valproic acid in relation to UGT2B7 genetic polymorphism in healthy subjects. Clin Pharmacol Ther 83:595–600
Anderson GD (1998) A mechanistic approach to antiepileptic drug interactions. Ann Pharmacother 32:554–563
Zaccara G, Messori A, Moroni F (1988) Clinical pharmacokinetics of valproic acid—1988. Clin Pharmacokinet 15:367–389
Reith DM, Andrews J, Parker-Scott S et al (2000) Urinary excretion of valproate metabolites in children and adolescents. Biopharm Drug Dispos 21:327–330
Dickinson RG, Hooper WD, Dunstan PR et al (1989) Urinary excretion of valproate and some metabolites in chronically treated patients. Ther Drug Monit 11:127–133
Argikar UA, Remmel RP (2009) Effect of aging on glucuronidation of valproic acid in human liver microsomes and the role of UDP-glucuronosyltransferase UGT1A4, UGT1A8, and UGT1A10. Drug Metab Dispos 37:229–236
Burchell B, Brierley CH, Monaghan G et al (1998) The structure and function of the UDP-glucuronosyltransferase gene family. Adv Pharmacol 42:335–338
Mackenzie PI, Owens IS, Burchell B et al (1997) The UDP-glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 7:255–269
Ethell BT, Anderson GD, Burchell B (2003) The effect of valproic acid on drug and steroid glucuronidation by expressed human UDP-glucuronosyltransferases. Biochem Pharmacol 65:1441–1449
Green MD, King CD, Mojarrabi B et al (1998) Glucuronidation of amines and other xenobiotics catalyzed by expressed human UDP-glucuronosyltransferase 1A3. Drug Metab Dispos 26:507–512
Krishnaswamy S, Hao Q, Al-Rohaimi A et al (2005) UDP glucuronosyltransferase (UGT) 1A6 pharmacogenetics: II. Functional impact of the three most common nonsynonymous UGT1A6 polymorphisms (S7A, T181A, and R184S). J Pharmacol Exp Ther 313:1340–1346
Caillier B, Lepine J, Tojcic J et al (2007) A pharmacogenomics study of the human estrogen glucuronosyltransferase UGT1A3. Pharmacogenet Genomics 17:481–495
Chen Y, Chen S, Li X et al (2006) Genetic variants of human UGT1A3: functional characterization and frequency distribution in a Chinese Han population. Drug Metab Dispos 34:1462–1467
Riedmaier S, Klein K, Hofmann U et al (2010) UDP-glucuronosyltransferase (UGT) polymorphisms affect atorvastatin lactonization in vitro and in vivo. Clin Pharmacol Ther 87:65–73
Kodama Y, Koike Y, Kimoto H et al (1992) Binding parameters of valproic acid to serum protein in healthy adults at steady state. Ther Drug Monit 14:55–60
Kagaya H, Inoue K, Miura M et al (2007) Influence of UGT1A8 and UGT2B7 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. Eur J Clin Pharmacol 63:279–288
Iwuchukwu OF, Ajetunmobi J, Ung D et al (2009) Characterizing the effects of common UDP glucuronosyltransferase (UGT) 1A6 and UGT1A1 polymorphisms on cis- and trans-resveratrol glucuronidation. Drug Metab Dispos 37:1726–1732
Tan L, Yu JT, Sun YP et al (2010) The influence of cytochrome oxidase CYP2A6, CYP2B6, and CYP2C9 polymorphisms on the plasma concentrations of valproic acid in epileptic patients. Clin Neurol Neurosurg 112:320–323
Iwai M, Maruo Y, Ito M et al (2004) Six novel UDP-glucuronosyltransferase (UGT1A3) polymorphisms with varying activity. J Hum Genet 49:123–128
Ehmer U, Vogel A, Schutte JK et al (2004) Variation of hepatic glucuronidation: novel functional polymorphisms of the UDP-glucuronosyltransferase UGT1A4. Hepatology 39:970–977
Xing Y, Yang L, Wang L et al (2009) Systematic screening for polymorphisms within the UGT1A6 gene in three Chinese populations and function prediction through structural modeling. Pharmacogenomics 10:741–752
Saeki M, Saito Y, Jinno H et al (2005) Genetic polymorphisms of UGT1A6 in a Japanese population. Drug Metab Pharmacokinet 20:85–90
Lampe JW, Bigler J, Horner NK et al (1999) UDP-glucuronosyltransferase (UGT1A1*28 and UGT1A6*2) polymorphisms in Caucasians and Asians: relationships to serum bilirubin concentrations. Pharmacogenetics 9:341–349
Menard V, Girard H, Harvey M et al (2009) Analysis of inherited genetic variations at the UGT1 locus in the French-Canadian population. Hum Mutat 30:677–687
Lin GF, Guo WC, Chen JG et al (2005) An association of UDP-glucuronosyltransferase 2B7 C802T (His268Tyr) polymorphism with bladder cancer in benzidine-exposed workers in China. Toxicol Sci 85:502–506
Saeki M, Saito Y, Jinno H et al (2006) Haplotype structures of the UGT1A gene complex in a Japanese population. Pharmacogenomics J 6:63–75
Kohle C, Mohrle B, Munzel PA et al (2003) Frequent co-occurrence of the TATA box mutation associated with Gilbert's syndrome (UGT1A1*28) with other polymorphisms of the UDP-glucuronosyltransferase-1 locus (UGT1A6*2 and UGT1A7*3) in Caucasians and Egyptians. Biochem Pharmacol 65:1521–1527
Maruo Y, Iwai M, Mori A et al (2005) Polymorphism of UDP-glucuronosyltransferase and drug metabolism. Curr Drug Metab 6:91–99
Burchell B (2003) Genetic variation of human UDP-glucuronosyltransferase: implications in disease and drug glucuronidation. Am J Pharmacogenomics 3:37–52
Kwara A, Lartey M, Boamah I et al (2009) Interindividual variability in pharmacokinetics of generic nucleoside reverse transcriptase inhibitors in TB/HIV-coinfected Ghanaian patients: UGT2B7*1c is associated with faster zidovudine clearance and glucuronidation. J Clin Pharmacol 49:1079–1090
Gutierrez K, Walter H, Bankier B (1999) Valproic acid and Carbamazepine: a successful antipsychotic medication? The problem of diagnosis and its relevance for therapy. Psychopathology 32:235–241
Chung JY, Cho JY, Yu KS et al (2005) Effect of the UGT2B15 genotype on the pharmacokinetics, pharmacodynamics, and drug interactions of intravenous lorazepam in healthy volunteers. Clin Pharmacol Ther 77:486–494
Kiang TK, Ensom MH, Chang TK (2005) UDP-glucuronosyltransferases and clinical drug–drug interactions. Pharmacol Ther 106:97–132
Funding
This work was supported by the National Natural Science Foundation (Grants 91029746, 81072695), Jiangshu province Natural Science Foundation (Grant BK 2010066), and a funding for innovative research team in institutions of Jiangsu higher education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiao-Man Chu and Li-Fang Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 113 kb)
Rights and permissions
About this article
Cite this article
Chu, XM., Zhang, LF., Wang, GJ. et al. Influence of UDP-glucuronosyltransferase polymorphisms on valproic acid pharmacokinetics in Chinese epilepsy patients. Eur J Clin Pharmacol 68, 1395–1401 (2012). https://doi.org/10.1007/s00228-012-1277-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1277-7