Abstract
Purpose
To derive estimates of CYP1A2 abundance as a function of daily cigarette consumption and use these values to predict the clearances of CYP1A2 substrates in smokers.
Methods
Smoking-induced changes in hepatic CYP1A2 abundance were extrapolated from reported in vivo caffeine clearance data for sub-groups of a smoking population that were categorized according to their daily cigarette consumption. These abundance values together with in vitro–in vivo extrapolation (IVIVE) within the Simcyp population-based Simulator were used to predict the clearances of caffeine, theophylline, and clozapine in smokers. The model was used subsequently to predict differences in oral clearance between smoker and non-smoker cohorts in a Phase 1 clinical trial involving PF-2400013, a drug metabolized by CYP1A2.
Results
Estimated hepatic CYP1A2 abundance values were 52, 64, 79, 90, and 94 pmol/mg microsomal protein for subjects smoking 0, 1–5, 6–10, 11–20, and >20 cigarettes/day respectively. Predicted -fold increases in oral clearance of caffeine, theophylline and clozapine in smokers relative to non-smokers were consistent with observed data. The validated model was able to recover the smoking-induced increase in oral clearance of PF-2400013; predicted and observed mean (CV%) values in male nonsmokers and smokers were 90 L/h (40%) and 141 L/h (34%) respectively, and 100 L/h (58%) and 131 L/h (33%) respectively.
Conclusions
This study demonstrates that it may be possible to predict the clearance of CYP1A2 substrates in smoking populations using quantitative estimates of CYP1A2 abundance based on daily cigarette consumption in conjunction with an IVIVE approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Human cytochrome P450 1A2 (CYP1A2) is the main enzyme involved in the metabolism of many clinically important drugs, including clozapine, olanzapine, theophylline, flutamide, lidocaine, tacrine, and caffeine [1]. CYP1A2 is a member of the CYP1A superfamily and accounts for approximately 15% of total hepatic P450. It is highly inducible by 3-methylcholanthrene-like inducers (e.g., polycyclic aromatic hydrocarbons, heterocyclic aromatic amines, and dioxins) through the aryl hydrocarbon receptor/Ah receptor nuclear translocator (AhR/Arnt) induction mechanism [2].
The high degree of inter-individual variability of CYP1A2 expression and activity in human liver is probably due to both genetic and environmental factors [3, 4]. Although more than 20 CYP1A2 allelic variants have been identified (www.cypalleles.ki.se/cyp1a2.htm), the existence of clinically relevant genetic polymorphisms, especially those that confer a significant reduction in enzyme activity, is still controversial [5]. Pharmacogenetic studies investigating the population distribution of CYP1A2 metabolic activity have reported conflicting results. Unlike polymorphic enzymes such as CPY2D6 or CYP2C19, no reproducible multimodal distribution patterns have been observed for CYP1A2 [6–8]. In a recent study, CYP1A2*1 F, CYP1A2*1 D, and CYP1A2*1 C polymorphisms in smokers were found to influence CYP1A2 activity, but had no effect on CYP1A2 inducibility [9]. Although CYP1A2 polymorphisms may have some influence on CYP1A2 activity, it appears that a number of other host-dependent and environmental factors may be more important in determining CYP1A2 expression; these include gender, BMI, oral contraceptive use, alcohol consumption, caffeine intake, diets containing cruciferous vegetables or grilled meat, and cigarette use [1, 3, 5].
The polycyclic aromatic hydrocarbon fraction of cigarette smoke is known to induce hepatic CYP1A2 activity in smokers, which can lead to enhanced clearance and altered plasma concentrations of drugs metabolized primarily by CYP1A2 [1, 10, 11]. The clinical implication of this is reduced drug efficacy. Alternatively, in the case of smoking cessation, toxicity due to elevated drug concentrations may occur as a result of the CYP1A2 abundance returning to constitutively expressed levels [5, 12]. This is of particular importance for the antipsychotic drugs clozapine and olanzapine, which have narrow therapeutic indices [11, 13].
Although there are no guidelines specific to the assessment of the pharmacokinetics of drugs in a smoking population, regulatory authorities in both the USA (CDER) [14] and Europe (EMEA) [15] recommend investigating the impact of extrinsic factors (including smoking) on the pharmacokinetics of drugs. Semi-mechanistic physiologically based pharmacokinetic (PBPK) models that account for changes in metabolism, blood flow, organ size, and excretion have been developed for other special populations, including those with cirrhosis to predict the impact of liver impairment on the clearance of drugs [16, 17]. It may be possible to extend this approach to a smoking population to predict the change in drug exposure in smokers vs nonsmokers and if necessary, facilitate the design of more efficient clinical trials. Quantitative assessment of CYP1A2 induction in smokers is essential for evaluating the impact of smoking on the pharmacokinetics of drugs metabolized by this enzyme. Thus, the main aim of this study was to derive estimates of hepatic CYP1A2 abundance as a function of daily cigarette consumption. In order to validate the model, these values were used together with mechanistic in vitro–in vivo extrapolation (IVIVE) within the Simcyp population-based Simulator (version 8.2) to perform a comparison of predicted and observed oral clearances of caffeine, theophylline, and clozapine in smokers. Once validated, the model was used to predict the kinetic differences between smokers and nonsmokers for an in-house compound (PF-2400013).
Materials and methods
In vitro–in vivo extrapolation
Prediction of drug clearance from in vitro enzyme kinetic data is performed in two steps and has been described in detail previously [18, 19]. Initially, intrinsic clearance per unit enzyme is converted to a whole organ intrinsic metabolic clearance using relevant scaling parameters (enzyme abundance—the amount (pmol P450) per milligram of microsomal protein; MPPGL—the amount (mg) of microsomal protein per gram of liver; and the liver weight). This value is then combined with binding parameters and liver blood flow to extrapolate to whole organ clearance.
Population parameters
The Simcyp population-based Simulator allows facile extrapolation of in vitro enzyme kinetic data to predict pharmacokinetic changes in vivo in virtual populations. Genetic, physiological, and demographic variables relevant to the prediction of clearance are generated for each individual using correlated Monte Carlo methods and equations derived from population databases are obtained from literature sources [20]. The equations relating height to age, weight to height, body surface area (BSA) to height and weight, liver size to BSA, cardiac output to age and BSA, and liver blood flow to cardiac output have been described previously [19, 21]. Key parameters of the clearance prediction model include enzyme abundance [22], MPPGL [23], liver volume [24], plasma protein levels, hematocrit, and liver blood flow, and default values are available in a north European Caucasian population [19, 21]. Although extensive reviews of the literature were conducted to obtain corresponding values in a smoking population, limited data were found.
Estimation of hepatic CYP1A2 abundance
The default hepatic CYP1A2 abundance of 52 pmol/mg microsomal protein (coefficient of variability [CV] = 67%) in a north European Caucasian population was based on a meta-analysis of four independent studies in the literature with data on 119 liver samples from Caucasian subjects who were nonsmokers [22]. Although there were no reports of absolute or relative abundance data for CYP1A2 in hepatocytes or human liver microsomes derived from the livers of smokers, activity data were available [25]. However, the smoking history of the donors of the livers was not always well characterized. Therefore, estimates of CYP1A2 abundance were derived from an in vivo study where caffeine clearance was determined in 401 nonsmokers and 385 smokers [26]. Daily cigarette consumption was determined to be an important covariate of caffeine clearance and accounted for increased clearance in male smokers relative to male nonsmokers; values were 1.22-, 1.47-, 1.66-, and 1.72-fold for subjects consuming 1–5, 6–10, 11–20, and >20 cigarettes/day respectively. These data were the basis for estimating hepatic CYP1A2 abundance as a function of daily cigarette consumption.
For a population (n = 100) of equal numbers of male and female nonsmokers (age: 25–60 years, body weight: 35–140 kg), the mean caffeine clearance using default parameters of the compound file in the Simcyp Simulator (version 8.2) was predicted to be 5.8 L/h. This value was adjusted by the relative change in caffeine clearance for each of the smoker subgroups reported by Tantcheva-Poór et al. [26]. Assuming a bioavailability of 1.0 [27] and negligible renal clearance, the clearance values were converted to a total unbound hepatic metabolic intrinsic clearance CLuint,H (L/h) using the “reverse” well-stirred equation (Eq. 1):
where CLH,B is the hepatic blood clearance (L/h), QH is hepatic blood flow (90 L/h), and fuB is the unbound fraction of caffeine in the blood (0.69). The resultant CLuint,H was converted to units of mL/min/kg using the average population body weight of 74 kg. The latter was not corrected for the proportion cleared via CYP1A2 as it was approximately 1. A key assumption of the model was that the main impact of smoking was increased expression of CYP1A2 [2]. Hence, ratios of the CYP1A2-mediated CLuint,H values for smokers relative to nonsmokers were applied directly to the default CYP1A2 abundance in the Simcyp Simulator (52 pmol/mg microsomal protein) to derive the corresponding values for each of the cigarette consumption subgroups (Table 1). Values of CV for CYP1A2 abundance in the subgroups of smokers were estimated from the product of the corresponding CV in nonsmokers (67%) and the ratio of caffeine clearance for each sub-group relative to that of nonsmokers.
Model validation
Studies involving estimation of clearance of the CYP1A2 substrates, caffeine, clozapine, and theophylline in smokers were identified from the literature. Simulations were run using default values for the compound files within the Simcyp Simulator (version 8.2) and the static model to predict the clearances of the three substrates in a north European Caucasian population (default) and a smoking population. To ensure that the characteristics of the virtual subjects were matched closely to those of the subjects studied in vivo, numbers, age range, and gender ratios were replicated in the simulations. For each simulation 10 separate trials were generated to assess variability across groups. Predicted clearances and -fold increases in the clearance of smokers for each subgroup relative to nonsmokers were compared with reported data.
Simulation of caffeine clearance in smokers and nonsmokers
Simulations based on the in vivo study described by Tantcheva-Poór et al. [26] were run to ensure that the derived CYP1A2 abundances based on daily cigarette consumption were able to recover the observed clearance data in each of the four sub-groups. A second dataset (Terziivanov et al. [28]), including 20 nonsmokers and 14 smokers, was used to validate the smoking model. Because of the small number of smokers in this study, it was not feasible to stratify the smokers into the same daily cigarette consumption groups as shown in Table 1. Therefore, the following categories were assigned; nonsmokers (n = 20), light smokers (<10 cigarettes/day, n = 5), heavy smokers (>10 cigarettes/day, n = 9), and all smokers (any cigarette consumption, n = 14). CYP1A2 abundance values of 52, 64, 94, and 94 pmol/mg microsomal protein were used for each group respectively.
Simulation of theophylline clearance in smokers and nonsmokers
Smoking-induced changes in the clearance of theophylline were evaluated using datasets from Hunt et al. [29], Gardner et al. [30], and Jennings et al. [31]. Individual subject data were not reported in the former two studies. Therefore, these data were extracted from the original reference figures using DigitizeIt (version 1.5.8) and descriptive statistics were generated using Origin (version 7.5). Based on the small number of subjects and limited cigarette consumption history in these studies, it was only possible to simulate nonsmoker and heavy smoker groups (>1–2 packs/day); values of 52 (CV, 67%) and 94 (CV, 43%) pmol/mg microsomal protein respectively were used for CYP1A2 abundance.
Simulation of steady-state clozapine concentrations in smokers and nonsmokers
Smoking-induced changes in the steady-state plasma concentrations of clozapine were evaluated using datasets from Seppella et al. [32], Rostami et al. [33], and Palego et al. [34]. The literature reports represent a diverse range of study designs, subject demographics, and daily doses (see Table 4). To allow for a cross-study comparison of differences in average steady-state plasma clozapine concentration (Css) between nonsmokers and smokers, concentration units were normalized for dose and body weight to common units of μg/mL/mg/kg/day. All concentration data represent plasma samples taken approximately 12 h after the last dose. Clozapine pharmacokinetics for nonsmokers and heavy smokers (>20 cigarettes/day) were simulated as 10 trials of 10 subjects with an equal number of men and women receiving 6.0 mg/kg/day. The average age and body weight (range) of the simulated population subjects were 28.6 years (18–78 years) and 75 kg (44–125 kg) respectively. CYP1A2 abundance values of 52 and 94 pmol/mg microsomal protein were used for nonsmokers and smokers respectively. The predicted percentage decreases in Css values of clozapine were compared with observed data.
Model application
Model input parameters of PF-2400013
PF-2400013 is a selective 5-HT2c agonist that was under development for the treatment of schizophrenia. In vitro metabolism studies with human liver microsomes and specific chemical inhibitors of cytochrome P450s indicate that PF-2400013 is a substrate of both CYP1A2 and CYP3A, with an estimated fractional metabolism (fm) through these pathways of 0.45 and 0.55 respectively. The compound-specific model input parameters for PF-2400013 are listed in Table 2.
Clinical study design: PF-2400013
Based on the significant contribution of CYP1A2 to the metabolism of PF-2400013, the potential impact of smoking on CYP1A2 induction to affect the exposure of PF-2400013 was investigated as part of a Phase 1 single-dose safety and toleration clinical study. The trial was an investigator- and subject-blind (sponsor open) crossover study of within subject ascending single oral doses of PF-2400013 with randomized placebo substitution. Only the study details for the smoker and nonsmoker comparator cohorts are described here.
Eleven subjects (5 nonsmokers and 6 smokers) were given a single 50-mg oral dose of PF-2400013 under fasted conditions. The mean (range) age and body weight of the nonsmoker group were 32 years (21–38 years) and 87 kg (72–96 kg) respectively. The mean (range) age and body weight of the smoker group were 35 years (23–45 years) and 72 kg (60–81 kg) respectively and consumed an average of 11.3 cigarettes/day (5–20 cigarettes/day). Blood samples for pharmacokinetic analysis were collected on day 1 at hour 0 (just prior to dosing) and 0.5, 1, 1.5, 2, 3, 4, 8, 12, 24, 48, 72, and 96 h following dosing. The resulting serum was analyzed for PF-2400013 concentrations using a validated LC/MS method. Individual serum concentration–time data for PF-2400013 were analyzed by standard noncompartmental methods. This study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Conference on Harmonization (ICH) Good Clinical Practice (GCP) Guidelines and following all local regulatory requirements.
Prediction of PF-2400013 clearance in smokers and nonsmokers
Oral clearance values of PF-2400013 for nonsmokers and smokers (5–20 cigarettes/day) were simulated as 10 trials of 5 or 6 male subjects respectively, receiving a single oral dose of 50 mg. CYP1A2 abundance values of 52 and 94 pmol/mg microsomal protein were used for nonsmokers and smokers respectively.
Power of detecting clearance differences of PF-2400013 in smokers and nonsmokers
Using the derived hepatic CYP1A2 abundance values and associated variability for nonsmokers and smokers consuming 1–5, 6–10, 11–20 and >20 cigarettes/day, simulations were performed for 1,000 subjects in each group for a hypothetical compound that was cleared exclusively by hepatic metabolism and had an fmCYP1A2 = 0.45. A statistical comparison of the predicted clearance values for each of the smoker groups compared with nonsmokers was performed using Origin V7.5 (OriginLab Corp, Northampton, MA, USA) and were then applied to estimate the power of hypothetical sample sizes of 5, 10, 25, 50, 100, and 200 subjects at a significance level (α) of 0.05. A second set of simulations was used to explore hypothetical compounds that were cleared exclusively by hepatic metabolism and had fmCYP1A2 values of 0.1, 0.2, 0.45, and 0.9. Simulations performed for 1,000 subjects in each fmCYP1A2 group and a statistical comparison of the predicted clearance values for nonsmokers and heavy smokers (>20 cigarettes/day) using Origin version 7.5 were then applied to estimate the power of hypothetical sample sizes of 5, 10, 25, 50, 100, and 200 subjects at a significance level (α) of 0.05.
Results
Caffeine clearance in smokers and nonsmokers
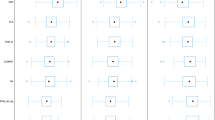
Simulations based on the in vivo study described by Tantcheva-Poór et al. [26] confirmed that the derived CYP1A2 abundances were able to recover the observed clearance data in each of the four sub-groups (Table 3). As expected, predicted -fold increases in the clearance of smokers for each subgroup relative to nonsmokers were also consistent with in vivo data (Fig. 1; Table 3).
Caffeine clearance in smokers as a function of daily cigarette consumption. The bar represents the upper and lower quartiles, median, mean (square), and 90% confidence interval (whiskers) of data from 10 replicate trials using a study design that was consistent with the original published reference. The filled circles represent the mean observed caffeine clearance reported or derived from data within the publication by Tantcheva-Poór et al. [26]
Predicted clearances, including associated variability over the 90% confidence intervals (Table 3; Fig. 2), and -fold increases (Table 3) in caffeine clearance for heavy smokers and all smokers relative to nonsmokers were in good agreement with observed data reported by Terziivanov et al. [28]. However, for light smokers, both parameters were over-predicted by 1.5-fold when compared with corresponding in vivo data (Table 3).
Predicted (open bars) and observed (hatched bars) caffeine clearance and associated variability for nonsmokers, light smokers, and heavy smokers (Terziivanov et al. [28]). Results are displayed as box-and-whisker plots where the bar represents the upper and lower quartiles, median, mean (squares), and 90% confidence interval (whiskers) of data from 10 replicate trials using a study design consistent with the original published reference
Theophylline clearance in smokers and nonsmokers
Although absolute values of theophylline clearance for both non-smokers and smokers were generally over-predicted compared with corresponding data in each subgroup of the three in vivo studies, -fold increases in clearance for the smokers relative to nonsmokers were more consistent with reported data (Table 3). However, the latter values were variable, ranging from 1.4- to 2.3-fold. Larger deviations between observed and predicted data occurred in studies where a relatively small number of subjects were used.
Steady-state clozapine concentrations in smokers and nonsmokers
Predicted steady-state concentrations of clozapine in smokers and nonsmokers and observed data for three independent studies are presented in Table 4. Predicted values adjusted for dose and body weight were 0.083 and 0.131 μg/mL/mg/kg for smokers and nonsmokers respectively. Across the three studies, steady-state clozapine concentrations ranged from 0.057 to 0.068 μg/mL/mg/kg for smokers and 0.086 to 0.115 μg/mL/mg/kg for non-smokers. The relative difference in steady-state clozapine concentrations was predicted to be −36.8% for smokers compared with nonsmokers and is consistent with reported differences for three literature studies that ranged from −33.6 to −40.1%.
Clearance of PF-2400013 in smokers and nonsmokers
Although the difference was not statistically significant, the mean oral clearance of PF-2400013 in male smokers was higher than that observed in nonsmokers; values were 130 and 100 L/h respectively (Fig. 3). Similarly, predicted mean oral clearances of PF-2400013 in male smokers and non-smokers were 141 and 100 L/h (Fig. 3). Although the mean and median values of predicted and observed clearances were similar for the respective groups, the variability in the smokers was under-predicted when compared with observed data (Fig. 3).
Predicted (open bars) and observed (hatched bars) clearance of PF-2400013 and associated variability for smokers and nonsmokers. Results are displayed as box-and-whisker plots where the bar represents the upper and lower quartiles, median, mean (squares) and 90% confidence intervals (whiskers) of data from 10 replicate trials using a study design consistent with the clinical study
Discussion
Application of smoking-induced changes in hepatic CYP1A2 abundance as a function of daily cigarette consumption together with mechanistic IVIVE within the Simcyp Simulator allowed the prediction of changes in oral clearance from in vitro data with reasonable accuracy for three different CYP1A2 probe substrates, namely, caffeine, theophylline, and clozapine. Once validated, the model was used retrospectively to estimate the increase in oral clearance of PF-2400013, a drug that had a significant contribution of CYP1A2 to its metabolism. Despite the fact that the latter was lower than is accepted for that of a probe drug (fmCYP1A2 of 0.45 versus 0.9), the model was able to capture with reasonable accuracy, differences in the mean value of oral clearance between smokers and non-smokers (130 versus 100 L/h). Although this difference was not statistically significant, a trend was observed. As the impact of smoking on clearance was not the primary aim, the clinical study was not powered sufficiently to detect a difference in clearance between the small number of smokers and non-smokers that were included as part of the sub-study.
The ability to detect an increase in drug clearance in smokers due to CYP1A2 induction is a function of the number of cigarettes smoked per day, the fmCYP1A2 in the non-induced state and the number of subjects studied (Fig. 4). For a compound cleared exclusively by metabolism with an fmCYP1A2 of 0.45, the power to detect an effect on clearance in smokers increases with cigarette consumption (Fig. 4: top panel). Light smokers (1–5/day) are not expected to exhibit altered clearance even for substrates with a very high fmCYP1A2 (>0.9; Fig. 4: bottom panel). In heavy smokers (>20 cigarettes/day) the greatest increase in clearance relative to nonsmokers will be observed for compounds with a high fmCYP1A2, thus requiring fewer subjects to detect the effect (Fig. 4: bottom panel). Figure 4 illustrates quite clearly that a prospective study involving more subjects than were used in the clinical study described in this report is required to determine the impact of smoking on the clearance of PF-240013. Application of the estimates of hepatic CYP1A2 abundance derived in the current study to other substrates, can be considered as long as daily cigarette consumption is well characterized and the extent of CYP1A2 contribution to overall clearance (fmCYP1A2) is known.
The ability to detect an effect of increased drug clearance due to CYP1A2 induction is a function of both the number of cigarettes smoked per day (top panel), the fractional total clearance due to CYP1A2 metabolism (fmCYP1A2) in the non-induced state (lower) and the number of subjects studied. For a hypothetical compound cleared exclusively by metabolism with an fmCYP1A2 = 0.45, the power to detect an effect on clearance in smokers increases with cigarette consumption (top panel). In heavy smokers (>20 cigarettes/day) the greatest increase in clearance relative to nonsmokers will be seen for compounds with a high fmCYP1A2, thus requiring fewer subjects to detect the effect (lower panel)
The estimates of CYP1A2 abundance were based on data from a single literature study where quantitative assessment of the contributors to the high variability in CYP1A2 in vivo was investigated using a saliva-based caffeine test in 863 healthy Caucasians, including 385 smokers [26]. Caffeine clearance values were estimated from molar concentration ratios of paraxanthine to caffeine measured in saliva prior to and 5–7 h after a test dose of caffeine. Theoretical analysis has confirmed that the latter is an even more stable marker of CYP1A2 activity than any of the urine ratios that are often used [35]. Daily cigarette consumption was determined to be a significant covariate of oral caffeine clearance and accounted for increased clearance in smokers relative to nonsmokers; values were 1.22-, 1.47-, 1.66-, and 1.72-fold for subjects consuming 1–5, 6–10, 11–20, and >20 cigarettes/day respectively [26]. These values were used to derive the estimates of hepatic CYP1A2 abundance based on daily cigarette consumption in smokers relative to nonsmokers. As the number of subjects in each sub-group of smokers ranged from 70 to 140, it is likely that estimates of the abundance are representative of a population. A more recent study has reported that in smokers undergoing de-induction at the end of a 4-week smoking cessation study, inducibility, as measured by the ratio of plasma concentrations of paraxanthine to caffeine at week 0 relative to week 4 was 1.46 (1.15–1.65) and 1.67 (1.35–1.98) for subjects consuming 10–19 and 20–29 cigarettes per day respectively [9]. These induction values were consistent with those reported by Tantcheva-Poór et al. [26], which were used in the current analysis for extrapolation to estimates of CYP1A2 abundance based on daily cigarette consumption. Smokers consuming less than 10 cigarettes per day were not evaluated in the more recent study.
One of the main limitations of the current study is that it was not possible to assess the robustness of the abundance values derived for each of the sub-categories using the “validation set” of clinical studies that were identified in the literature or that involving PF-2400013. The accuracy of any prediction is also dependent on the quality of the in vivo data that is being recovered. Because of the logistical problems in recording accurate smoking histories of subjects recruited into drug studies, only a limited number of subjects could be assigned to the sub-categories, particularly those involving “light smokers.” Therefore, with the exception of the study reported by Terziivanov et al. [28] subjects were categorized as nonsmokers or smokers.
Potential changes in protein binding or liver blood flow due to smoking were not considered when extrapolating the in vivo caffeine clearance data back to estimates of hepatic CYP1A2 abundance. However, as the fu value of caffeine is 0.68 and the extraction ratio is low, it is likely that any changes in protein binding or liver blood flow due to smoking will have a negligible impact on the clearance. Similarly, these factors were not accounted for during IVIVE, as the data were not available in the literature.
The induction of CYP1A2 by heavy coffee consumption in Serbs and Swedes was reported recently [36]. It was suggested by the authors that polycyclic aromatic hydrocarbons, present in roasted coffee beans, are responsible for the effect. Among nonsmokers and non-oral contraceptive users, consumption of at least three cups of coffee per day led to significant increases in CYP1A2 activity in Serbs (mean difference 0.11; 95% CI of the mean difference 0.04, 0.18; p = 0.003) and Swedes (mean difference 0.07; 95% CI of the mean difference 0.01, 0.12; p = 0.02). It may be possible to extend the approach presented in the current paper, to estimate changes in hepatic CYP1A2 abundance as a function of daily coffee consumption.
Although there are no guidelines specific to the assessment of the pharmacokinetics of drugs in a smoking population, regulatory authorities, including the FDA and EMEA recommend investigating the impact of extrinsic factors (including smoking) on the pharmacokinetics of drugs. Despite the obvious limitations of the current model to investigate the impact of smoking on the clearance of drugs metabolized by CYP1A2, it may still provide a useful tool to help in the design of clinical studies during drug development and clinically, in the assessment of likely dosage adjustment in smokers. Alternatively, in the case of smoking cessation, where toxicity due to elevated drug concentrations may occur as a result of the CYP1A2 abundance returning to constitutively expressed levels, dosage adjustment may be required.
References
Faber MS, Jetter A, Fuhr U (2005) Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin Pharmacol Toxicol 97(3):125–134
Ma Q, Lu AY (2003) Origins of individual variability in P4501A induction. Chem Res Toxicol 16(3):249–260
Gunes A, Dahl ML (2008) Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics 9(5):625–637
Schweikl H, Taylor JA, Kitareewan S, Linko P, Nagorney D, Goldstein JA (1993) Expression of CYP1A1 and CYP1A2 genes in human liver. Pharmacogenetics 3(5):239–249
Carrillo JA, Herraiz AG, Ramos SI, Gervasini G, Vizcaino S, Benitez J (2003) Role of the smoking-induced cytochrome P450 (CYP)1A2 and polymorphic CYP2D6 in steady-state concentration of olanzapine. J Clin Psychopharmacol 23(2):119–127
Obase Y, Shimoda T, Kawano T, Saeki S, Tomari SY, Mitsuta IK, Matsuse H, Kinoshita M, Kohno S (2003) Polymorphisms in the CYP1A2 gene and theophylline metabolism in patients with asthma. Clin Pharmacol Ther 73(5):468–474
Sachse C, Bhambra U, Smith G, Lightfoot TJ, Barrett JH, Scollay J, Garner RC, Boobis AR, Wolf CR, Gooderham NJ, Colorectal Cancer Study G (2003) Polymorphisms in the cytochrome P450 CYP1A2 gene CYP1A2 in colorectal cancer patients and controls: allele frequencies, linkage disequilibrium and influence on caffeine metabolism. Br J Clin Pharmacol 55(1):68–76
Van der Weide J, Steijns LS, van Weelden MJ (2003) The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics 13(3):169–172
Dobrinas M, Cornuz J, Oneda B, Kohler Serra M, Puhl M, Eap CB (2011) Impact of smoking, smoking cessation, and genetic polymorphisms on CYP1A2 activity and inducibility. Clin Pharmacol Ther 90 (1):117–125
Bondolfi G, Morel F, Crettol S, Rachid F, Baumann P, Eap CB (2005) Increased clozapine plasma concentrations and side effects induced by smoking cessation in 2 CYP1A2 genotyped patients. Therapeutic Drug Monit 27(4):539–543
Haslemo T, Eikeseth PH, Tanum L, Molden E, Refsum H (2006) The effect of variable cigarette consumption on the interaction with clozapine and olanzapine. Eur J Clin Pharmacol 62(12):1049–1053
McCarthy RH (1994) Seizures following smoking cessation in a clozapine responder. Pharmacopsychiatry 27(5):210–211
Bigos KL, Pollock BG, Coley KC, Miller DD, Marder SR, Aravagiri M, Kirshner MA, Schneider LS, Bies RR (2008) Sex, race, and smoking impact olanzapine exposure. J Clin Pharmacol 48(2):157–165
FDA (2003) Guidance document: exposure-response relationships—study design, data analysis and final regulatory applications
EMA (2010) Draft guidance on drug interactions
Edginton AN, Willmann S (2008) Physiology-based simulations of a pathological condition: prediction of pharmacokinetics in patients with liver cirrhosis. Clin Pharmacokinet 47(11):743–752
Johnson TN, Boussery K, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A (2010) A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet 49(3):189–206
Houston JB (1994) Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem Pharmacol 47(9):1469–1479
Howgate EM, Rowland Yeo K, Proctor NJ, Tucker GT, Rostami-Hodjegan A (2006) Prediction of in vivo drug clearance from in vitro data. I. Impact of inter-individual variability. Xenobiotica 36(6):473–497
Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A (2009) The Simcyp population-based ADME simulator. Expert Opinion Drug Metab Toxicol 5(2):211–223
Jamei M, Dickinson GL, Rostami-Hodjegan A (2009) A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: A tale of ‘bottom-up’ vs ‘top-down’ recognition of covariates. [Review] [150 refs] [Erratum appears in Drug Metab Pharmacokinet. 2009;24(5):488]. Drug Metab Pharmacokinet 24(1):53–75
Rowland-Yeo K, Rostami-Hodjegan A, Tucker GT (2004) Abundance of cytochrome P450 in human liver: a meta-analysis. Br J Clin Pharmacol 57(5):687
Barter ZE, Bayliss MK, Beaune PH, Boobis AR, Carlile DJ, Edwards RJ, Houston JB, Lake BG, Lipscomb JC, Pelkonen OR, Tucker GT, Rostami-Hodjegan A (2007) Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: reaching a consensus on values of human microsomal protein and hepatocellularity per gram of liver. [Review] [80 references]. Curr Drug Metabol 8(1):33–45
Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A (2005) Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl 11(12):1481–1493
Parkinson A, Mudra DR, Johnson C, Dwyer A, Carroll KM (2004) The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol 199(3):193–209
Tantcheva-Poór I, Zaigler M, Rietbrock S, Fuhr U (1999) Estimation of cytochrome P-450 CYP1A2 activity in 863 healthy Caucasians using a saliva-based caffeine test. Pharmacogenetics 9(2):131–144
Blanchard J, Sawers SJ (1983) The absolute bioavailability of caffeine in man. Eur J Clin Pharmacol 24(1):93–98
Terziivanov D, Bozhinova K, Dimitrova V, Atanasova I (2003) Nonparametric expectation maximisation (NPEM) population pharmacokinetic analysis of caffeine disposition from sparse data in adult Caucasians: systemic caffeine clearance as a biomarker for cytochrome P450 1A2 activity. Clin Pharmacokinet 42(15):1393–1409
Hunt SN, Jusko WJ, Yurchak AM (1976) Effect of smoking on theophylline disposition. Clin Pharmacol Ther 19(5 Pt 1):546–551
Gardner MJ, Tornatore KM, Jusko WJ, Kanarkowski R (1983) Effects of tobacco smoking and oral contraceptive use on theophylline disposition. Br J Clin Pharmacol 16(3):271–280
Jennings TS, Nafziger AN, Davidson L, Bertino JS Jr (1993) Gender differences in hepatic induction and inhibition of theophylline pharmacokinetics and metabolism. J Lab Clin Med 122(2):208–216
Seppälä NH, Leinonen EV, Lehtonen ML, Kivistö KT (1999) Clozapine serum concentrations are lower in smoking than in non-smoking schizophrenic patients. Pharmacol Toxicol 85(5):244–246
Rostami HA, Amin AM, Spencer EP, Lennard MS, Tucker GT, Flanagan RJ (2004) Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: a predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients. J Clin Psychopharmacol 24(1):70–78
Palego L, Biondi L, Giannaccini G, Sarno N, Elmi S, Ciapparelli A, Cassano GB, Lucacchini A, Martini C, Dell OL (2002) Clozapine, norclozapine plasma levels, their sum and ratio in 50 psychotic patients: influence of patient-related variables. Prog Neuropsychopharmacol Biol Psychiatry 26(3):473–480
Rostami-Hodjegan A, Kroemer HK, Tucker GT (1999) In-vivo indices of enzyme activity: the effect of renal impairment on the assessment of CYP2D6 activity. Pharmacogenetics 9(3):277–286
Djordjevic N, Ghotbi R, Bertilsson L, Jankovic S, Aklillu E (2008) Induction of CYP1A2 by heavy coffee consumption in Serbs and Swedes. Eur J Clin Pharmacol 64(4):381–385
Acknowledgements
We would like to thank the PF-2400013 clinical team for providing the internal validation data sets for smoker and nonsmokers from the Phase 1 study for PF-2400013.
Competing interests
KRY is an employee of and a shareholder of the company Simcyp Limited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plowchalk, D.R., Rowland Yeo, K. Prediction of drug clearance in a smoking population: modeling the impact of variable cigarette consumption on the induction of CYP1A2. Eur J Clin Pharmacol 68, 951–960 (2012). https://doi.org/10.1007/s00228-011-1189-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1189-y