Abstract
Objective
To compare the effect of metformin and sulphonylureas on the risks of switching to insulin therapy, hospitalisation for macrovascular disease and all-cause mortality.
Methods
The 70,437 residents of the Italian Region of Lombardy aged 40 to 90 years who started diabetes treatment with metformin or sulphonylureas during 2001–2003 entered the study and were followed until July 2007. We estimated the effects of the first-line agent, early compliance, and persistence with first-line therapy on the risks of switching to insulin, hospitalisation for macrovascular disease and all-cause mortality, by fitting a multistate model and adjusting for age, gender and selected clinical factors.
Results
Compared with patients who started on metformin, those who started on sulphonylureas were at a higher risk of switching to insulin (adjusted hazard ratio and 95% CI, 1.55; 1.43, 1.68), hospitalisation (1.15; 1.08, 1.21), and death (1.37; 1.26, 1.49). Compared with patients who stayed on sulphonylureas for 3 months or less, those on sulphonylureas for more than 9 months had an adjusted hazard ratio of 1.24 (1.13, 1.35) for switching to insulin and 1.14 (1.05, 1.23) for hospitalisation. The risks of switching to insulin and hospitalisation were both increased among patients who switched from metformin to another oral hypoglycaemic agent or combined initial monotherapy with another agent.
Conclusions
Our study provides evidence that the risks of switching to insulin, hospitalisation because of macrovascular events and death changes according to the first prescribed oral hypoglycaemic agent, as well as to the early compliance and persistence with such agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus has reached epidemic proportions, and doubling of the worldwide diabetic population is predicted over the next 25 years [1]. Cardiovascular disease is the major cause of mortality among patients with diabetes, accounting for 60–80% of the deaths [2]. Large randomised clinical trials provide robust evidence that improving glucose control reduces long-term microvascular and possibly macrovascular complications of diabetes [3–9]. However, concerns about the potential adverse cardiovascular effects of oral hypoglycaemic agents that increase insulin exposure, specifically sulphonylurea medications, have been raised since 1970 by the University Group Diabetes Project (UGDP) [10, 11]. The UGDP study showed increased cardiovascular mortality in the group randomised to receive tolbutamide compared with the placebo group. Despite the UGDP findings, treatment with sulphonylurea has been widely prescribed to treat patients with type 2 diabetes probably because of multiple methodological flaws in this study [12], and perhaps because a plausible mechanism remains unclear [13]. More recently, the United Kingdom Prospective Diabetes Study (UKPDS) reported that treatment with sulphonylureas was not associated with an increased risk of death [6]. However, the lack of beneficial effects of sulphonylureas on the incidence of macrovascular events, despite the documented improvement of glycaemic control, may suggest that this class of drugs might have a harmful effect.
Several observational studies have shown that the use of metformin is associated with a reduced risk of morbidity and death compared with sulphonylurea monotherapy [14–18]. Two observational studies, however, have reported a significant increase in mortality associated with the use of metformin [19, 20].
Results from the UKPDS showed that in patients with suboptimal glycaemic control despite the maximum dose of sulphonylurea, the early addition of metformin improved glycosylated haemoglobin (HbA1c) [21]. However, this early addition of metformin was associated with an increase in diabetes-related mortality compared with sulphonylureas alone [7]. An increased risk of death associated with the combined treatment with metformin and sulphonylurea has been also reported [20–22]. In contrast, a more recent study did not find increased mortality among patients who were prescribed a combination of sulphonylurea and metformin, compared with those on monotherapy [23].
With increasing disease duration, patients with type 2 diabetes often experience secondary failure to oral hypoglycaemic agents due to the loss of β-cell mass and the worsening of insulin resistance caused by the persistent exposure to hyperglycaemia [24, 25]. When secondary failure occurs patients eventually require exogenous insulin to avoid marked hyperglycaemia [6]. Therefore, when studying the effectiveness of oral hypoglycaemic agents, the switch to insulin therapy may be considered an intermediate outcome [26]. To our knowledge, no study has yet investigated the full range of secondary failure of oral hypoglycaemic agents, such as the switching to insulin therapy, and primary clinical outcomes such as the onset of macrovascular events.
We conducted a large population-based prospective cohort study to compare the effects of the use of metformin and sulphonylurea as monotherapy for the treatment of type 2 diabetes on the risk of switching from oral hypoglycaemic agents to insulin therapy, hospitalisation for macrovascular disease and all-cause mortality.
Materials and methods
Setting
The data used for this study were obtained from the health service databases of Lombardy, one of the 20 regions of Italy. It has a population of approximately 9 million residents, i.e. 16% of the Italian population. This population is entirely covered by the National Health Service (NHS). An automated system of databases has been used since 1997 to collect data on the use of health services, including demographic and administrative data, hospital discharge and outpatient prescription drug benefits. Different information is recorded for the beneficiaries of the NHS (practically the entire resident population) and can be linked together for each individual using a unique personal identification code.
Procedures aimed at protecting personal data were enforced in order to protect patients’ privacy. Briefly, each identification code was automatically converted to a unique and anonymous code and the inverse process was prevented by the deletion of the conversion table. Full details describing data sources and their use in the field of pharmacoepidemiology have been reported elsewhere [27].
Study cohort
All beneficiaries of the NHS who were Lombardy residents and aged 40 to 90 years represented the source population. According to the 2001 Italian Census, this population comprised 4,697,096 individuals. Those who received at least one prescription for an oral hypoglycaemic agent (i.e. metformin or sulphonylureas) from 1 January 2001 until 31 December 2003 were identified, and the date of the first dispensation was considered as the index date.
Patients were excluded if they:
-
1.
Received one or more prescriptions for oral hypoglycaemic agents in the year before the index date (in order to ensure the inclusion of only newly treated individuals)
-
2.
Started diabetes treatment using a combination of metformin and sulphonylureas or switched to insulin during the year after the index date (in order to ensure homogeneity of disease severity at entry)
-
3.
Received only one prescription for any medication to treat diabetes during follow-up (based on the assumption that long-term drug treatment might not have been indicated for these patients)
-
4.
Were hospitalised for macrovascular disease or received one or more prescriptions for drugs used for heart failure or coronary heart disease (i.e. digitalis glycosides and organic nitrates) in the year prior to the index date (in order to ensure the inclusion of only incident events during follow-up)
-
5.
Did not reach at least 1 year of follow-up (in order to ensure at least 1 year of potential exposure to the medications of interest)
All the remaining patients constituted our study cohort.
Each member of the cohort accumulated person-years of follow-up from the index date until the earliest among either of the following dates: hospitalisation for macrovascular disease, death, emigration, or 31 July 2007 (i.e. end of the follow-up period).
Assessing persistence and compliance with first-line treatment
The duration of the prescriptions for oral hypoglycaemic agents dispensed to each member of the cohort during the first year of follow-up was estimated by dividing the total amount of drug prescribed by the defined daily dose. Starting from the index date, consecutively refilled prescriptions were assigned to a unique therapeutic cycle if the length of time between finishing the previous prescription and starting the next one with the same drug type did not exceed an interval equal to 2.5-fold the time supply of that previous fill [28]. Modification of the initial therapy was assumed otherwise, and three reasons for modification were considered:
-
1.
Switching to another monotherapy was assumed if another oral hypoglycaemic agent replaced the medication prescribed first
-
2.
Change from monotherapy to combination therapy was assumed if another oral hypoglycaemic agent different from that dispensed initially was added on or before its refilling
-
3.
Discontinuation was assumed if no oral hypoglycaemic agent was prescribed within the considered period
Therefore, patients were classified into one of the following categories of persistence with first-line therapy: continuers, patients continuing the first-line therapy for at least 1 year; switchers, patients changing from first-line to another oral hypoglycaemic agent; combiners, patients receiving an additional type of oral hypoglycaemic agent other than the first-line drug; and discontinuers, patients stopping the first-line therapy without having a prescription for another oral hypoglycaemic agent [29].
Furthermore, patients were also classified according to their refill compliance with first-line therapy during the first year of follow-up. Refill compliance was measured by summing the number of days with first-line medication available until modification of the initial therapy and expressed according to progressively increasing categories of exposure duration (≤3 months, 4–6 months, 7–9 months, >9 months).
Outcomes definition
Three outcomes were considered, i.e. switching to insulin therapy, hospitalisation for macrovascular disease, and death from any cause. The date of the first insulin prescription was considered as the date of onset of the corresponding outcome. The date of the first hospitalization for myocardial infarction (ICD-9 code 410-412.99 and 414.xx), cerebrovascular disease (430–437.1 and procedure code 38.12), coronary artery bypass graft (36.10–36.16 and 36.2–36.9), percutaneous transluminal coronary angioplasty (36–36.02 and 36.05), or peripheral vascular disease (443-444.xx, 250.7x or 39.29, 39.25, 39.59, and 84.10-84.17) was considered as the date of hospitalisation for macrovascular disease [30]. Finally, we considered the recorded date of death, whereas no data about the cause of death were reported in the databases. It should be noted that patients who experienced any of these outcomes within 1 year of the index date were excluded from the cohort (please see exclusion criteria 2 and 5 of the “Study cohort” section).
Data analysis
The idea underlying our analysis was that the clinical course of chronic diseases, including type 2 diabetes, could be conceptualised as a sequence of episodes concerning drug treatment and treatment failures. The evolution of a patient over time can be modelled by fitting multistate models [31]. With such an approach a number of states is defined (i.e. first-line therapy, failures onset) and the focus is placed on the process of transition from one state to another [32, 33]. Estimating the transition rates is interesting in itself, since their comparison may provide useful insight into the relationships between endpoints. Moreover, the influence of prognostic factors on each of the transition rates can be estimated and compared. The tutorial reviewing statistical methods for the analysis of multistate models recently published in Statistics in Medicine [32], and a recent paper by the same author of the tutorial [33], describe the method used in the current paper.
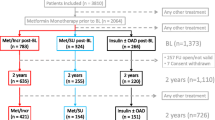
The multistate model that is useful for our purpose is illustrated in Fig. 1. Four different states were identified. In the first one (state 1) all the patients entered at the start of therapy with an oral hypoglycaemic agent and stayed in this state until the onset of one of the considered outcomes; again, because of exclusion criteria, all patients stayed in state 1 for at least 1 year. In the second state (state 2) only patients who experienced treatment with insulin entered and stayed in this state until the hospitalisation for macrovascular disease or death. Finally, two absorbing states of hospitalisation for macrovascular disease (state 3) and death (state 4) were considered. (Please note that hospitalisation for macrovascular disease is only artificially considered an absorbing state, since transition from hospitalisation to death was not of interest for the current study). The focus of this four-state model is on the process of transition from one state to another, as represented by the five arrows connecting boxes in Fig. 1. We assumed that the model represented in Fig. 1 is a Markov model. Loosely speaking, the Markov property states that the future depends on history only through the present [32].
A four-state, five-transition representation of clinical course of patients recruited in the study, including treatment with oral hypoglycaemic agents, insulin therapy, hospitalisation for macrovascular disease and death. Boxes represent the four states, and arrows represent the five transitions between states. The number of patients who reached each state and transition rates (and corresponding 95% confidence intervals) per 10,000 person-months at risk are also reported
Rates of each transition between states per 10,000 person-months at risk were calculated. The corresponding 95% confidence intervals (CI) were computed under the assumption that the observed number of events follows a Poisson distribution.
The effect of the covariates on the transition hazards was modelled using the multistate extension of the Cox’s proportional hazards model and expressed as hazard ratios (HR) and corresponding 95% CI. The extension concerned the following two devices. First, the models were fitted considering the transition variable as a stratification factor in order to obtain separate estimates of the effects of interest for each transition. Second, the Lin and Wei robust sandwich estimate of the covariance matrix was used in order to take into account the dependence of the data [34].
The covariates of interest were the factors related to the exposure to oral hypoglycaemic agents (first-line drug type, categories of compliance and persistence with first-line treatment). Differences in HR were evaluated by testing their homogeneity between transitions or over ordinal categories within transition according to the z test or a test for a linear trend, respectively. This last test was based on statistical significance of the regression coefficient obtained by scoring the corresponding categories. Differences between regression coefficients were tested according to a z test.
Estimates were adjusted for age (continuous), gender, and use of drugs commonly used for treatment of heart failure, coronary heart disease, hypertension and hyperlipidaemia during the first year of follow-up. In addition, the Charlson comorbidity index score [35] was calculated for each patient using diagnostic information from inpatient encounters 1 year prior to and 1 year after the index date, and expressed according to progressively increasing categories (0, 1, ≥2). These last were included as covariates in the fitted model.
Calculations were carried out using the PHREG procedure of the SAS package, 8.2 release [36], using the STRATA option to obtain separate estimates of the effects of interest for each transition and the COVSANDWICH option to produce the Lin and Wei estimate of the covariance matrix.
For all hypotheses tested two-tailed p values less than 0.05 were considered to be significant.
Results
We identified 70,437 patients aged 40–90 years in whom the initial treatment for type 2 diabetes consisted of a single prescription for an oral hypoglycaemic agent and who satisfied the inclusion criteria detailed above during 2001–2003. Table 1 summarises their general characteristics according to the first-line oral hypoglycaemic agent. At entry, sulphonylureas were prescribed to more than two thirds of the patients. Short-acting agents, such as gliclazide (20,979) and glimepiride (18,133) were the most frequently prescribed sulphonylurea drugs. At baseline, the groups significantly differed with regard to age, gender and concomitant treatments, the patients on sulphonylureas being preferentially older men who were treated more frequently with digitalis glycosides and organic nitrates and less commonly with antihypertensive and lipid-lowering agents than those on metformin. Conversely, the proportion of patients with a Charlson comorbidity index ≥2 was similar among those who initiated treatment with metformin (7.7%) and those who initiated treatment with sulphonylureas (7.6%). The initial monotherapy was maintained in only a minority of the patients. Most of the patients modified their initial treatment through discontinuation, rather than through combining with or switching to another class of oral hypoglycaemic agents. The groups also significantly differed with regard to refill compliance and persistence. In particular, patients who started on metformin had lower refill compliance, more frequently discontinued or switched, and less often combined, than those on sulphonylureas.
During follow-up, 3,760 patients switched to insulin, 7,014 experienced at least one hospital admission because of macrovascular events and 4,692 died (Fig. 1). As expected, the rates of both hospitalisation and death among patients who switched to insulin were much higher than those among patients who were not prescribed insulin therapy.
Figure 2 shows that, compared with patients who started on metformin (reference group), those on sulphonylureas were at a significantly higher risk of switching to insulin (1→2 transition; HR, and corresponding 95% CI, 1.55; 1.43, 1.68), hospitalisation for macrovascular disease (1→3 transition; 1.15; 1.08, 1.21) and death (1→4 transition; 1.37; 1.26, 1.49). There was no statistical evidence that initial monotherapy affected transitions 2→3 (1.18; 0.86, 1.62) and 2→4 (1.12; 0.77, 1.63). Furthermore, the HRs of the 1→3 and 2→3 transitions, as well as the HRs of the 1→4 and 2→4 transitions, did not significantly differ between patients starting on metformin or sulphonylureas (p values 0.5737 and 0.1517 respectively). This justifies why transitions 2→3 and 2→4 were not considered in the following analyses.
Multistate hazard ratios (HR), and 95% confidence intervals (95% CI), estimating the association between starting diabetes treatment with sulphonylureas (compared with starting with metformin) and the risk of experiencing the considered transitions. Transitions are: 1→2 from starting first-line oral hypoglycaemic agent to beginning treatment with insulin; 1→3 from starting first-line oral hypoglycaemic agent to hospitalisation for macrovascular disease; 2→3 from starting treatment with insulin to hospitalisation for macrovascular disease; 1→4 from starting first-line oral hypoglycaemic agent to death from any cause; 2→4 from starting treatment with insulin to death from any cause. Estimates are adjusted for age at entry (continuous), gender, concomitant use of medications (digitalis glycosides, organic nitrates, antihypertensive agents and lipid-lowering drugs) and categories of the Charlson comorbidity index score
Figure 3 shows that the risks of switching to insulin and of hospitalisation for macrovascular disease progressively increased with increasing refill compliance with sulphonylureas (tests for trend: p < 0.0001 for switching to insulin and p = 0.0082 for hospitalisation). Indeed, compared with patients who stayed on sulphonylurea for 3 months or less, those who accumulated more than 9 months having sulphonylurea available had HRs (95% CIs) of 1.24 (1.13, 1.35) for switching to insulin and 1.14 (1.05, 1.23) for hospitalisation. There was no statistical evidence that refill compliance with sulphonylureas modified the risk of all-cause mortality, nor that refill compliance with metformin was associated with the transitions of interest. Finally, there was statistical evidence that refill compliance with sulphonylureas and metformin acted differently in increasing the risk of both switching to insulin (p = 0.0020) and hospitalisation for macrovascular disease (p = 0.0451), but not the risk of death (p = 0.4247).
Multistate hazard ratios (HR), and 95% confidence intervals (95% CI), estimating the relationship between progressively increasing categories of refill compliance with first-line hypoglycaemic agent and the risk of transitions to insulin therapy, hospitalisation for macrovascular disease and all-cause mortality. Estimates are adjusted for age at entry (continuous), gender, concomitant use of medications (digitalis glycosides, organic nitrates, antihypertensive agents and lipid-lowering drugs) and categories of the Charlson comorbidity index score
Figure 4 shows that, compared with patients who, during the first year of follow-up, continued first-line therapy with metformin, those who switched from metformin to another oral hypoglycaemic agent were at a significantly higher risk of switching to insulin and hospitalisation for macrovascular disease, the corresponding HRs (95% CIs) being 2.53 (2.00, 3.19) and 1.27 (1.05, 1.54) respectively. Conversely, there was no statistical evidence that switching from sulphonylureas to another oral hypoglycaemic agent modified the risk of the outcomes of interest. Patients who combined initial monotherapy with another oral hypoglycaemic agent were at a higher risk of switching to insulin, the HRs (and 95% CI) being 3.03 (2.43, 3.79) and 2.15 (1.93, 2.39) for patients who started on metformin and sulphonylurea respectively. Combiners were at a higher risk of hospitalisation for macrovascular disease, with HRs of 1.40 (1.16, 1.69) and 1.25 (1.12, 1.39) for patients on metformin and sulphonylurea respectively. Compared with combiners who started with sulphonylurea, those who started on metformin had significantly higher HRs for switching to insulin (p < 0.0001) and being hospitalised for macrovascular disease (p < 0.0001). Finally, there was no statistical evidence that discontinuers were at a different risk of experiencing each of the outcomes of interest than patients who continued with first-line oral diabetes treatment.
Multistate hazard ratios (HR), and 95% confidence intervals (95% CI), estimating the relationship between categories of persistence with first-line hypoglycaemic agent and the risk of transitions to insulin therapy, hospitalisation for macrovascular disease and all-cause mortality. Estimates are adjusted for age at entry (continuous), gender, concomitant use of medications (digitalis glycosides, organic nitrates, antihypertensive agents and lipid-lowering drugs) and categories of the Charlson comorbidity index score
Discussion
This is one of the largest population-based cohort studies evaluating the association between oral hypoglycaemic agents for the treatment of type 2 diabetes and the risk of intermediate and clinical outcomes in general practice.
We found that, compared with patients who used metformin as first-line therapy, those who started with sulphonylureas were at higher risk of switching to insulin therapy, hospitalisation for macrovascular disease and death. This confirms the results of previous studies showing increased cardiovascular morbidity and/or mortality associated with the use of sulphonylureas [14–18, 37–44]. This evidence supports the hypothesis of a direct effect of sulphonylureas on the risk of the considered outcomes, possibly because of the cardiovascular toxicity of this class of agents [10, 45]. The observation that the risk of hospitalisation for macrovascular disease progressively increased as the compliance with sulphonylureas increases, while no evidence of such a trend was found among metformin users (Fig. 3), seems to confirm such an hypothesis.
The observed differences in the risk of macrovascular disease, as well as in all-cause mortality, might be mediated by the exposure to hyperglycaemia [46]. Metformin and sulphonylureas do improve glycaemic control to a similar degree [47]. However, patients treated with sulphonylurea may have had higher glucose levels than those treated with metformin. At least four findings in our study support this hypothesis:
-
1.
Patients who started with sulphonylureas were at a higher risk of switching to insulin, hospitalisation for macrovascular disease and mortality than patients who started with metformin (Fig. 2)
-
2.
The early addition of another oral hypoglycaemic agent was more common among patients who started with sulphonylureas than among those who started with metformin (Table 1)
-
3.
The risk of switching to insulin progressively increased as refill compliance with sulphonylureas increases, while there was no evidence of such a trend among metformin users (Fig. 3)
-
4.
Compared with combiners who started with sulphonylureas, those who started with metformin were at higher risk of both switching to insulin and hospitalisation for macrovascular diseases (Fig. 4)
All these observations taken together suggest that patients who started with sulphonylureas, as well as those who started with metformin and switched to, or added sulphonylureas, had more severe disease and more rapid worsening of glycaemic control.
Some other results of our study deserve to be mentioned. First, despite international guidelines in the past 10 years recommending metformin as the first-line therapy for the treatment of type 2 diabetes, in our setting only 1 in 3 patients were prescribed metformin as the starting oral hypoglycaemic agent [48].
Second, discontinuation of diabetes treatment was recorded among 45% of the patients after 1 year of follow-up. This confirms the results of previous studies that initial monotherapy is frequently abandoned just a few months after prescription [28, 49–52]. Among patients who experienced at least one episode of discontinuation during the first year of treatment, the risks of switching to insulin, hospitalisation for macrovascular disease and death were similar to those of patients who continued oral hypoglycaemic agents for at least 1 year. The finding confirms the results of a recent case–control study that did not find statistical evidence of an association between refill compliance and the risk of switching to insulin therapy [26]. This evidence supports the hypothesis that patients who had an early discontinuation of oral hypoglycaemic agents are those with milder diabetes where lifestyle modifications (diet and physical activity) rather than drug therapy, are indicated to control hyperglycaemia. However, regardless of the underlying reason, a 45% of treatment discontinuation among patients with a disease requiring lifetime treatment is of great concern and should be addressed by health care providers because it may lead to a high rate of inappropriate treatment and/or an unnecessary drug costs.
Third, the first prescribed monotherapy was changed to a combination treatment in 8% of the patients in our cohort, while 6% of them switched to another oral agent within 1 year of the first prescription. We found that the early combination of oral agents was a negative prognostic factor for switching to insulin therapy. This finding supports the “stepping stone theory”, which is intrinsic to secondary failure. Before the ultimate switch to insulin, intermediate steps are taken, like the addition of other oral hypoglycaemic agents in response to the worsening of metabolic control [26]. If this explanation is true, the higher risk of macrovascular complications among patients who combined initial monotherapy with another oral agent may be due to worse metabolic control among these patients. Perhaps a similar mechanism might explain the higher risk in sulphonylurea users.
Our study has several limitations, including selection bias, misclassification and confounding. As patients with less than 1 year of follow-up were excluded from the analyses, our conclusions apply only to patients who do not experience any of the considered outcomes during the first year after starting therapy (likely patients with better clinical profile), and do not take into account the early effects of oral hypoglycaemic agents. However, the observation that patients on sulphonylureas more frequently began treatment for heart failure and/or coronary heart disease during the first year after starting oral hypoglycaemic agents (Table 1) suggests that sulphonylureas might exert an early negative effect on macrovascular outcomes. On the other hand, as data on the macrovascular events have been drawn from the hospital discharge database, our conclusions apply only to non-fatal cardiovascular disease.
To some extent the patients included in our cohort may have been misclassified because of errors in the medication and diagnosis codes. However, good diagnostic performances of the hospital discharge database were recently reported by our group for both coronary heart and cerebrovascular outcomes (the positive predictive values being 88% and 84% respectively) [53]. In spite of this, the lack of information on the extent of exposure misclassification is a main source of systematic uncertainty in our estimates.
Because in our study the allocation to an oral hypoglycaemic agent was not randomised, the results may be affected by confounding. That is, the observed associations might have in fact been generated by disparities between the patients first treated with metformin or sulphonylureas. Disparities might concern patients’ clinical characteristics (e.g. glycaemic level, severity of diabetes, microvascular complications of diabetes, co-morbidities) and modifiable cardiovascular risk factors (e.g. physical inactivity, obesity/overweight, hypertension, hyperlipidaemia and smoking). We attempted to limit confounding by adjusting estimates for age, gender, co-treatment and co-morbidity profiles. However, because our analysis was based entirely on administrative databases, we cannot fully account for the possible preferential prescribing pattern of sulphonylureas to patients at higher risk of the outcomes of interest (i.e. confounding by indication). At the time of the cohort recruitment, international and national guidelines recommended the use of metformin at least for overweight or obese patients [54]. On the other hand, there was no evidence from our cohort that patients at increased cardiovascular risk, e.g. those with the worse clinical profile, were preferentially prescribed sulphonylureas. Rather, patients on metformin more frequently received treatment for hypertension and hyperlipidaemia. All of these observations taken together suggest that patients on metformin are expected to be at higher cardiovascular risk than those on sulphonylureas, at least for being overweight or obese, and likely for their higher prevalence of hypertension and hyperlipidaemia. Notwithstanding these reassuring observations, however, we cannot exclude the possibility that the general prescribing pattern in Lombardy could still be a surrogate for other unmeasured characteristics, such as preferring treatment with sulphonylureas among patients with higher glucose levels. Even the use of a defined daily dose for estimating the duration of a single drug prescription covering might have engendered confounding by indication. Patients who received a higher dosage prescription, appear to have higher compliance, but, on the other hand, are expected to have a worse clinical profile. Therefore, the observed association between compliance and risk of hospitalization might be explained, at least in part, by the confounding effect of the unmeasured prescribed dosage. The fact that such an association was not found for metformin users, may be due to the fact that sulphonylureas are much more titrated compared with metformin.
Even in a study with such a large sample size as ours, the interpretation of the lack of statistical evidence for some of the associations may be limited by a relatively low statistical power. For example, in our setting only a few patients modified the initially prescribed oral hypoglycaemic agent. This implies that the lack of statistical evidence that initial monotherapy affects the risk of hospitalisation and death among patients who switched to insulin (Fig. 2), or that refill compliance with both sulphonylurea and metformin affects the risk of death (Fig. 3), may be explained by the limited chance to detect significant effects among patients who switched to insulin or experienced a given category of exposure duration.
In conclusion, our cohort of 70,437 patients with a first prescription of a single oral hypoglycaemic agent were at a higher risk of switching to insulin therapy, hospitalisation for macrovascular diseases and all-cause mortality if they started with sulphonylurea than if they started with metformin, if they started with metformin and switched to another oral hypoglycaemic agent and/or if they combined their first-line therapy with another oral hypoglycaemic agent during the first year after starting therapy. The risks of switching to insulin therapy and hospitalisation for macrovascular disease progressively increased with increasing refill compliance with sulphonylurea treatment. These findings suggest that sulphonylureas should be prescribed with caution in patients with type 2 diabetes at higher risk of cardiovascular events.
Abbreviations
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- NHS:
-
National Health Service
- UGDP:
-
University Group Diabetes Project
- UKPDS:
-
United Kingdom Prospective Diabetes Study
References
King H, Aubert RE, Herman WH (1998) Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 21:1414–1431
Turner R, Cull C, Holman R (1996) United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann Intern Med 124:136–145
Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M (1995) Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 28:103–117
Vijan S, Hofer TP, Hayward RA (1997) Estimated benefits of glycemic control in microvascular complications in type 2 diabetes. Ann Intern Med 127:788–795
Gaster B, Hirsch IB (1998) The effects of improved glycemic control on complications in type 2 diabetes. Arch Intern Med 158:134–140
United Kingdom Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS33). Lancet 352:837–853
United Kingdom Prospective Diabetes Study (UKPDS) Group (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS34). Lancet 352:854–865
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS35): prospective observational study. BMJ 321:405–412
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359:1577–1589
Meinert CL, Knatterud GL, Prout TE, Klimt CR (1970) A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes 19:785–830
Knatterud GL, Klimt CR, Levin ME, Jacobson ME, Goldner MG (1978) Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA 240:37–42
Kilo C, Miller JP, Williamson JR (1980) The crux of the UGDP: spurious results and biologically inappropriate data analysis. Diabetologia 18:179–185
Schwartz TB, Meinert CL (2004) The UGDP controversy: thirty-four years of contentious ambiguity laid to rest. Perspect Biol Med 47:564–574
Johnson JA, Simpson SH, Majumdar SR, Toth EL (2002) Decreased mortality associated with the use of metformin compared with sulphonylurea monotherapy in type 2 diabetes. Diabetes Care 25:2244–2248
Johnson JA, Simpson SH, Toth EL, Majumdar SR (2005) Reduced cardiovascular morbidity and mortality associated with metformin use in subjects with type 2 diabetes. Diabet Med 22:497–502
Simpson SH, Majumdar SR, Tsuyuki RT, Eurich DT, Johnson JA (2006) Dose-response relation between sulphonylurea drugs and mortality in type 2 diabetes mellitus: a population-based cohort study. CMAJ 174:169–174
Evans JMM, Ogston SA, Emslie-Smith A, Morris AD (2006) Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia 49:930–936
Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, Ng A, Hughes RI, Khunti K, Wilkins MR, Majeed A, Elliott P (2009) Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. doi:10.1136/bmj.b4731
Fisman EZ, Tenenbaum A, Benderly M, Goldbourt U, Behar S, Motro M (1999) Antihyperglycemic treatment in diabetics with coronary disease: increased metformin-associated mortality over a 5-year follow-up. Cardiology 91:195–202
Olsson J, Lindberg G, Gottsäter M, Lindwall K, Sjöstrand A, Tisell A, Melander A (2000) Increased mortality in type II diabetic patients using sulphonylurea and metformin in combination: a population-based observational study. Diabetologia 43:558–560
United Kingdom Prospective Diabetes Study (UKPDS) Group (1998) A randomized trial of efficacy of early addition of metformin in sulfonylurea-treated type 2 diabetes (UKPDS28). Diabetes Care 21:87–92
Fisman EZ, Tenenbaum A, Boyko V, Benderly M, Adler Y, Friedensohn A, Kohanovski M, Rotzak R, Schneider H, Behar S, Motro M (2001) Oral antidiabetic treatment in patients with coronary disease: time-related increased mortality on combined glyburide/metformin therapy over a 7.7 year follow-up. Clin Cardiol 24:151–158
Gulliford M, Latinovic R (2004) Mortality in type 2 diabetic subjects prescribed metformin and sulphonylurea drugs in combination: cohort study. Diabetes Metabol Res Rev 20:239–245
Polonsky KS, Sturis J, Bell GI (1996) Non-insulin dependent diabetes mellitus—a genetically programmed failure of the beta cell to compensate for insulin resistance. N Engl J Med 334:777–783
Groop LC, Pelkonen R, Koskimies S, Bottazzo GF, Doniach D (1986) Secondary failure to treatment with oral antidiabetic agents in non-insulin dependent diabetes mellitus. Diabetes Care 9:129–133
Spoelstra JA, Stolk RP, Heerdink ER, Klungel OH, Erkens JA, Leufkens HGM, Groobbe DE (2003) Refill compliance in type 2 diabetes mellitus: a predictor of switching to insulin therapy? Pharmacoepidemiol Drug Saf 12:121–127
Corrao G, Cesana G, Merlino L (2008) Pharmacoepidemiological research and the linking of electronic healthcare databases available in the Italian region of Lombardy. Biomed Stat Clin Epidemiol 2:117–125
Boccuzzi SJ, Wogen J, Fox J, Sung JCY, Shah AB, Kim J (2001) Utilization of oral hypoglycemic agents in a drug-insured US population. Diabetes Care 24:1411–1415
Mazzaglia G, Mantovani L, Sturkenboom MC, Filippi A, Trifirò G, Cricelli C, Brignoli O, Caputi AP (2005) Patterns of persistence with antihypertensive medications in newly diagnosed hypertensive patients in Italy: a retrospective cohort study in primary care. J Hypertens 23:2093–2100
Hillier TA, Pedula KL (2003) Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care 26:2999–3005
Andersen PK, Keiding N (2002) Multi-state models for event history analysis. Stat Meth Med Res 11:91–115
Putter H, Fiocco M, Geskus RB (2007) Tutorial in biostatistics: competing risks and multi-state models. Stat Med 26:2389–2430
Putter H, van der Hage J, de Bock GH, Elgalta R, van de Velde CJH (2006) Estimation and prediction in a multi-state model for breast cancer. Biom J 3:366–380
Lin DY, Wei LJ (1989) The robust inference for the Cox proportional hazards model. J Am Stat Assoc 84:1074–1079
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619
SAS Institute Inc. Release 8.1 enhancements to SAS/STAT software. Available at: http://support.sas.com/rnd/app/da/new/dastat81.html
Boyle D, Hadden DR, Bhatia SK, Montgomery DA, Weaver JA (1972) Ischaemic heart-disease in diabetics. A prospective study. Lancet 7746:338–339
Hadden DR, Montgomery DA, Weaver JA (1972) Myocardial infarction in maturity-onset diabetics. A retrospective study. Lancet 7746:335–338
Soler NG, Pentecost BL, Bennett MA, FitzGerald MG, Lamb P, Malins JM (1974) Coronary care for myocardial infarction in diabetics. Lancet 7856:475–477
Garratt KN, Brady PA, Hassinger NL, Grill DE, Terzic A, Holmes DR Jr (1999) Sulfonylurea drugs increase early mortality in patients with diabetes mellitus after direct angioplasty for acute myocardial infarction. J Am Coll Cardiol 33:119–124
Gustafsson I, Hildebrandt P, Seibaek M, Melchior T, Torp-Pedersen C, Køber L, Kaiser-Nielsen P (2000) Long-term prognosis of diabetic patients with myocardial infarction: relation to antidiabetic treatment regimen. The TRACE Study Group. Eur Heart J 21:1937–1943
Aronow WS, Ahn C (2001) Incidence of new coronary events in older persons with diabetes mellitus and prior myocardial infarction treated with sulfonylureas, insulin, metformin, and diet alone. Am J Cardiol 88:556–557
O’Keefe JH, Blackstone EH, Sergeant P, McCallister BD (1998) The optimal mode of coronary revascularization for diabetics. A risk-adjusted long-term study comparing coronary angioplasty and coronary bypass surgery. Eur Heart J 19:1696–1703
Thisted H, Johsen SP, Rungby J (2006) Sulfonylureas and the risk of myocardial infarction. Metabolism 55:S16–S19
Leibowitz G, Cerasi E (1996) Sulphonylurea treatment of NIDDM patients with cardiovascular disease: a mixed blessing? Diabetologia 39:503–514
Coutinho M, Wang Y, Gerstein HC, Yusuf S (1999) The relationship between glucose and incident cardiovascular events. Diabetes Care 22:233–240
Bolen S, Feldman L, Vassy J, Wilson L, Yeh H-C, Marinopulos S, Wiley C, Selvin E, Wilson R, Bass EB, Brancati FL (2007) Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 147:386–399
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B (2009) Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy. A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32:193–203
Skaer TL, Selar DA, Markowski DJ, Won JKH (1993) Effect of value-added utilities on prescription refill compliance and Medicaid health care expenditures—a study of patients with non-insulin-dependent diabetes mellitus. J Clin Pharm Ther 18:295–299
Paes AHP, Bakker A, Soe-Agnie CJ (1997) Impact of dosage frequency on patient compliance. Diabetes Care 20:1512–1517
Paes AHP, Bakker A, Soe-Agnie CJ (1998) Measurement of patient compliance. Pharm World Sci 20:73–77
Venturini F, Nichol MB, Sung JCY, Bailey KL, Cody M, McCombs JS (1999) Compliance with sulfonylureas in a health maintenance organization: a pharmacy-record based study. Ann Pharmacother 33:281–288
Corrao G, Zambon A, Nicotra F, Bagnardi V, Fornari C, La Vecchia C, Mezzanzanica M, Nappi RE, Merlino L, Cesana G (2007) Persistence with oral and transdermal hormone replacement therapy and hospitalisation for cardiovascular outcomes. Maturitas 57:315–324
Burgers KS, Bailey JV, Klazinga NS, Van Der Bij AK, Grol R, Feder G, for the Agree Collaboration (2002) Comparative analysis of recommendations and evidence in diabetes guidelines from 13 countries. Diabetes Care 25:1933–1939
Acknowledgements
This study was funded by grants from the Italian Minister for University and Research (“Fondo d’Ateneo per la Ricerca” portion, year 2008).
Conflict of interest
The authors have no conflicts of interest to declare in reference to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corrao, G., Romio, S.A., Zambon, A. et al. Multiple outcomes associated with the use of metformin and sulphonylureas in type 2 diabetes: a population-based cohort study in Italy. Eur J Clin Pharmacol 67, 289–299 (2011). https://doi.org/10.1007/s00228-010-0939-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0939-6