Abstract
Purpose
Rivaroxaban is a newly developed oral medicine that direct inhibits factor Xa for the prevention and treatment of thromboembolic disorders. The objective of this study was to compare the efficacy and safety of rivaroxaban versus enoxaparin, a medicine routinely used for thromboprophylaxis after total hip or knee arthroplasty.
Methods
We performed a meta-analysis of relevant randomized controlled trials (RCTs) identified in PubMed, Cochrane library, and Embase. The primary efficacy outcome for our meta-analysis was total venous thromboembolism (VTE) and all-cause mortality. The primary safety outcome was bleeding events, which were categorized as major, clinically relevant non-major, or minor events.
Results
Eight RCTs, involving 15,586 patients, were included in our meta-analysis. Compared to enoxaparin, thromboprophylaxis with rivaroxaban was associated with significantly fewer VTE and all-cause mortality [9,244 patients, risk ratio (RR) 0.56, 95% confidence interval (CI) 0.39–0.80] cases and a similar incidence of bleeding cases (major bleeding events: 13,384 patients, RR 1.65, 95% CI 0.93-2.93; clinically relevant non-major bleeding events: 13,384 patients, RR 1.21, 95% CI 0.98–1.50; total bleeding events, 13,384 patients, RR 1.10, 95% CI 0.97–1.24). The total hip or knee arthroplasty subgroup analysis revealed consistent efficacy and safety findings.
Conclusions
Rivaroxaban was more effective than the recommended dose of enoxaparin and had a similar safety profile for thromboprophylaxis after hip and knee arthroplasty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Venous thromboembolism (VTE) is a major and potentially fatal complication after total hip or knee arthroplasty [1]. Without prophylactic anticoagulation, the frequency of VTE after total hip or knee arthroplasty is high, and anticoagulant therapy has the potential to substantially reduce the frequency [2–4].
At the present time, low-molecular-weight heparins (LMWHs) and vitamin K antagonists are routinely used for thromboprophylaxis after major orthopedic surgery. Although they effectively reduce the incidence of VTE, they are associated with a number of limitations. Vitamin K antagonists, such as warfarin, have unpredictable pharmacologic effects and numerous food and drug interactions, and they require frequent monitoring that is difficult to manage [5]. LMWHs need to be administered subcutaneously, which often results in discontinuation after discharge from hospital, and they are cost-effective only when patients or caregivers can be taught to inject the drug at home [6–8]. Consequently, the search for a new and improved agent is a continuing clinical challenge.
Rivaroxaban (BAY 59-7939) is a newly approved, oral direct inhibitor of factor Xa that can be used for the prevention and treatment of thromboembolic disorders. It inhibits the active site of factor Xa directly (without the need for the cofactor antithrombin), which distinguishes its mechanism of action from that of the indirect factor Xa inhibitors, such as the LMWHs (e.g., the pentasaccharides fondaparinux and idraparinux) [9].
Several randomized controlled trials (RCTs) have been carried out to compare the efficacy and safety of rivaroxaban versus enoxaparin, a LMWH that is usually given for thromboprophylaxis after total hip or knee arthroplasty [10–17]. Results from these RCTs have indicated that rivaroxaban is an efficacious and promising agent for thromboprophylaxis after total hip or knee arthroplasty and superior to—or at least as effective as—enoxaparin. In the study reported here, our aim was to compare more conclusively the efficacy and safety of rivaroxaban versus enoxaparin for thromboprophylaxis after hip or knee arthroplasty by performing a meta-analysis of relevant RCTs.
Methods
Data sources
The study was performed using a prespecified search strategy and study eligibility criteria. We performed an extensive search of PubMed (up to March 2010), the Cochrane Central Register of Controlled Trials (Cochrane Library Issue 2, 2010), and Embase (1980 to March 2010) to identify relevant RCTs for our meta-analysis. We restricted the search to RCTs. Search term combinations were “rivaroxaban”, “enoxaparin”, “low-molecular-weight heparins”, “total knee arthroplasty” and similar, “total hip arthroplasty” and similar, “thromboprophylaxis” and similar, and “venous thromboembolism” and similar. The language of the research papers was not restricted to English. All reference lists from the relevant articles and reviews were hand searched for additional eligible studies. Experts in the field were also consulted. The articles that were not available to us were requested from the authors.
Study selection
Two reviewers (YBC and JDZ) independently searched the literature and examined relevant RCTs for further assessment. The criteria for including a study in our meta-analysis was: (1) it was a RCT; (2) it included patients of all ages undergoing total hip or knee arthroplasty; (3) it compared the efficacy and safety of rivaroxaban versus enoxaparin for thromboprophylaxis. Trials with a blinded and unblinded design were both included; abstracts in scientific conferences were not included. Experimental trials and trials focusing on pharmacokinetic or pharmacodynamic variables were excluded.
Qualitative assessment
Evaluation of the methodological quality of the RCTs included in the meta-analysis was performed independently by the two reviewers (MMA and ZZ) using the Jadad scoring system as follows [18]. One point is awarded for the presence of randomization, blinding, and data on study withdrawals, respectively. Also, if the randomization or blinding procedures are appropriate, one point is awarded for each procedure; no points are awarded if no data are provided on the methodology of the above-mentioned procedures. Finally, if any of these procedures is not deemed appropriate, one point is deducted for each one. The maximum score that can be attributed to an RCT is 5. An RCT with a score >2 is considered to be an RCT of adequately good quality [19, 20].
Data extraction
The two reviewers (YBC and JDZ) independently extracted data from the included trials. Data were extracted from each study with a predesigned review form. In the case of disagreement between the two reviewers, a third reviewer extracted the data, and the results were attained by consensus. We contacted the authors of trials for missing data when necessary. Data on study characteristics (methodology, included population, study design and drugs, and publication details), endpoint data (efficacy outcomes and safety outcomes), and adverse events during treatment and follow up were extracted.
Analyzed outcomes
The primary efficacy outcome of this meta-analysis was total VTE and all-cause mortality, defined as the composite of VTE (any deep-vein thrombosis or nonfatal pulmonary embolism) and death from any cause. The secondary efficacy outcome included major VTE (defined as the composite of proximal deep vein thrombosis, nonfatal pulmonary embolism, or death from VTE), deep vein thrombosis (any thrombosis, including both proximal and distal), and symptomatic VTE.
The primary safety outcome of the meta-analysis was bleeding events, which were categorized as major events, clinically relevant non-major bleeding events, or minor events, beginning after the first dose of the study drug and remaining up to 2 days after the last dose of the study drug. A major bleeding event was defined as bleeding that was fatal, that occurred in a critical organ, or that required a re-operation, or as extrasurgical-site bleeding that was clinically overt and associated with a fall in the hemoglobin level of at least 2 g/dl or that required the transfusion of ≥2 U of whole blood or packed cells. The secondary safety outcome was drug-related adverse event.
Data analysis and statistical methods
Statistical analyses were done with Review Manager ver. 5.0.20 (Cochrane Collaboration, Oxford, UK). We assessed the heterogeneity of the trial results by calculating a chi-square test of heterogeneity and the I 2 measure of inconsistency. The publication bias was assessed by examining the funnel plot. We used a random-effects model by using the DerSimonian and Laird method for pooling risk ratios (RRs) and 95% confidence intervals (CIs) of all primary and secondary outcomes throughout the meta-analysis. Heterogeneity was investigated through subgroup analyses as defined above.
Results
Study selection process
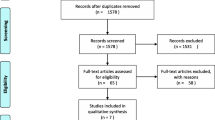
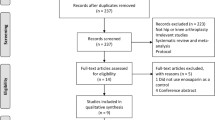
The flow diagram (Fig. 1) shows the detailed screening and selection process that we applied before including trials in our meta-analysis. The search was performed in PubMed, the Cochrane Central Register of Controlled Trials, and Embase. We obtained 18 full papers from 84 studies for detailed evaluation. We ultimately identified eight RCTs that fulfilled all of the criteria for inclusion in the meta-analysis.
Study characteristics
The main characteristics of the eight included RCTs (type of study design, characteristics of the included population, drug tested, number of patients randomized, and Jadad score) are presented in Table 1. The total population of the included trials was 15,586 patients. All of the included RCTs were performed exclusively in adult patients undergoing total hip arthroplasty (five RCTs) or knee arthroplasty (three RCTs), and all RCTs were assessed to be good in terms of methodology (seven trials with appropriate double blinding and double-dummy protocols). The high Jadad scores (Five RCTs had a score of 5, two had 4, and one had 3) also indicated the high quality of the RCTs included in the meta-analysis. We examined the funnel plot [standard error (SE) of log RR plotted against RRs] to estimate publication bias and obtained a symmetric inverse funnel distribution.
Treatment schedules for thromboprophylaxis were comparable between the included trials. All of the patients in the rivaroxaban group received the first dose after 6–8 h of wound closure. For dose-ranging studies, only the group treated with a total daily dose of 10 mg was included in the analysis to avoid clinical heterogeneity. In the patients included in our meta-analysis, rivaroxaban was administrated orally once daily with a dose of 10 mg in five RCTs, and orally twice daily with total daily dose of 10 mg in the other three dose-ranging RCTs. The trials included in our meta-analysis used the enoxaparin dose and regimen approved for use in Europe (six RCTs; 40 mg once daily, first dose received 12 h or the evening before surgery and medication resumed 6–8 h after wound closure) or in the USA (two RCTs: 30 mg twice daily, first dose received on the morning after surgery or 12–24 h after wound closure).
Efficacy outcomes
Data on primary outcome and secondary outcomes were provided in all eight relevant RCTs. Compared to enoxaparin, thromboprophylaxis with rivaroxaban was associated with significantly fewer total VTE and all-cause mortality (9,244 patients, RR 0.56, 95% CI 0.39–0.80), but a similar mortality (9,622 patients, RR 0.58, 95% CI 0.24–1.37). All secondary efficacy outcomes were fewer in the rivaroxaban groups than in the enoxaparin groups (major VTE: 10,086 patients, RR 0.42, 95% CI 0.20–0.84; deep-vein thrombosis: 9,244 patients, RR 0.54, 95% CI 0.37–0.79; symptomatic VTE: 12,500 patients, RR 0.49, 95% CI 0.34–0.72). The RRs for the development of the primary outcome and secondary efficacy outcomes in individual RCTs as well as the pooled RRs are given in Figs. 2 and 3, respectively. Our meta-analysis of the primary outcome and secondary outcomes reveals the superiority of rivaroxaban over enoxaparin for thromboprophylaxis after total hip or knee arthroplasty.
In terms of the total hip arthroplasty subgroup, there were significantly fewer total VTE and all-cause mortality (5,491 patients, RR 0.49, 95% CI 0.25-0.98), deep-vein thrombosis (5,491 patients, RR 0.46, 95% CI 0.22-0.99), and symptomatic VTE (6,921 patients, RR 0.38, 95% CI 0.19-0.75) cases but a similar number of major VTE (5,892 patients, RR 0.33, 95% CI 0.10–1.07) cases in the rivaroxaban groups compared to the enoxaparin groups (Figs. 2 and 3). In the total knee arthroplasty subgroup, there were significantly fewer total VTE and all-cause mortality (3,753 patients, RR 0.66, 95% CI 0.48-0.91) and all secondary efficacy outcomes (deep-vein thrombosis, 3,753 patients, RR 0.65, 95% CI 0.50–0.85; major VTE, 4,194 patients, RR 0.53, 95% CI 0.33–0.86; symptomatic VTE, 5,579 patients, RR 0.55, 95% CI 0.35–0.88) in rivaroxaban group compared to enoxaparin group (Figs. 2 and 3). The separate analyses of the total hip and knee arthroplasty subgroups produced findings similar to those of the overall meta-analysis.
Safety outcomes
All eight RCTs provided the relevant safety outcomes. There were no significant differences in the incidence of bleeding events between the rivaroxaban groups and enoxaparin groups (major bleeding events, 13,384 patients, RR 1.65, 95% CI 0.93-2.93; clinically relevant non-major bleeding events, 13,384 patients, RR 1.21, 95% CI 0.98–1.50; minor bleeding events, 1,001 patients, RR 1.15, 95% CI 0.63–2.08), while numerically higher bleeding events occurred in rivaroxaban groups (Fig. 4). We have performed a pooled analysis of bleeding in order to completely exclude the significance of this difference between rivaroxaban and LMW heparins. Our analysis of total bleeding events also revealed that there were no significant differences in the incidence of bleeding events between the rivaroxaban groups and enoxaparin groups (13,384 patients, RR 1.10, 95% CI 0.97–1.24; Fig. 4c). The results of the total hip or knee arthroplasty subgroup analysis of the primary and secondary safety outcomes suggest the same findings as with the overall meta-analysis (Fig. 4). The safety analysis revealed that the benefits of rivaroxaban for thromboprophylaxis were not obtained at the expense of a significant increased risk of bleeding, although numerically higher bleeding events did occur in the rivaroxaban groups. Rivaroxaban and enoxaparin were also associated with an overall similar number of drug-related adverse events (12,383 patients, RR 1.00, 95% CI 0.92–1.09; Fig. 5).
Sensitivity analysis
The sensitivity analysis limited to double-blind RCTs did not change the efficacy and safety findings for the review overall. Removal of each individual study or those studies of lower quality also did not significantly affect our primary outcome. The sensitivity analysis of the alternative inclusion of other rivaroxaban dosage groups in the dose-ranging studies again did not significantly change the overall efficacy and safety findings of the study. The results of the analysis that only included those trials using 40 mg of enoxaparin once daily were similar to those of the overall meta-analysis in terms of efficacy and safety.
Discussion
The use of oral anticoagulants may lead to more convenient and safe antithrombotic therapies with increased patient compliance compared to LMWHs and vitamin K antagonists. These oral agents have the potential to act as alternatives to treatment with LMWHs and vitamin K antagonists after total hip or knee arthroplasty and in atrial fibrillation. The major oral anticoagulants in current use are the newly developed direct thrombin inhibitors (e.g., dabigatran) and factor Xa inhibitors (e.g. rivaroxaban, apixaban).
A previous systematic review with a meta-analysis suggested that dabigatran possesses an efficacy and safety similar to those of the LMWH agent enoxaparin after total hip or knee arthroplasty. Our study is a systematic review with a meta-analysis that compared the efficacy and safety of rivaroxaban, a newly developed oral direct factor Xa inhibitor, with enoxaparin for thromboprophylaxis in patients undergoing total hip and knee arthroplasty. The overall finding of our meta-analysis suggests that thromboprophylaxis with rivaroxaban (total daily dose of 10 mg) was superior to that with the recommended dose of enoxaparin (Figs. 2, 3).The safety outcome analysis revealed that the benefit of rivaroxaban for thromboprophylaxis was not associated with an increasing risk of both bleeding (Fig. 4) and drug-related adverse events (Fig. 5).
The conclusion of this study is based on a pooled analysis of both total hip and knee arthroplasty trials. To avoid the influence of clinical heterogeneity, we performed a subgroup meta-analysis on the total hip and knee arthroplasty trials, respectively. The subgroup analysis also demonstrated that rivaroxaban had superior effect and a similar incidence of bleeding for thromboprophylaxis, showing findings that were unchanged with those of the overall review.
One widely recognized impediment to effective thromboprophylaxis in patients undergoing total hip or knee arthroplasty is concern about the risk of bleeding [4]. In this regard, our meta-analysis, based on currently available data, is reassuring in that even though it was found to be superior to enoxaparin for thromboprophylaxis, rivaroxaban was not associated with significant increases in bleeding events (Fig. 4) and drug-related adverse events (Fig. 5). However, well-designed head-to-head RCTs focusing on the safety of rivaroxaban compared with enoxaparin are warranted because of the numerically higher bleeding events associated with the former, although our meta-analysis indicated the difference between the rivaroxaban and enoxaparin groups was not statistically significant. We also stress that rivaroxaban should be contraindicated for patients susceptible to hemorrhage.
Recent evidence-based guidelines issued by the American College of Chest Physicians (ACCP) and the UK National Institute for Health and Clinical Excellence (NICE) recommend extended prophylaxis against VTE for patients undergoing total hip or knee arthroplasty [1, 21]. The patients are generally required to continue anticoagulants after hospital discharge, but the present-day shortness of stays in the hospital often result in fewer patients receiving the duration of prophylaxis recommended by the guidelines [1, 21]. Similar to enoxaparin, rivaroxaban can be administered in a fixed, unmonitored dose, but it also can be given orally, which may lead to more convenient and safe antithrombotic therapies with increased compliance. Therefore, in addition to its superior efficacy with a similar safety profile, the fixed, unmonitored, oral dose also makes rivaroxaban an attractive alternative to enoxaparin for thromboprophylaxis after total hip or knee arthroplasty.
In contrast to an earlier pooling analysis of three RCTs [22], in the study reported here, we examined eight RCTs using a wider range of clinically relevant outcome variables. The main strength of our meta-analysis is that it focused on high-quality RCTs with large number of patients (n = 155,86). The efficacy and safety outcomes were defined in a similar manner in both our meta-analysis and the individual included trials. In the included treatment arms, the same administration route and doses of rivaroxaban (total daily dose of 10 mg were given orally, once or twice daily) were compared with the enoxaparin dose and regimen approved for use in Europe (40 mg once daily, six RCTs) or in the USA (30 mg twice daily, two RCTs). Deep-vein thrombosis in all of the included trials was assessed by means of systematic ascending, bilateral venography with the use of the Rabinov and Paulin technique [23, 24]. The similar treatment schedule and evaluation criteria of the included trials provided greater statistical confidence for our meta-analysis.
This meta-analysis is not without limitations. First, despite obtaining analysis efficacy outcomes by pooling results from all the available properly randomized trials with large number patients, our meta-analysis lacked statistical power to provide precise estimates of the frequency and treatment effect for clinically important outcomes such as pulmonary embolism. However, VTE and pulmonary embolism represent clinical manifestations of the same underlying disease process. Therefore, strategies that are effective for the VTE, especially those proximally located, are also likely to be effective for the prevention of non-fatal and fatal pulmonary embolism. Second, there was some heterogeneity between the RCTs included in our meta-analysis, such as different prophylactic duration, treatment schedule. Six of the RCTs included in our meta-analysis used the enoxaparin 40 mg once daily regimen, while two RCTs used the 30 mg twice daily regimen. In the knee-arthroplasty subgroup, two RCTs used the 30 mg twice daily regimen and one RCTs used the 40 mg once daily regimen. However, differences among trials are inevitable since each individual trial comprises different populations and uses different treatment protocols, and there is always some heterogeneity, even within individual trials [25, 26]. Heterogeneity did not preclude the pooling of their results because individual patients are directly compared only with other patients within the same trial, and not across the trials. The validity of our approach was also supported by the sensitivity analysis, which obtained the similar efficacy and safety findings as the overall analysis. Third, another point that should be considered is that four RCTs excluded premenopausal women, and all of the trials excluded adolescent and pediatric patients. So the finding of this meta-analysis should be interpreted with caution with regards to premenopausal women, adolescents, and young children. Fourth, all of the eight included trials, including the one unblinded study, were supported by the pharmaceutical company who hold the patent for rivaroxaban, which may generate bias in the assessment of outcomes. Nevertheless, the sensitivity analysis performed in our meta-analysis obtained findings similar to those of the primary analysis.
In conclusion, despite the limitations of our meta-analysis, we suggest that rivaroxaban appears to be more effective than enoxaparin for thromboprophylaxis after hip and knee arthroplasty and that the benefits of rivaroxaban in VTE prevention were not gained at the expense of an increased risk of bleeding. Current evidence suggests that rivaroxaban, an effective direct factor Xa inhibitor which is given in a fixed, unmonitored oral dose, is an alternative to enoxaparin for preventing VTE after hip and knee arthroplasty. However, well-designed head-to-head RCTs focusing on the bleeding risk of rivaroxaban compared with enoxaparin are warranted.
References
Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW (2008) Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines, 8th edn. Chest 133:381S–453S
Mohr DN, Silverstein MD, Murtaugh PA, Harrison JM (1993) Prophylactic agents for venous thrombosis in elective hip surgery: meta-analysis of studies using venographic assessment. Arch Intern Med 153:2221–2228
Friedman RJ (2003) Extended thromboprophylaxis after hip or knee replacement. Orthopedics 26[2 Suppl]:s225–s230
Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA Jr, Wheeler HB (2001) Prevention of venous thromboembolism. Chest 119:132S–175S
Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E (2004) The pharmacology and management of the vitamin K antagonists. In: 7th ACCP Conf Antithrombotic and Thrombolytic Therapy. Chest 126[Suppl]:204S–233S
Davies LM, Richardson GA, Cohen AT (2000) Economic evaluation of enoxaparin as postdischarge prophylaxis for deep vein thrombosis (DVT) in elective hip surgery. Value Health 3:397–406
Dahl OE, Pleil AM (2003) Investment in prolonged thromboprophylaxis with dalteparin improves clinical outcomes after hip replacement. J Thromb Haemost 1:896–906
Friedman RJ, Gallus AS, Cushner FD, Fitzgerald G, Anderson FA Jr (2008) Physician compliance with guidelines for deep-vein thrombosis prevention in total hip and knee arthroplasty. Curr Med Res Opin 24:87–97
Perzborn E, Strassburger J, Wilmen A, Pohlmann J, Roehrig S, Schlemmer KH, Straub A (2005) In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct Factor Xa inhibitor. J Thromb Haemost 3:514–521
Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel TJ, Benson A, Misselwitz F, Fisher WD (2009) Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 373:1673–1680
Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 358:2765–2775
Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AG (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 358:2776–2786
Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap AF, Misselwitz F, Haas S (2008) Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 372:31–39
Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, Misselwitz F, Muehlhofer E, Kälebo P (2007) Dose-escalation study of rivaroxaban (BAY 59–7939)—an oral, direct Factor Xa inhibitor for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Res 120:685–693
Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, Muehlhofer E, Dierig C, Misselwitz F, Kälebo P (2006) A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement. Circulation 114:2374–2381
Eriksson BI, Borris L, Dahl OE, Haas S, Huisman MV, Kakkar AK, Misselwitz F, Kälebo P (2006) Direct Factor Xa inhibition with BAY 59-7939 for the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost 4:121–128
Turpie AG, Fisher WD, Bauer KA, Kwong LM, Irwin MW, Kälebo P, Misselwitz F, Gent M (2005) BAY 59-7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement. A phase II dose-ranging study. J Thromb Haemost 3:2479–2486
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Khan KS, Daya S, Jadad A (1996) The importance of quality of primary studies in producing unbiased systematic reviews. Arch Intern Med 156:661–666
Moher D, Jadad AR, Tugwell P (1996) Assessing the quality of randomized controlled trials. Current issues and future directions. Int J Technol Assess Health Care 12:195–208
National Collaborating Centre for Acute Care (2007) Venous thromboembolism. Reducing the risk of venous Thromboembolism (deep vein thrombosis and pulmonary embolism) in inpatients undergoing surgery. National Collaborating Centre for Acute Care, The Royal College of Surgeons of England, London. Available at: http://www.rcseng.ac.uk/surgical_research_units/nccac/guidelines/venous_thrombo_guideline.html
Eriksson BI, Kakkar AK, Turpie AG, Gent M, Bandel TJ, Homering M, Misselwitz F, Lassen MR (2009) Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J Bone Joint Surg Br 91:636–644
Rabinov K, Paulin S (1972) Roentgen diagnosis of venous thrombosis in the leg. Arch Surg 104:134–144
Kälebo P, Ekman S, Lindbratt S, Eriksson BI, Pauli U, Zachrisson BE, Close P (1996) Percentage of inadequate phlebograms and observer agreement in thromboprophylactic multicenter trials using standardized methodology and central assessment. Thromb Haemost 76:893–896
Lau J, Ioannidis JPA, Schmid CH (1997) summing up the evidence: one answer is not always enough. Lancet 351:123–127
Thompson SG (1994) Why sources of heterogeneity in meta-analysis should be investigated. Br Med J 309:1351–1355
Acknowledgments
We are indebted to the authors of the primary studies; without their contributions, this work would have been impossible.
Conflict of interest
The authors state that they have no conflict of interest.
Funding of study
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, Y.B., Zhang, J.D., Shen, H. et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 66, 1099–1108 (2010). https://doi.org/10.1007/s00228-010-0889-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0889-z