Abstract
Background
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used in the elderly. NSAIDs may cause a series of adverse drug reactions, of which gastrointestinal, renovascular/cardiovascular and bleeding complications are of particular concern. Concomitant use of several other drugs may further increase the risk of these adverse effects.
Objective
To examine the extent of chronic use of NSAIDs and co-prescription of drugs which may seriously interact with NSAIDs in elderly subjects, by using data from the Norwegian Prescription Database.
Results
A total of 7.3% of all individuals (71,681/984,457) over 60 years of age filled at least one prescription for reimbursed NSAIDs during the 1-year study period (2006). Co-prescription of medications which may interact with NSAIDs was prevalent for drugs used for hypertension and/or heart failure (59.5%), antithrombotic drugs (35.1%), systemic glucocorticoids (12.9%) and SSRI antidepressants (8.3%). As many as 4.8 and 3.8% of NSAID users were co-prescribed warfarin or oral methotrexate respectively.
Conclusion
The frequent co-prescription of medications which may cause detrimental interactions in elderly chronic NSAID users adds to safety concerns regarding this widely prescribed class of drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used, in particular by the elderly. By inhibiting prostanoid and thromboxane synthesis, NSAIDs interfere with essential regulatory mechanisms and may cause a series of adverse events, either induced by the NSAID acting alone or in concert with other, and thus interacting, drugs. The most important adverse events associated with NSAID use are gastrointestinal complications, including ulcers and bleeding [1, 2], and renovascular/cardiovascular reactions with fluid retention and in some cases deteriorated renal function, resulting in elevated blood pressure and an increased risk for heart failure [3–6]. NSAIDs also affect coagulation and blood vessel homeostasis with an ensuing propensity for either bleeding or thromboembolic complications [7]. The old and infirm are disproportionally over-represented as far as all of these complications are concerned [1–7].
Concomitant use of drugs which alter the risk of gastric ulceration and/or bleeding [gastroprotective agents, antithrombotic drugs, selective serotonin reuptake inhibitors (SSRIs), systemic glucocorticoids], drugs used for hypertension and/or congestive heart failure agents [digitalis glycosides, diuretics, beta blockers, calcium antagonists, angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists] and potentially toxic drugs which are critically dependent on intact renal function for their elimination (methotrexate, lithium) may modify the risks involved [8]. The aim of this study was to explore the extent of chronic use of NSAIDs and the frequency of co-prescription of potentially interacting drugs in subjects aged 60 years or older.
Materials and methods
Prescription database
The Norwegian Prescription Database (NorPD) is a record of all prescriptions filled by individual patients outside institutions from January 1, 2004, and covers the entire Norwegian population of approx. 4.7 million inhabitants [9]. NSAID prescriptions are reimbursed by the government when used for chronic (duration >3 months annually) gout, rheumatoid arthritis, ankylosing spondylitis (Bekhterev’s disease) and severe forms of cox- and gonarthrosis; these prescriptions can be specifically identified in the database by drug reimbursement codes.
The data collected from NorPD and used in this study included unique patient identifiers (encrypted), age, dispensing date and drug information, including the code according to the Anatomical Therapeutic Chemical (ATC) classification system, the defined daily dose (DDD) and the appropriate drug reimbursement code. For this study, the ATC/DDD version valid from 2009 was used [10].
Study population
All subjects born in 1946 or before who during 2006 received at least one reimbursed (i.e., having a treatment duration of at least 3 months) oral NSAID prescribed for either chronic gout (reimbursement code 3), rheumatoid arthritis or ankylosing spondylitis (code 17) or cox- or gonarthrosis (code 35) were included in the study. The drugs defined as NSAIDs were those belonging to ATC group M01A excluding M01A X, which encompasses glucosamine (which is not an NSAID) and nabumetone (which was negligibly used). By 2006, the selective COX-2 inhibitors had been omitted from the Norwegian reimbursement regime. In addition we retrieved information on all the other drugs these subjects had received during the same year. Individuals having at least one prescription for any of the potentially interacting agents dispensed from the pharmacy during 2006 were included in further analysis. These drugs were chosen on the basis of the possible significance of the interactions with NSAIDs [8]. The following groups and agents were included:
-
Gastroprotective drugs
-
Histamine H2 receptor antagonists (ATC group A02B A)
-
Misoprostol (ATC group A02B B)
-
Proton pump inhibitors (ATC group A02B C)
-
-
Antithrombotic drugs
-
Warfarin (ATC code B01A A03)
-
Clopidogrel (ATC code B01A C04)
-
Ticlopidine (ATC code B01A C05)
-
Acetylsalicylic acid, low dose (ATC code B01A C06)
-
Dipyridamole (ATC code B01A C07)
-
-
Drugs used for hypertension and/or congestive heart failure
-
Digitalis glycosides (ATC group C01A A)
-
Thiazide diuretics (ATC group C03A)
-
Loop diuretics (ATC group C03C)
-
Beta blockers, including combination preparations (ATC group C07)
-
Calcium antagonists (ATC group C08)
-
ACE inhibitors and angiotensin II receptor antagonists, including combination preparations (ATC group C09)
-
-
Glucocorticoids for systemic use (ATC group H02A B)
-
Methotrexate, tablets (ATC code L04A X03)
-
Lithium (ATC code N05A N01)
-
SSRI antidepressants (ATC group N06A B)
If a subject received an NSAID which was reimbursed in accordance with one of the reimbursement codes listed above, she or he was considered to be a chronic NSAID user. At least one co-prescription of any of the potentially interacting drugs during 2006 was interpreted as co-administration of the drugs in question. Patients with an incomplete person identifier represented <1% of prescriptions of interest and were excluded from further analysis.
Statistical comparisons were performed using chi-squared tests. When various age groups were compared, chi-squared tests for trend were applied. P values less than 0.05 were considered statistically significant.
Results
The number of individuals ≥60 years of age prescribed NSAIDs for chronic gout, rheumatoid arthritis/ankylosing spondylitis and/or severe cox- and gonarthrosis amounted to 71,681, corresponding to 7.3% of the total population of 984,457 of this age. In the age groups 60–69, 70–79 and ≥80 years, the proportions were 7.6, 7.9 and 5.9% respectively.
The median and mean numbers of DDDs of NSAIDs dispensed to the 71,681 chronic users during 2006 were 133 and 179 respectively. Of all users, 22,944 (32.0%) received 90 DDDs or less, 20,259 (28.3%) received 91–180 DDDs, 11,794 (16.5%) received 181–270 DDDs, 7,414 (10.3%) received 271–360 DDDs and 9,271 (12.9%) received more that 360 DDDs during the year. Using co-prescription of warfarin (ATC code B01A A03), low dose acetylsalicylic acid (ATC code B01A C06) and ACE inhibitors and angiotensin II receptor antagonists (ATC group C09) as sentinels, we found no major differences in the distribution of annual NSAID use in DDDs as a function of whether the patients had received co-prescribed interacting drugs or not (data not shown).
The degree of co-prescription of potentially interacting agents is shown in Table 1. Co-prescription was prevalent for gastroprotective drugs, antithrombotic drugs and drugs used for hypertension and/or congestive heart failure, whereas systemic glucocorticoids, methotrexate, SSRIs and lithium were co-prescribed to a lower degree.
Gastrointestinal safety and co-prescription
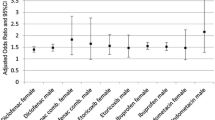
Antithrombotic medication was prescribed to 35.1% of chronic NSAID users 60 years or older (Table 1). The two drugs most widely used concomitantly were acetylsalicylic acid and warfarin, which were prescribed to 28.4 and 4.8% of the NSAID users respectively (Table 2). Clopidogrel, ticlopidine and dipyridamole were used by <2% each. The frequency of co-prescription of antithrombotic medication increased significantly with age (P < 0.001) (Table 1, Fig. 1).
Of the patients prescribed NSAIDs, 12.9 and 8.3% were also prescribed a systemic glucocorticoid or an SSRI antidepressant drug respectively (Table 1).
In total, 20.6% filled prescriptions for a gastroprotective drug, either a proton pump inhibitor (16.9%) or a histamin H2 receptor antagonist (4.7%). Although the use of antithrombotic drugs increased with age, this pattern was not found for the prescription of gastroprotective medications (Fig. 1).
Cardiovascular safety and co-prescription
Prescription of drugs which lower blood pressure or are used to treat congestive heart failure was highly prevalent, with a total co-prescription rate of 59.5% (Table 1). Notably, the use increased considerably with age, from 50.9% in the age group 60–69 years, to 72.4% in the age group 80 years and above (P < 0.001). The drugs most often used in concert with NSAIDs were ACE inhibitors/angiotensin II receptor antagonists (36.4%), beta blockers (28.0%), calcium antagonists (19.5%) and loop diuretics (15.6%). Thiazide diuretics and digitalis glycosides were prescribed to 4.8 and 2.3% respectively (Table 3).
We separately analysed the frequency of co-prescription of cardiovascular agents in our group of NSAID users compared to the rest of the population in the same age group. With the exception of digitalis glycosides, the NSAID users were significantly overrepresented as far as co-prescribed cardiovascular drugs were concerned (Table 4).
Methotrexate and lithium
Oral methotrexate was prescribed to 3.8% of our group of presumably chronic NSAID users aged 60 or above. Just 204 individuals (i.e., 0.3%) were prescribed lithium, and 121 of these were in the 60–69 years age group (Table 1).
Discussion
The study population comprised patients aged 60 years or above who had at least one reimbursed prescription of NSAIDs for either cox- or gonarthrosis, rheumatoid arthritis/alkylosing spondylitis or chronic gout, dispensed at Norwegian pharmacies during 2006. Since less than half of the NSAID use in a community, irrespective of age, is related to arthritic conditions [1], our study population represents a minority as far as NSAID users in general are concerned. However, the risk of gastrointestinal [1, 2], cardiovascular [3–5] and renovascular [6] adverse events associated with NSAID use increases massively as a function of age [1–7], and drug interactions are clearly a more common problem in advanced age. The study population thus represents the relevant age group. Reimbursement criteria for all NSAID prescriptions in the current analysis include chronicity in the sense that patients must require drug therapy over a period exceeding 3 months per year. Assuming that one DDD corresponds to an average daily dose, 1/3 of patients in our cohort were dispensed NSAIDs corresponding to up to 3 months of treatment and about 40% were dispensed NSAIDs corresponding to a treatment period of 6 months or more. As far as ascertained, there were no major differences between amounts of NSAID prescribed to patients given the potentially interacting drugs and patients not co-prescribed such drugs. Thus, the population largely meets the requirement of being chronic NSAID users. It is also important to notice that data on drugs given to patients in health institutions are not available at the individual level in the NorPD. Thus, we have probably underestimated the number of users, particularly among individuals of advanced age, where an increasing proportion will be institutionalised.
In addition, we only know that the prescriptions have been filled at the pharmacies; with the current methodology, there is no way to ascertain that any of the drugs in question were actually consumed. Moreover, although the NSAID and the potentially interacting drug were prescribed during the same year, we do not know that they were actually used simultaneously. The amount of NSAIDs dispensed, with a mean number of DDDs per patient of 179, is nevertheless considerable. With a few exceptions, most notably gastroprotective agents and to some extent SSRIs, the potentially interacting agents are used for chronic conditions. It may also be argued that the prescription of two or more drugs with a propensity for serious interactions within the same time frame nevertheless reflects a potential health hazard for the patients involved.
We had no access to outcome data such as death or hospitalisation in our cohort of patients. The facts that many of the interactions investigated may evade diagnosis in these patients, that many of the patients are treated outside of hospitals, and that the rate of autopsies and postmortem toxicological analyses is low in Norway raise the additional issue of reliability in outcome data.
The time period for the study (the year 2006) was chosen because of the assumed heightened awareness about adverse events associated with use of NSAIDs in general in the aftermath of the COX-2 inhibitor debacle brought about by the worldwide withdrawal of rofecoxib (Vioxx) in late 2004. Thus, it seems reasonable to assume that the prescriptions have been made by physicians who at least must have had some notion about NSAID-related safety issues, and that the data do not reflect a ”worst case” scenario.
Gastrointestinal safety and co-prescription
Gastrointestinal adverse reactions to NSAIDs are reported to account for in excess of 70,000 hospitalisations and 7,000 deaths in the US [11] and 1,200 deaths from complications from ulcers in the UK [12] annually. Gastric complications, including deaths, from NSAIDs are rare in younger individuals, but increase sharply after the age of 60 [1].
Almost one-third of our cohort of chronic NSAID users was co-prescribed low-dose acetylsalicylic acid, and approximately 5% of the patients were co-prescribed warfarin. Co-administration of acetylsalicylic acid and an NSAID has been associated with an increased risk for gastric ulceration in both case control [13] and cohort [14] studies. Warfarin and a NSAID should only be co-prescribed with extreme caution, as concluded from a retrospective cohort study [15]. A more recent case control analysis [16] has suggested that the risk may be smaller than originally perceived [15], but this particular drug combination is nevertheless to be strongly discouraged. Of special concern in our study group is the fact that the co-prescription of these drugs increased significantly with age, with almost 8% of the chronic NSAID users ≥80 years of age being prescribed warfarin.
About 1/10 of the NSAID-exposed elderly patients were co-medicated with either an SSRI or a systemic glucocorticoid, both of which have been shown to significantly enhance the risk of upper gastrointestinal toxicity [1, 17].
Prescriptions of gastroprotective medication, most frequently proton pump inhibitors, were given to approximately 20% of our chronic NSAID users. Whereas exposure to antithrombotic agents increased with age, this trend was not seen with gastroprotective agents (Fig. 1). Further analyses revealed that of the patients co-exposed to both NSAIDs and warfarin (n = 3,439), only 24.0% (n = 825) were given gastroprotective drugs. This seems at odds with good clinical practice.
Cardiovascular safety and co-prescription
Epidemiological data have shown an association between NSAID use and hypertension [18, 19], and meta-analyses of clinical data have confirmed that NSAIDs may increase blood pressure in both normotensive and hypertensive individuals [3, 4] and antagonise the effect of antihypertensive drugs [4]. Moreover, several observational studies suggest an association between the use of NSAIDs and heart failure [20–25], although there is controversy as to whether this association relates to the onset or worsening of the condition.
Almost 60% of the NSAID users in our study population were co-prescribed drugs used for hypertension and/or congestive heart failure. A large fraction (28–36%) of patients filled prescriptions for calcium antagonists, beta blockers, ACE inhibitors or angiotensin II receptor antagonists, whereas as few as 4.8% were prescribed thiazide diuretics. Moreover, 2.3 and 15.6% were prescribed digitalis glycosides or loop diuretics respectively, which amongst other indications may reflect congestive heart failure. Some of the other co-prescribed drugs (beta blockers, ACE inhibitors and angiotensin II receptor antagonists) are also used in the treatment of heart failure. Data from the observational investigations cited above [18–25] should, in our view, be considered a major caveat against the use of NSAIDs in patients with congestive heart failure. ACE inhibitors and angiotensin II receptor antagonists also have an increased propensity for causing renal dysfunction in combination with NSAIDs [8].
Data in Table 4 show that the relative risk for the use of cardiovascular drugs except for digitalis glycosides is higher in the NSAID group than in the general population of the same age. NSAID therapy may in itself be a risk factor for receiving treatment with antihypertensives [18, 19], or NSAIDs may worsen an established state of hypertension or congestive heart failure and thus increase the need for pharmacotherapy. However, it must be emphasised that no consideration is given to duration of treatment or sequence of and time period between prescriptions filled. Furthermore, our study group includes only users who received an NSAID in accordance with one of the reimbursement codes, whereas users who filled non-reimbursed prescriptions or purchased NSAIDs over the counter are included in the control group. The relative risk for exposure to cardiovascular drugs among NSAID users may thus be even more pronounced than calculated.
Methotrexate and lithium
Although the detailed mechanism(s) remain subject to speculation, the interactions between NSAIDs on one hand and lithium or methotrexate on the other are well established [8]. In both instances, co-medication may lead to accumulation of the latter compounds and enhanced lithium or methotrexate toxicity. As both of these drugs display renal toxicities, have narrow therapeutic indices and are cleared by the kidneys, these interactions may precipitate a “vicious circle” with rapid deterioration and, occasionally, fatal outcomes. Elderly patients, and particularly individuals with an already compromised renal function, appear to be especially at risk. In our study group, the co-prescription of NSAIDs and methotrexate was relatively frequent, as would be expected from the overlapping indications for these drugs, whereas NSAIDs and lithium were not often used concomitantly.
Conclusion
In our study group, which included all Norwegians aged 60 or above prescribed chronic NSAID therapy for gout, rheumatoid arthritis, ankylosing spondylitis or cox- and gonarthrosis in primary healthcare, there was a high incidence of co-prescriptions of potentially interacting drugs. Antithrombotic drugs, drugs used for hypertension and congestive heart failure, SSRIs, glucocorticoids and oral methotrexate were all frequently co-prescribed to the elderly NSAID users. In particular, it is noteworthy that as many as 5% of the patients were prescribed an NSAID and warfarin within the same time frame. Monotherapy with NSAIDs may confer serious toxicities, and the elderly—and especially infirm individuals with compromised cardiovascular and renal function—are particularly at risk. On the other hand, available data suggest that NSAIDs are not very effective when used in chronic conditions. For example, a meta-analysis of NSAIDs in gonarthrosis suggested that a majority of individuals would be unable to discern the effect of NSAID therapy from that of placebo [26]. This raises the worrying possibility that for many old and chronic users, NSAIDs actually do more harm than good. When these drugs in addition are co-prescribed with other agents that in combination can cause severe and possibly life-threatening interactions, the assumed benefit may further deteriorate. Although we did not have access to outcome data in the present study, our results suggest that co-prescription of NSAIDs and interacting drugs in the elderly constitutes a major problem. Improved educational initiatives directed towards prescribers and enhanced vigilance during all stages of prescription handling may improve this state.
References
Seager JM, Hawkey CJ (2001) Indigestion and non-steroidal antiinflammatory drugs. BMJ 323:1236–1239
Hawkey CJ, Langman MJS (2003) Non-steroidal antiinflammatory drugs: overall risks and management. Complementary roles for COX-2 inhibitors and proton pump inhibitors. Gut 52:600–608
Pope JE, Andersson JJ, Felson DT (1993) A meta-analysis of the effects of NSAIDs on blood pressure. Arch Intern Med 153:477–484
Johnson AG, Nguyen TV, Day RO (1994) Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med 121:289–300
Garcia Rodriguez LA, Hernandez-Diaz S (2003) Nonsteroidal antiinflammatory drugs as a trigger of clinical heart failure. Epidemiology 14:240–246
Whelton A, Hamilton CW (1991) Non-steroidal anti-inflammatory drugs: effects on kidney function. J Clin Pharmacol 31:588–598
Patrono C, Patrignani P, Garcia Rodriguez LA (2001) Cyclooxygenase-selective inhibition of prostanoid formation: transducing biochemical selectivity into clinical read-outs. J Clin Invest 108:7–13
Baxter K (ed) (2008) Stockley’s drug interactions, 8th edn. Pharmaceutical Press, London
Furu K, Strøm H, Rønning M, Skurtveit S, Engedal A, Tverdal A (2005) The Norwegian Prescription Database (NorPD): new register for pharmacoepidemiological research covering a whole nation. Pharmacoepidemiol Drug Saf 14(Suppl 2):S48
WHO Collaborating Centre for Drug Statistics Methodology (2008) ATC classification index with DDDs 2009. WHO, Oslo
Fries JF (1991) NSAID gastropathy: the second most deadly rheumatic disease? Epidemiology and risk appraisal. Rheumatology 28(Suppl):6–10
Hudson N, Hawkey CJ (1993) Non-steroidal anti-inflammatory drug-associated upper gastointestinal ulceration and complications. Eur J Gastroenterol Hepatol 5:412–419
Weil J, Colin-Jones D, Langman M, Lawson D, Logan R, Murphy M, Rawlins M, Vessey M, Wainwright P (1995) Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ 310:827–830
Sorensen HT, Mellomkjær L, Blot WJ, Nielsen GL, Steffensen FH, McLaughlin JK, Olsen JH (2000) Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterol 95:2218–2224
Shorr RI, Ray WA, Daugherty JR, Griffin MR (1993) Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med 153:1665–1670
Battistella M, Mamdami MM, Juurlink DN, Rabeneck L, Laupacis A (2005) Risk of upper gastrointestiunal hemorrhage in warfarin users treated with nonselective NSAIDs or COX-2-inhibitors. Arch Intern Med 165:189–192
Helin-Salmivaara A, Huttunen T, Gronroos JM, Klaukka T, Huupponen R (2007) Risk of serious upper gastrointestinal events with concurrent use of NSAIDs and SSRIs: a case-control study in the general population. Eur J Clin Pharmacol 63:403–408
Johnson AG, Simons LA, Simons J, Friedlander Y, McCallum J (1993) Nonsteroidal anti-inflammatory drugs and hypertension in the elderly: a community-based cross-sectional study. Br J Clin Pharmacol 35:455–459
Gurwitz JH, Avorn J, Bohn RL, Glynn RJ, Monane M, Mogun H (1994) Initiation of antihypertensive treatment during nonsteroidal anti-inflammatory drug therapy. JAMA 272:781–786
Heerdink ER, Leufkens HG, Herings RMC, Ottervanger JP, Stricker BH, Bakker A (1998) NSAIDs associated with increased risk of congestive heart failure in elderly patients taking diuretics. Arch Intern Med 158:1108–1112
Page J, Henry D (2000) Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an unrecognized public health problem. Arch Intern Med 160:777–784
Merlo J, Broms K, Lindblad U, Björck-Linné A, Liedholm H, Östergren PO, Erhardt L, Råstam L, Melander A (2001) Association of outpatient utilisation of non-steroidal antiinflammatory drugs and hospitalised heart failure in the entire Swedish population. Eur J Clin Pharmacol 57:71–75
Feenstra J, Heerdink ER, Grobbe DE, Stricker BH (2002) Association of nonsteroidal anti-inflammatory drugs with first occurrence of heart failure and with relapsing heart failure: the Rotterdam study. Arch Intern Med 162:265–270
Mamdani M, Juurlink DN, Lee DS, Rochon PA, Kopp A, Naglie G, Austin PC, Laupacis A, Stukel TA (2004) Cyclo-oxygenase-2 inhibitors versus non-selective non-steroidal anti-inflammatory drugs and congestive heart failure outcomes in elderly patients: a population-based cohort study. Lancet 363:175–176
Hudson M, Richard H, Pilote L (2005) Differences in outcomes of patients with congestive heart failure prescribed celecoxib, rofecoxib, or non-steroidal anti-inflammatory drugs: population based study. BMJ 330:1370–1375
Bjordal JM, Ljunggren AE, Klovning A, Slørdal L (2004) Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo-controlled trials. BMJ 329:1317–1322
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vandraas, K.F., Spigset, O., Mahic, M. et al. Non-steroidal anti-inflammatory drugs: use and co-treatment with potentially interacting medications in the elderly. Eur J Clin Pharmacol 66, 823–829 (2010). https://doi.org/10.1007/s00228-010-0825-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0825-2