Abstract
Aim
To describe the pattern of drug use among Chinese women during the first trimester and to examine the impact of maternal diseases on the choice of drugs.
Method
This drug utilisation study of pregnant women was performed using data from the ABCD cohort study. A total of 4,290 women were enrolled in the analysis. Information was collected by self-completion questionnaire combined with the “Maternal health handbook”.
Results
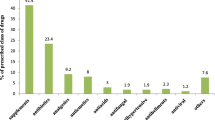
Of the 4,290 women interviewed, 75.9% of women took at least one drug during the first trimester. Users took a mean number of 1.43 drugs. The most frequently used drugs were folic acid (65.2%), vitamins (14.6%), calcium (12.0%), minerals (11.1%), Chinese traditional patent medicine (CTPM; 10.1%) and anti-infectives (6.5%). Among the women having used CTPM, influenza/cold and threatened abortion were the most commonly reported indications. Logistic regression analysis of drug use (excluding nutritional and haematological drugs) shows that CTPM and Western medicine are both associated with the use of drugs for occasional diseases and against threatened abortion. Maternal chronic diseases were not associated with the use of CTPM.
Conclusion
This analysis of pregnant women showed that drugs were prescribed to most women, even when nutritional and haematological drugs were excluded. Our data reflect, except for drugs used for chronic diseases, a general reluctance among Chinese women to use Western medicine and resorting to CTPM during pregnancy
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the thalidomide disaster, many studies have been performed to establish drug exposure during pregnancy [1]. International studies have shown that drugs have been used extensively during pregnancy in recent years [2–11]. Nonetheless, there were no data on the pattern of drug consumption in Chinese women.
Pharmacoepidemiological studies have been performed on non-Chinese pregnant women. Since traditional ideas of avoiding drug use during pregnancy may be a prevailing dogma, fear of failure and having a negative result in epidemiological studies were widespread. Also, China as a developing country lacked a large-scale or well-defined register system, especially pregnancy exposure registries. Thus, other methodologies were worth learning to open up the field of research on drug use during pregnancy, such as those used in the ALSPAC cohort in the UK [3] and the Perinatal Project in the US [12], etc.
In the light of easy accessibility of drugs, including prescription and over-the-counter (OTC) drugs obtained from drugstores, coupled with the inadequate health care system in China [13], self-medication has probably increased the proportions of drug use, especially for common complaints. Previous studies have shown that drug consumption behaviours during pregnancy are socially conditioned patterns that are associated with socio-cultural factors, religions, customs and beliefs [14]. However, we have not yet discovered the influence of Oriental culture on drug utilisation during pregnancy, especially in China.
Traditional Chinese herbal medicine is an integral part of Chinese civilisation and one of the major systems of contemporary medicine in practice in China. It consists of three categories: Chinese medicinal materials, Chinese herbal pieces and CTPM. Although these drugs are often promoted as “being of plant origin” and “risk free”, the use of herbal medicines during pregnancy has raised concerns worldwide in recent years [15].
First, information on the potential teratogenic effect is limited. In addition, many CTPM contain high levels of heavy metals, Western medicines or prohibited ingredients that are not demonstrated in the specification [16].
The present study reports data from a well-defined Chinese cohort, in which birth outcomes and long-term child development are still being followed. The study aims to examine the pattern of drug utilisation in the first trimester in Chinese women, with particular attention focussed on CTPM. The aim was also to investigate whether different maternal diseases had an influence upon maternal choice of CTPM or Western medicine.
Materials and methods
The ABCD cohort study
The ABCD cohort study (Anhui Birth Defects and Child Development Cohort Study) is an on-going prospective longitudinal cohort study in Anhui Province of China starting in October 2008 to investigate the relationship between prenatal environmental exposure and birth defects/child development. Women who receive prenatal care at maternal and child health (MCH) centres are eligible for inclusion in the cohort. After providing written informed consent, the data on social demographic characteristics, occupational experience, chemicals exposure, including drug- and life style-related, are collected by questionnaire from the first trimester of pregnancy. Eligible women also had their serum samples collected at their first prenatal visit, which were frozen at −80°C for future analysis. This research is the key programme for the China National Key Technology Research and Development Programme in 11th Five-Year Plan Period.
Eligible pregnant women
The data for this study were collected as part of the ABCD cohort study. Pregnant women resident in Hefei and Maanshan regions in the north and south of the Yangtze River respectively were eligible, covering 766,743 women of childbearing age. The data were collected in seven MCH centres from October 2008 to June 2009, three in Hefei and four in Maanshan respectively, covering an average of 18,437 pregnant women per year. The study population comprised 4,290 women, and the dates of the last menstrual period fell between 21 April 2008 and 27 March 2009. The seven MCH centres provide community-based prenatal care and establish the “Maternal health handbook” service. After uniform training, the health doctors recruited women and managed all the questionnaires in the MCH centres. Women who were recruited into the cohort were given the questionnaire after the first trimester. Thus, the sample was considered to be representative of the whole regions of Hefei and Maanshan, which contain a population of women from varied socio-economic backgrounds.
The study was approved by the institutional review board of the Anhui Medical University (Approval No. 2008020). Ensuring the realisation of the women’s right to informed consent, eligible women provided written informed consent.
Drug exposure ascertainment
Information was collected by a self-completion questionnaire combined with the “Maternal health handbook”, which established a feedback system between the client and the authorities via the care provider. Pregnant women who received their first prenatal care at MCH centres were given the questionnaire. Thus, all the information was collected before child birth. Floating population was excluded from the study, and whose Hukou, the system of residency permits in mainland China, is not in Hefei or Maanshan. A structured questionnaire was specifically designed for the purposes of the cohort study to collect:
-
1.
Basic socio-demographic questions included maternal age, body mass index, race, place of residence, education level, socio-economic status, gestational age, last menstrual period and previous pregnancy history
-
2.
A mixture of information about drug use and also inpatient and outpatient diagnoses and pregnancy complications in detail
-
3.
If and where available, a review of each participant’s “Maternal health handbook” to provide additional information and details
To improve the quality of data on drug use, our questionnaire contained open-ended questions with indication-orientated and drug-orientated questions, and also we combined the recall of drug use with maternal diseases. Questionnaires contained the following three questions asked in sequence. The first question was open-ended: “please describe which kind of medicines you have taken during the first trimester?”; the second asked about drug use for selected indications; and the third asked: “if you have forgotten, can you describe to which type the medicine belonged?’
First trimester was defined as between the date of the last menstrual period and completion of 12 weeks’ gestation. We also divided drugs according to the Anatomical Therapeutic Chemical (ATC) codes into 12 mutually exclusive therapeutic groups as follows: haematological drugs; nutritional drugs; drugs against threatened abortion; gastrointestinal drugs; cardiovascular drugs; anti-infectives; CNS drugs except analgesics; analgesics; respiratory drugs; other drugs; unknown code. This classification of the drug categories is similar to that adopted by an Italian report [10]. All drugs were also divided into two main groups: CTPM and Western medicine.
Information on maternal chronic diseases, occasional diseases and threatened abortion were obtained from the interview questionnaire and elicited from the “Maternal health handbook”. Epilepsy, diabetes mellitus, inflammatory bowel disease. asthma, chronic gastroduodenal ulcer, chronic bronchitis, hyperthyroidism, hypothyroidism, hypertension, heart disease, and allergy diseases were counted as maternal chronic diseases. Occasional diseases included infection, fever and influenza/cold during pregnancy.
Statistical analysis
The impact of three kinds of disease on two types of drug use, controlling for the other independent variables, was estimated as adjusted odds ratios with 95% confidence intervals (CI) in binary logistic regression. For each of the two types of drug use (yes/no), a separate model was employed. The reference category consisted of women who did not use any kind of drugs. P values were two-tailed, and all tests were conducted at the P < 0.05 level of statistical significance. Crude and adjusted odds ratios (OR) and the corresponding 95% confidence intervals (CI) were computed. The statistical analyses were performed using the Statistical Package for Social Sciences (SPSS), version 10.0.
Results
A total of 4,290 pregnant women of any gestational age attending antenatal visits at the MCH centres in Hefei and Maanshan from October 2008 to June 2009 were recruited to elicit information about their pattern of medication use during the first trimester. Two thousand four hundred and eighty-seven (58.0%) women came from Hefei, and the remaining 1,803 (42.0%) from Maanshan. The mean number of drugs used in Hefei was 1.36, and in Maanshan it was 1.48. One thousand an thirty-two (24.1%) women received no drugs and 2,244 (52.3%) only haematological and nutritional drugs (minerals, calcium, vitamins, iron preparations and folic acid). Three thousand two hundred and fifty-eight (75.9%) used at least one drug. Three thousand and four (70.0%) women used haematological and nutritional drugs. One thousand and fourteen (23.6%) women consumed therapeutic drugs (including CTPM and Western medicines).
Table 1 shows the characteristics of three groups of women who were recruited in this study according to their drug consumption. The mean ages of women who did not use any drugs and women who used at least one drug were 26.7 and 27.2 years respectively. The majority of women (79.8%) were between 22 and 30 years of age. The women’s ages of the whole sample (n = 4,290) ranged from 18 to 46 years with a mean age of 27.1 years (SD 3.36 years).
Table 2 presents the percentages of all drug groups used in pregnancy. The mean number of drugs per women was 1.43.The most commonly used drug was folic acid (65.2%).
Table 3 presents the most commonly reported indications for use of CTPM in the first trimester. In all, 471 indications for use were reported. The most commonly reported indications were influenza/cold (n= 204), but only 18 (4%) women chose CTPM to treat chronic medical problems.
Table 4 shows the most commonly reported CTPM used, and the CTPM containing multiple products, including herbs, plants, animal parts, minerals, which are formulated into tablets or pills for ease of use. In our study, CTPM were reportedly used by 434 pregnant women (10.1%). Among 434 women, the most frequently used CTPM were Ban Lan Gen, which was used by 24.4% of the women.
Table 5 shows the binary logistic regression model of the risk of using two groups of drugs (except for haematological and nutritional drugs) according to maternal reported diseases. Single factor analysis showed maternal chronic diseases (OR 1.97; 95% CI 1.40–2.77), occasional diseases (OR 12.75; 95% CI 10.12–16.06) and threatened abortion (OR 2.73; 95% CI 2.23–3.35) were all associated with maternal CTPM use. In multivariate logistic regression analyses, only occasional diseases (OR 13.23; CI 10.37–16.88) and threatened abortion (OR 2.86; 95% CI 2.25–3.63) were included in the model. With regard to Western medicine, after adjustment for all other variables, maternal chronic diseases (OR 4.43; 95% CI 3.21–6.10), occasional diseases (OR 10.24; 95% CI 8.40–12.48) and threatened abortion (OR 2.52; 95% CI 2.05–3.10) were significantly associated with Western medicine use. In addition, women who had occasional diseases were more inclined to consume CTPM and Western medicine.
Discussion
Methodological differences in worldwide studies of data collection have made it difficult to compare drug consumption during pregnancy directly. There are manufacturers’ registries, observational studies based on self-completion questionnaires or personal interview, database linkage studies and medical records [17]. The pros and cons of these methods are discussed [1, 17]. Although data from register-based studies were considered to be the most accurate, detailed comparisons of prescribed drugs were also difficult [3, 10].
The reason was the lack of uniform reporting standards and different reimbursement regulations for prescribed drugs. As pointed out by Egen-Lappe and Hasford [4], white-collar employees with more qualifications take out medical insurance, so selection bias in a register-based large cohort study also existed.
The mean or median number of prescription drugs computed from a prescription database was often higher than the data originating from a questionnaire-based study for the same drug or sub-category [10].
Although the name and dosage of drugs prescribed to pregnant women are very clear from the data, they cannot confirm that medications have been actually consumed. Consequently, the true drug use pattern is not reflected; drug use may have been overestimated. Conversely, self-administration and OTC drugs data cannot be collected in the database; thus, maternal risk exposure may be underestimated. For example, only 1.2% of women used vitamin B12 and folic acid during the first trimester in a population-based cohort study of 106,000 pregnancies in Norway [11].
Reliability of recall can be a problem in studies based on personal interviews and subject questionnaires, especially if collection of the data has been delayed until some time after delivery [18].
Despite this, only a few studies collect information before childbirth [3, 6]. Sometimes women reported only the medical problem for which they requested a drug; therefore, multiple drugs may have been considered as one medication under the pertinent drug category. In our study, we used open-ended questions including indication-orientated and drug-orientated questions to aid women to recall their true drug consumption, and some specific questions were to establish only the use of nutritional and haematological drugs [19].
Although it is difficult to make a comprehensive comparison of many studies, some distinction by comparison may also reflect a number of issues such as drug use pattern and the risk factors of drug use. Previous reports showed that drug use varies between countries, even in the same country it varies over time. Except for haematological and nutritional drugs, 23.6% of women used therapeutic drugs including Western medicine and CTPM. This was lower than 36.2% of the women using at least one therapeutic drug in a study of four European countries [6]. Including haematological and nutritional drugs, most women (75.9%) consumed at least one drug in our study. This was higher than 41% of all the women who received at least one drug in the first trimester in a recent study in Italy [2], because 65.2% of women added folic acid in our study.
The mean number of drugs per woman was 1.43, which was mainly due to haematological and nutritional drugs, because 70% of women received these. The mean number of drugs per woman was lower than a study in France which was 5.2 per woman, based on the records of the French Health Insurance Service in the South West of France [7]. This higher prescription rate during pregnancy resulted from compensation for drugs with different reimbursement mechanisms and overestimated drug exposure [8].
In addition, it was consistent with similar studies in Italy [10] and in north India [5]. Although folic acid alone was the most frequently prescribed substance, the Ministry of Health recommended that all women who were newly married or planning pregnancy should take a pill containing 400 mg of folic acid daily, and women who were receiving prenatal care were advised to take folic acid [20]. Thus, the intervention of preventing neural tube defects (NTD) is not carried out effectively.
In comparison, other haematological or nutritional drugs are taken relatively infrequently compared with other studies [10], such as iron preparations, vitamins and minerals. Calcium supplementation was advised by doctors to be taken during pregnancy in MCH centres in China. Iron preparations have been frequently prescribed in some European countries [2, 7, 10], but only 5.9% of women took them in our study. Iron deficiency, which increases over the period of gestation, are of great public health concern in China. The fact that iron deficiency is not obvious in early pregnancy may be the reason, and socio-demographic factors also influence the use of iron supplements and prophylactically prescribed iron was low. One study from Germany [4] reported that 31% of the women received iodide during pregnancy to avoid diseases of the thyroid gland in both mother and child. In our study, none of the women used iodide during pregnancy, because womens’ dietary intake of iodide is sufficient in China.
Anti-infective drugs, drugs against threatened abortion and analgesics were the most frequently used Western medicines, but these were lower than in other similar studies [3, 5, 6, 10]. Two possible explanations were as follows: women avoided the use of Western medicine; women found CTPM as alternative therapy for fear of teratogenicity caused by drugs.
In the United States, the FDA still classifies herbs as dietary supplements and they are not regulated like conventional drugs. However, in China, CTPM are classed as therapeutic drugs. In traditional Chinese beliefs, Chinese herbal medicines were seen to be safe for use during pregnancy [21].
The utility rate of CTPM was 10.1% in the first trimester. As far as we know, no literature has reported CTPM use in pregnant women in mainland China. Studies from Hong Kong [22] and Taiwan [23] have shown only the use of Chinese medicinal materials or Chinese herbal pieces during pregnancy. In a recent study in Taiwan, 33.6% reported using Chinese herbal medicines during pregnancy [23]. The most commonly used Chinese herbal medicines during pregnancy were an-tai-yin (13.5%). Under the circumstances of complementary medicine booming worldwide [15], data from most studies pay close attention to the use of traditional Chinese herbal medicine as complementary and alternative drugs. Thus, the use of CTPM as a therapeutic drug should be a matter of widespread concern, especially in mainland China.
Previous studies have reported an association between socio-demographic factors and traditional Chinese herbal medicine [23] or Western medicine [10]. However, predictors of CTPM use have not been analysed before. In the current study, maternal chronic diseases were not associated with CTPM use, but Western medicine was. CTPM were not effective therapeutic drugs in treating chronic diseases and many pregnant women avoiding using drugs for chronic conditions may be main reason. Women with occasional diseases used more CTPM and Western medicine than those with maternal chronic diseases and threatened abortion.
Threatened abortion is an independent risk factor for using CTPM, as reported by Chuang et al. [21]. Because of a lack of public awareness of the risks of consuming traditional Chinese herbal medicine, the possibility of any teratogenic or fetopathic effects connected with herbal medication, especially when consumed during first trimester of pregnancy, is seldom even considered.
Limitations of the study
As a study based on questionnaires, we have ignored some of the factors associated with drug use, such as occupation, marital status (living with husband or not) and treatment before conception. Also, recall bias and uncontrollable factors such as seasonal variation were ineluctable. China as a multi-racial country, diversities in traditional medicinal knowledge, experience, culture, local economic development, beliefs and the procurability of health care have influenced the drug use pattern. Obviously, the present data cannot be representative of all Chinese women.
Conclusions
This drug utilisation study found medication use to be very common during the first trimester of pregnancy in Chinese women, even when nutritional and haematological drugs were excluded. It also observed that CTPM are used extensively in the first trimester and our data reflect a general reluctance among Chinese women to use Western medicine and thus resorting to CTPM. Because of the limited safety information on these drugs, CTPM use during pregnancy should raise more concern. In addition, it is necessary for healthcare personnel to discuss the use of herbal drugs with their pregnant patients. Our results may be useful as a comparison for further studies.
References
Källén BA (2005) Methodological issues in the epidemiological study of the teratogenicity of drugs. Congenit Anom (Kyoto) 45:44–51
Gagne JJ, Maio V, Berghella V, Louis DZ, Gonnella JS (2008) Prescription drug use during pregnancy: a population-based study in Regione Emilia-Romagna, Italy. Eur J Clin Pharmacol 64:1125–1132
Headley J, Northstone K, Simmons H, Golding J, ALSPAC Study Team (2004) Medication use during pregnancy: data from the Avon Longitudinal Study of Parents and Children. Eur J Clin Pharmacol 60:355–361
Egen-Lappe V, Hasford J (2004) Drug prescription in pregnancy: analysis of a large statutory sickness fund population. Eur J Clin Pharmacol 60:659–666
Sharma R, Kapoor B, Verma U (2006) Drug utilization pattern during pregnancy in North India. Indian J Med Sci 60:277–287
De Vigan C, De Walle HE, Cordier S, Goujard J, Knill-Jones R, Aymé S, Calzolari E, Bianchi F (1999) Therapeutic drug use during pregnancy: a comparison in four European countries. OECM Working Group. Occupational Exposures and Congenital Anomalies. J Clin Epidemiol 52:977–982
Lacroix I, Damase-Michel C, Lapeyre-Mestre M, Montastruc JL (2000) Prescription of drugs during pregnancy in France. Lancet 356:1735–1736
Bakker MK, Jentink J, Vroom F, Van Den Berg PB, De Walle HE, De Jong-Van Den Berg LT (2006) Drug prescription pattern before, during and after pregnancy for chronic, occasional and pregnancy-related drugs in the Netherlands. BJOG 113:559–568
Lacroix I, Hurault C, Sarramon MF, Guitard C, Berrebi A, Grau M, Albouy-Cossard C, Bourrel R, Elefant E, Montastruc JL, Damase-Michel C (2009) Prescription of drugs during pregnancy: a study using EFEMERIS, the new French database. Eur J Clin Pharmacol 65:839–846
Donati S, Baglio G, Spinelli A, Grandolfo ME (2000) Drug use in pregnancy among Italian women. Eur J Clin Pharmacol 56:323–328
Engeland A, Bramness JG, Daltveit AK, Rønning M, Skurtveit S, Furu K (2008) Prescription drug use among fathers and mothers before and during pregnancy. A population-based cohort study of 106,000 pregnancies in Norway 2004–2006. Br J Clin Pharmacol 65:653–660
Hardy JB (2003) The Collaborative Perinatal Project: lessons and legacy. Ann Epidemiol 13:303–311
Blumenthal D, Hsiao W (2005) Privatization and its discontents—the evolving Chinese health care system. N Engl J Med 353:1165–1170
Checa MA, Peiró R, Pascual J, Carreras R (2005) Drug intake behaviour of immigrants during pregnancy. Eur J Obstet Gynecol Reprod Biol 121:38–45
[No authors listed] (1996) Complementary medicine is booming worldwide. BMJ 313:131–133
Ko RJ (1998) Adulterants in Asian patent medicines. N Engl J Med 339:847
Moretti M (2007) Collection and analysis of drug safety data in pregnancy. Can J Clin Pharmacol 14:e34–e36
Paganini-Hill A, Ross RK (1982) Reliability of recall of drug usage and other health-related information. Am J Epidemiol 116:114–122
Mitchell AA, Cottler LB, Shapiro S (1986) Effect of questionnaire design on recall of drug exposure in pregnancy. Am J Epidemiol 123:670–676
Li S, Moore CA, Li Z, Berry RJ, Gindler J, Hong SX, Liu Y, Mulinare J, Wong LY, Gu HQ, Erickson JD (2003) A population-based birth defects surveillance system in the People’s Republic of China. Paediatr Perinat Epidemiol 17:287–293
Chuang CH, Hsieh WS, Guo YL, Tsai YJ, Chang PJ, Lin SJ, Chen PC (2007) Chinese herbal medicines used in pregnancy: a population-based survey in Taiwan. Pharmacoepidemiol Drug Saf 16:464–468
Leung KY, Lee YP, Chan HY, Lee CP, Tang MH (2002) Are herbal medicinal products less teratogenic than Western pharmaceutical products. Acta Pharmacol Sin 23:1169–1172
Chuang CH, Chang PJ, Hsieh WS, Tsai YJ, Lin SJ, Chen PC (2009) Chinese herbal medicine use in Taiwan during pregnancy and the postpartum period: a population-based cohort study. Int J Nurs Stud 46:787–795
Acknowledgements
This research was supported financially by the China National Key Technology Research and Development Program 2006BAI05A03. We are extremely grateful to all the women who took part and to all the health doctors for their co-operation and help in recruitment. All authors contributed to the writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, X., Qi, X., Hao, J. et al. Pattern of drug use during the first trimester among Chinese women: data from a population-based cohort study. Eur J Clin Pharmacol 66, 511–518 (2010). https://doi.org/10.1007/s00228-009-0781-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-009-0781-x