Abstract
Background
Chronic migraine (CM) and medication-overuse headaches (MOH) are well-recognized disabling conditions affecting a significant portion of the headache population attending centers specialized in treating headaches. A dysfunctioning of the serotonergic system has been demonstrated in MOH and CM patients. Here we report on our assessment of the dysfunctioning of the endocannabinoid system as a potential underlying factor in pathogenic mechanisms involved in CM and MOH.
Method
To test the hypothesis of an impairment in the endocannabinoid system in patients with MOH and CM and to assess its relationship with any disruption of the serotonergic system, we determined the levels of the two main endogenous cannabinoids, anandamide (AEA) and 2-acylglycerol (2-AG), in platelets of 20 CM patients, 20 MOH patients and 20 control subjects and also measured the platelet serotonin levels in the same patients.
Results
We found that 2-AG and AEA levels were significantly lower in MOH patients and CM patients than in the control subjects, without significant differences between the two patient groups. Serotonin levels were also strongly reduced in the two patient groups and were correlated with 2-AG levels, with higher values for MOH patients.
Conclusion
These data support the potential involvement of a dysfunctioning of the endocannabinoid and serotonergic systems in the pathology of CM and MOH. These systems appear to be mutually related and able to contribute to the chronification of both CM and MOH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Around 40% of all patients attending Headache Centers complain of a chronic form of headache, mainly chronic migraine (CM), and 80% of these patients excessively use symptomatic drugs [1]. Although medication-overuse headache (MOH) has a prevalence of 1–2% in the general population, it represents a relevant health problem associated with considerable long-term morbidity and disability [2].
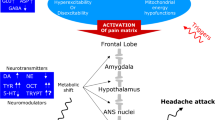
The pathophysiology of CM is poorly understood, and although several factors, such as analgesic and triptan overuse, increasing age and psychiatric disorders, are reported to play some role in the chronification of headache, the precise cascade of events leading to this condition is still unclear. This cascade may involve an impairment of nociceptive processing, associated with an increased excitability and sensitization of the dorsal horn neurons and neurons in the trigeminal nucleus caudalis and increased descending facilitation from the rostral ventromedial medulla, which may contribute to this increased excitability [3–5]. A dysfunctioning of the periaqueductal grey matter, which is the center of a powerful descending antinociceptive neuronal network projecting to the rostral ventromedial medulla and subsequently to the dorsal horn and which is also involved in the behavioral response to drug withdrawal, may explain why frequent analgesic use can result in MOH in migraineurs [6]. An integrative hypothesis for compulsive reward-seeking behavior has been proposed in MOH, similar that seen in cases of substance dependence and addiction. Medication-overuse headache has also been recently associated with reversible metabolic changes in some pain-processing structures and with persistent orbitofrontal hypofunction even after the withdrawal of analgesics [7, 8].
Human platelets share several receptors and transduction signaling pathways with neuronal cells, and previous findings suggest the possibility that platelets can be used as a cellular model of the central nervous system (CNS) [9, 10]. An alteration in serotonin platelet concentration has been found in patients with chronic headache consequent to analgesic abuse, as has an up-regulation of 5-HT2 platelet receptors, which has been correlated with chronification of the headache [11–14]. A recent investigation has focused on the endocannabinoid system, comprised of endocannabinoid receptors [the cannabinoid (CB) receptors], the endocannabinoids thenselves, the proteins involved in endocannabinoid synthesis and inactivation and the intracellular signaling pathways affected by endocannabinoids. Two lipidic mediators, acting through binding to both central and peripheral receptors (CB1 and CB2), have been demonstrated to be involved in the modulation of pain [15, 16]. Nevertheless, the role of endocannabinoids in primary headache is not clear. Using an in vivo model, Akerman et al. [17] demonstrated that anandamide (AEA), one of the best-studied endocannabinoids, is able to inhibit trigeminal neurons and dural blood vessel dilatation, mechanisms implicated in the pathogenesis of migraine via the binding to CB1 receptors on fibers of the spinal trigeminal tract and nucleus caudalis. CB2 receptor activation also inhibits inflammatory hyperalgesia, but no evidence is as yet available in this context for the trigemino-vascular system [18].
Anandamide has also been demonstrated to potentiate 5-hydroxytryptamine (5-HT)1A and inhibit 5-HT2A receptors, which are serotonin receptors, thereby supporting therapeutic efficacy in acute and preventive migraine treatment. It is also tonically active in the periaqueductal grey matter, which has been hypothesized to be a putative migraine generator [19–21].
The endocannabinoid system has been demonstrated to be involved in the neurobiological mechanism underlying drug addiction. The constituents of this system have in fact been recognized to modulate glutamatergic cortico-striatal transmission and influence the activity of the mesolimbic reward system [22, 23]. As such, it is possible that this system is active in drug-seeking and MOH patients.
Enzyme fatty acid amide hydrolase (FAAH), which is involved in endocannabinoid intracellular degradation, and AEA membrane transporter have been characterized recently in human platelets [24]. Human platelets have also been shown to bind and degrade 2-acylglycerol (2-AG), which activates these cellular elements through the CB1 cannabinoid receptor [25]. Anandamide also is able to induce platelet activation via its cleavage by specific hydrolase activity, leading to the release of arachidonic acid (AA) which, in turn, activates platelets [26]. Moreover, human platelets have been shown to respond to platelet-activating factor (PAF) stimulation by producing both monoacylglycerols [27].
Recent findings have demonstrated an increase in the activity of AEA hydrolase and AEA transporter in female but not male migraineurs, without any difference in cannabinoid receptor expression between genders. These results suggest that there is an increased degradation of AEA in the platelets of migraineur women and, consequently, a reduced concentration of AEA in the blood which, in turn, may contribute to a reduced pain threshold and possibly explain the prevalence of migraine in women [28]. Experimental evidence also indicates a positive relationship between the activation of the endocannabinoid and serotonin systems in platelets, suggesting that the two systems may also be working together in influencing the central serotonergic pathways [21].
Platelets have been widely used as a model of the CNS. Although there are some potential limitations in extrapolating findings obtained using this peripheral model to studies of the CNS, platelet models are a useful tool to study the relationship between the serotonergic and endocannabinoid systems in various pathological conditions, including CM and MOH.
The aim of this study was to test the hypothesis of a dysfunctioning (or part thereof) endocannabinoid system related to a change in the serotonergic system in CM patients with and without medication overuse. We therefore determined the levels of two endogenous cannabinoids, 2-AG and AEA, in platelets of 20 patients affected with CM without medication overuse, 20 patients with MOH, diagnosed according to the International Classification of Headache Disorders-II (ICHD-II), and 20 age-matched control nonheadache subjects. We also investigated the relationship between the intraplatelet levels of the two endocannabinoids and those of serotonin in both the patient and control groups.
Patients and methods
Patients and controls
The study protocol was approved by the Local Ethics Committee; all patients provided their written consent to participate in the study.
Twenty consecutive patients affected by CM and 20 MOH patients with a past history of migraine without aura, based on ICHD-II, all of whom were attending the Headache Center of the Neurologic Clinic of the University of Perugia, were admitted to the study. The latter group fulfilled the diagnosis of analgesic or combination analgesic overuse according to the revised criteria of ICHD-II [29–31].
Control values were obtained from 20 age-matched subjects free of CNS or systemic diseases, as determined by blood tests and various clinical examinations. All control subjects were drug-free for at least 2 months prior to the start of the study. In particular, they had not used illicit drugs and medications, including both prescription drugs and over-the-counter products. Moreover, none of the control subjects were taking any medication at the time of blood sampling or had a personal or family history of migraine or suffered from tension-type headache.
Biochemical analysis of AEA and 2-AG
For the identification of AEA, platelets were isolated from 30-ml blood samples. Cells from platelet-rich plasma (PRP; 2 × 108/mL pure platelets) were suspended in Thyroid buffer containing 200 μM phenylmethylsulfonylfluoride (PMSF) to prevent the breakdown of AEA and 2-AG. Pelleted cells were then extracted three times with 2 volumes of ethylacetate; the organic phase was dried under nitrogen and resuspended in ethanol. The extract was fractionated by reverse phase high-performance liquid chromatography (HPLC) in an acidified methanol:water gradient, as described elsewhere [32]. The fractions corresponding to the elution time of authentic AEA (95–103 min) were dried under nitrogen and then derivatized with bis[trimethylsilyl]trifluoroacetamide (BSTFA) for 1 h at 70°C.
The samples were dried again and resuspended in n-hexane. The trimethylsilylether derivatives were subjected to gas chromatography/mass spectrometry (GC/MS) as described previously [32]. Anandamide eluted from the GC (model 5890, Hewlett-Packard, Palo Alto, CA) column at approximately 23.2 min. Based on the electron impact spectrum of AEA, selected ion monitoring analyses were performed on m/z 85, m/z 116 and m/z 404. The amount of 2-AG in the GC elution peaks was estimated using a calibration curve generated with 0.5, 2, 5, 10, and 20 ng 2-AG. The limit of sensitivity for 2-AG was 100 pg.
Using the same reverse phase HPLC system as for AEA, authentic 2-AG eluted between 105 and 115 min. These fractions were pooled, dried under nitrogen and derivatized with BSTFA, as described above. The GC/MS analysis of 2-AG was carried out under the same conditions as that for AEA except that a different GC column (SPB-5; 30 m × 0.25 mm × 0.25 μm) was used. 2-Acylglycerol eluted from this GC column at approximately 26.9–27.0 min. Based on the electron impact spectrum of authentic 2-AG, selected ion monitoring analyses were performed on m/z 103, m/z 129, m/z 147, m/z 432, m/z 451 and m/z 507.
Data were expressed as picograms per milliliter PRP (≅2 × 108 platelets).
Platelet serotonin content
The platelet pellet was separated from platelet-poor plasma by centrifuging the PRP at 70,000 g for 10 min. The pellet was then washed twice in HEPES-thyroid buffer [145 mM NaCl, 5 mM KCl, 1 mM MgSo4, 10 mM N-2-hydroxyethilpiperazine-n-2-ethan sulfonic acid (HEPES) and 1 mM glucose/L, pH = 7.4]. All platelets were resuspended at a concentration of 1 × 109/ml and frozen at −80°C until the assay. Immediately prior to the assay, the samples were thawed at room temperature and sonicated. Platelet 5-HT was measured using HPLC with electrochemical detection. The chromatographic system included a Kontron instrument (model 420) with a 5-μm particle diameter, tracer analytic ODS and a Il C4b amperometric detector. The mobile phase consisted of 0.1 M phosphoric acid and 0.1 mM EDTA, adjusted to pH 2.85 with 4 M KOH solution. The internal standard was 3,4-dihydroxybenzylamine. Platelet 5-HT levels were expressed as nanograms per 109 platelets.
Statistical analysis
Data were expressed as the mean ± SD. The ANOVA and the least significant difference (LSD) as post-hoc test were used for the comparison of the means among the three groups. Correlation between clinical variables and endocannabinoid and serotonin levels in all groups were calculated using the Pearson correlation test. P values less than 0.05 were considered to be significant.
Results
Details of patient and control groups are reported in Table 1. Drugs abused by MOH patients are shown in Table 2.
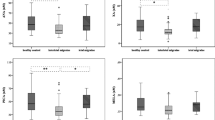
The content of 2-AG in the platelets of both the patients and control subjects was 20-fold greater than that of AEA. The levels of 2-AG and AEA were significantly lower in MOH patients and CM patients than in the controls (p < 0.0001 for both), but there were no significant differences between the two patient groups. Females in the CM and MOH groups showed significantly lower levels of AEA, 2-AG and serotonin than males (Table 3).
Serotonin levels were strongly reduced in the two patient groups (p < 0.001 and p < 0.002, respectively) with lower values detected in females than in males. They were also significantly correlated with 2-AG levels, with higher correlation coefficients for MOH (0.49, p < 0.02 and 0.82, p < 0.0001, respectively) (Fig. 1), but not with AEA levels.
No correlation was found between the intraplatelet levels of 2-AG, AEA and serotonin and age, age of headache onset (years), number of days with headache/month, headache index and number of drugs taken/month.
Discussion
In the study reported here, we used platelets as a peripheral model of some pathogenic mechanisms of CM and MOH. The choice of this model is based on the classical concepts developed from a comparison of the properties of blood platelets and serotonergic synaptosomes, which suggest that human platelets can serve as an appropriate model for the transport, metabolism and release of serotonin (5-HT) by CNS serotonergic neurons [32]. Although confirmation is necessary, it would appear that the relationship between different indices of 5-HT binding in the brain and 5-HT uptake characteristics in platelets is complex, nonuniform, and possibly gender-specific and that this observation can be extended to serotonin content. However, information from this model should be taken with caution when extrapolating the results to CNS serotonergic pathways [33].
Platelets have also classically been considered to be a suitable model for studying other forms of neurotransmitter and neuromodulator dysfunctioning, including endocannabinoids. Human platelets have been shown to possess the biochemical machinery to degrade AEA, which is a selective transporter of AEA and a fatty acid amide hydrolase (FAAH); they also bind and degrade 2-arachidonoylglycerol, which activates these cells through a cannabinoid receptor [24, 25].
However, to date, no studies have been conducted to compare data from platelets model and serotonergic pathways in terms of endocannabinoids and transporter and receptor expression with the aim of determining whether the findings from this peripheral model are reliably extendable to CNS pathways. Moreover, there are some recent data emphasizing the released of endocannabinoids, in particular anandamide, from blood cells ex vivo at a very high rate. Therefore, blood levels (in plasma and cellular elements) of these substances are strongly influenced by time and mode of storage until determination. This can influence their levels in blood cells, including platelets, which necessitates the application of strictly standardized pre-analytical protocols [34].
Despite the above limitations, platelets remain a useful tool to study both the serotonergic and endocannabinoid system due to the ease of collecting and separating these blood elements in several pathological conditions, including CM and MOH. We therefore hypothesize that our finding of reduced 2-AG and AEA levels in platelets related to reduced serotonin levels may reflect an imbalance in the endocannabinoid system which occurs in parallel with serotonergic dysfunctioning in these two chronic head pain conditions.
An increased degradation of AEA in platelets related to variations in the activity of AEA transporter and AEA hydrolase in peripheral platelets, compared with controls, consistently with a lowered endocannabinoid level has been recently shown in females with episodic migraine, but not in their male counterparts [28].
We confirmed the failure of the endocannabinoid system that had been previously demonstrated in episodic migraineurs, and in accordance with these previous findings we demonstrated significantly lower levels of 2-AG and AEA in females compared to males in both the CM and MOH groups. Values measured in males in both patients groups, however, were significantly reduced compared to those of the controls (when compared to only males or to the total group).
The failure of the endocannabinoid system observed at the peripheral level may be related to a dysfunctioning of the serotonergic system and can be hypothesized to occur within the CNS as well. To support this hypothesis, we refer to a recent finding of our group in which reduced levels of AEA and palmitylethanolamide were determined in the cerebrospinal fluid (CSF) of both CM and MOH patients, suggesting an imbalance in endogenous cannabinoid signaling that may contribute to chronic head pain through increased calcitonin gene-related peptide (CGRP) and nitric oxide (NO) production [35].
As found in previous CSF studies,, the reduced levels of both AEA and 2-AG do not seem to be specific to patients with CM because they are also evident – to an even greater extent – in patients with MOH. Consequently, it would appear that reduced levels of AEA and 2-AG are related to chronic head pain per se.
Based on previous CSF studies and actual platelet findings it is not possible to determine whether reduced endocannabinoid levels are a consequence of chronic pain in migraine patients. Although the precise cascade causing CM, both with and without medication overuse, is complex and has yet to be elucidated, the binding of endogenous cannabinoids to CB1 receptors is involved in the modulation of pain in general and chronic pain in particular. More specifically, the stimulation of the CB1 receptors is able to inhibit trigeminal neurons and dural blood vessel dilation, both mechanisms implicated in the pathogenesis of pain induction and maintenance in migraine [17, 36]. As such, it would seem that the chronic pain is likely stemming from a dysfunction in the CB system and not vice-versa. Moreover, the greater decrease in 2-AG in MOH patients relative to CM patients suggests that endocannabinoid dysfunctioning could also also play a potential role in the ‘addiction’ aspect in the drug-seeking behavior of MOH patients.
CB1 receptors are expressed in the mesolimbic reward circuit and modulate the dopamine-releasing brain reward systems. Endocannabinoids are also involved in the motivation to seek drugs by a dopamine-independent mechanism, known for psychostimulants and opioids but not yet demonstrated for other abused drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs) and triptans [37]. It can be hypothesized that in MOH patients, a dysfunctioning of the endocannabinoid system can result in a defect of endogenous brain reward processes and reward-related behaviors. This can underlie or reinforce motivational effects and relapse to the seeking behavior for abused drugs, such as NSAIDs and triptans [38]. These drugs may act, like other substances involved in addiction, as reward-enhancing drugs in that they increase neural firing and dopamine tone within the structures of mesolimbic reward systems. On the other hand, chronic exposure to such drugs can reduce endocannabinoid levels in different brain regions, such as the midbrain, hippocampus, striatum and cerebral cortex [39, 40]. This mechanism can further contribute to the decreased levels of AEA and 2-AG found in MOH patients. The above observation seems to be not true for AEA content in the limbic forebrain; some animal models of chronic exposure to habit-forming drugs have demonstrated that AEA content increases in this brain region [39]. Knowing that this region is a key area for the reinforcing properties of many abused drugs and taking into account the key importance of the CB1 receptor in the relapse and reinstatement of drug-seeking behavior, it remains to be established if similar modifications occur in the brain of MOH patients. Animal models aimed at studying this aspect should be developed as they could be helpful in investigating the usefulness of potential targeting of the endocannabinoid system in MOH, taking into account the complex intervention of this system in drug abuse mechanisms [41, 42].
On the basis of previous findings, it is possible to suggest that reduced endocannabinoid levels in platelets may be the consequence of an alteration in their degrading machinery. However, if this is the consequence of chronic pain maintenance, its cause remain to be established. Another question remaining to be answered is whether the reduction in AEA and 2-AG levels can be due to the expression of a particular genetic background in this subpopulation of headache patients that would influence both pain processing and threshold and/or be associated to drug abuse and dependence. This possibility is suggested by recent experimental findings [22, 35, 43].
This parallelism in the failure of endocannabinoids and serotonin is in line with experimental findings of a mutual interaction of 2-AG and 5-HT in modulating different signaling pathways involved in pain control. 2-Acylglycerol and 5-HT have been recognized to reinforce each other in terms of binding to their respective receptor on the platelet surface [25]. Therefore, altered expression of 5-HT2A receptors and reduced serotonin content, both of which are important mechanisms in the pathogenic mechanisms of CM, can themself contribute to the disruption of the endocannabinoid system. This impairment can further be impaired by chronic symptomatic exposure in MOH patients.
The mutual interaction between 2-AG and 5-HT is also supported by experimental data that demonstrate that 5-HT2A receptor stimulation leads to the release of 2-AG through the activation of phosphatidylinositol and phospholipase C. The consequence of this is the stimulation of a CB1 receptor by endocannabinoids released consequent to the 5-HT2A receptor activation; this step can represent a regulatory mechanism by which postsynaptic G-protein-coupled receptors can modulate presynaptic neurotransmitter release and neuronal plasticity [44]. A failure of this system can contribute to the abnormal modulation of brain nociceptive systems, leading to central sensitization which, in turn, may explain the shift in the migraine phenotype from episodic to chronic headache and to the maintenance of chronicity by the abuse of symptomatic medications.
Conversely, endogenous cannabinoids can surely affect the 5-HT system, as demonstrated by the finding that selective inhibition of the enzyme FAAH, which catalyzes the intracellular hydrolysis of anandamide, results in the increased firing activity of serotonergic neurons in the dorsal raphe nucleus and noradrenergic neurons in the nucleus locus ceruleus. This action is related to the increase in the endocannabinoid AEA and is prevented by CB1 antagonists [45]. Consequently, it cannot be excluded that a dysfunction in other endocannabinoids, such as 2-AG decrease, is ultimately causing the imbalance in the 5-HT system in CM and MOH. Further experimental studies aimed at targeting the specific enzyme participating in the degradation of this ester, the monoglyceride lipase, are needed [46].
Inhibitors of endocannabinoid degradation seem to be necessary tools for the development of endocannabinoid therapeutics, which is a subject attracting the growing interest of pharmacologists given their multiple potentials in terms of chronic pain and depression [47].
References
Pascual J, Colas R, Castillo J (2001) Epidemiology of chronic daily headache. Curr Pain Headache Rep 5:529–536
Diener HC, Limmroth V (2004) Medication-overuse headache: a worldwide problem. Lancet Neurol 3:475–483
Boes CJ, Black DF, Dodick DW (2006) Pathophysiology and management of transformed migraine and medication overuse headache. Semin Neurol 26:232–241
Srikiatkhachorn A (2001) Pathophysiology of chronic daily headache. Curr Pain Headache Rep 5:537–544
Welch KM (2003) Concepts of migraine headache pathogenesis: insights into mechanisms of chronicity and new drug targets. Neurol Sci 24(Suppl 2):S149–S153
Cupini LM, Calabresi P (2005) Medication-overuse headache: pathophysiological insights. J Headache Pain 6:199–202
Calabresi P, Cupini LM (2005) Medication-overuse headache: similarities with drug addiction. Trends Pharmacol Sci 26:62–68
Fumal A, Laureys S, Di Clemente L, Boly M, Bohotin V, Vandenheede M, Coppola G, Salmon E, Kupers R, Schoenen J (2006) Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain 129:543–550
D’Andrea G, Cananzi AR, Perini F, Hasselmark L (1995) Platelet models and their possibile usefulness in the study of migraine pathogenesis. Cephalagia 15:265–271
Gallai V, Floridi A, Mazzotta G, Codini M, Tognoloni M, Ambrosini P et al (1996) L-arginine/nitric oxide patway activation in platelets of migraine with and without aura patients. Acta Neurol Scand 94:151–160
Hering R, Glover V, Pattichis K, Catarci T, Steiner TJ (1993) 5HT in migraine patients with medication-induced headache. Cephalalgia 13:410–412
Srikiatkhachorn A, Govitrapong P, Limthavon C (1994) Up-regulation of 5-HT2 serotonin receptor: a possible mechanism of transformed migraine. Headache 34:8–11
Srikiatkhachorn A, Maneesri S, Govitrapong P, Kasantikul V (1998) Derangement of serotonin system in migrainous patients with analgesic abuse headache: clues from platelets. Headache 38:43–49
Sarchielli P, Alberti A, Russo S, Codini M, Panico R, Floridi A, Gallai V (1999) Nitric oxide pathway, Ca2+, and serotonin content in platelets from patients suffering from chronic daily headache. Cephalalgia 19:810–816
Calignano A, La Rana G, Giuffrida A, Piomelli D (1998) Control of pain initiation by endogenous cannabinoids. Nature 394:277–281
Walker JM, Hohmann AG (2005) Cannabinoid mechanisms of pain suppression. Handb Exp Pharmacol 168:509–554
Akerman S, Kaube H, Goadsby PJ (2004) Anandamide is able to inhibit trigeminal neurons using an in vivo model of trigeminovascular-mediated nociception. J Pharm Exp Ther 309:56–63
Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, Malan TP Jr (2003) Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology 99:955–960
Kimura T, Ohta T, Watanabe K, Yoshimura H, Yamamoto I (1998) Anandamide, an endogenous cannabinoid receptor ligand, also interacts with 5-hydroxytryptamine (5-HT) receptor. Biol Pharmacol Bull 21:224–226
Cheer JF, Cadogan AK, Marsden CA, Fone KC, Kendall DA (1999) Modification of 5-HT2 receptor mediated behaviour in the rat by oleamide and the role of cannabinoid receptors. Neuropharmacology 38:533–541
Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, Petrosino S, Guglielmotti V, Rossi F, Di Marzo V (2006) Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther 316:969–982
Parolaro D, Vigano D, Rubino T (2005) Endocannabinoids and drug dependence. Curr Drug Targets CNS Neurol Disord 4:643–655
Maccarrone M, Bari M, Battista N, Finazzi-Agro A (2002) Endocannabinoid degradation, endotoxic shock and inflammation. Curr Drug Targets Inflamm Allergy 1:53–63
Macarrone M, Bari M, Menichelli A, Giuliani E, Del Principe D, Finazzi-Agro A (2001) Human platelets bind and degrade 2-arachidonoylglycerol, which activates these cells through a cannabinoid receptor. Eur J Biochem 268:819–825, Feb
Maccarrone M, Bari M, Principe DD, Finazzi-Agro A (2003) Activation of human platelets by 2-arachidonoylglycerol is enhanced by serotonin. Thromb Haemost 89:340–347
Braud S, Bon C, Touqui L, Mounier C (2000) Activation of rabbit blood platelets by anandamide through its cleavage into arachidonic acid. FEBS Lett 471:12–16
Berdyshev EV, Schmid PC, Krebsbach RJ, Schmid HH (2001) Activation of PAF receptors results in enhanced synthesis of 2-arachidonoylglycerol (2-AG) in immune cells. FASEB J 15:2171–2178
Cupini LM, Bari M, Battista N, Argiro G, Finazzi-Agro A, Calabresi P, Maccarrone M (2006) Biochemical changes in endocannabinoid system are expressed in platelets of female but not male migraineurs. Cephalalgia 26:277–281
Headache Classification Subcommittee of the International Headache Society (2004) The international classification of headache disorders: 2nd edition. Cephalalgia 24(Suppl 1):9–160
Silberstein SD, Olesen J, Bousser MG, Diener HC, Dodick D, First M, Goadsby PJ, Göbel H, Lainez MJA, Lance JW, Lipton RB, Nappi G, Sakai F, Schoenen J, Steiner TJ, on behalf of the International Headache Society (2005) The international classification of Headache Disorders, 2nd Edition (ICHD-II)-revision of criteria for 8.2 Medication-overuse headache. Cephalalgia 25:460–465
Headache Classification Committee, Olesen J, Bousser MG, Diener HC, Dodick D, First M, Goadsby PJ, Gobel H, Lainez MJ, Lance JW, Lipton RB, Nappi G, Sakai F, Schoenen J, Silberstein SD, Steiner TJ (2006) New appendix criteria open for a broader concept of chronic migraine. Cephalalgia 26:742–746
Lingjaerde O (1990) Blood platelets as a model system for studying serotonergic dysfunction and effects of antidepressants. Pharmacol Toxicol 66(Suppl 3):61–68
Uebelhack R, Franke L, Herold N, Plotkin M, Amthauer H, Felix R (2006) Brain and platelet serotonin transporter in humans-correlation between [123I]-ADAM SPECT and serotonergic measurements in platelets. Neurosci Lett 406:153–158, Epub 2006 Aug 24
Vogeser M, Hauer D, Christina Azad S, Huber E, Storr M, Schelling G (2006) Release of anandamide from blood cells. Clin Chem Lab Med 44:488–491
Sarchielli P, Pini LA, Coppola F, Rossi C, Baldi A, Mancini ML, Calabresi P (2007) Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology 32:1384–1390
Akerman S, Holland PR, Goadsby PJ (2007) Cannabinoid (CB1) receptor activation inhibits trigeminovascular neurons. J Pharmacol Exp Ther 320:64–71
Maldonado R, Valverde O, Berrendero F (2006) Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29:225–232
Fattore L, Spano MS, Deiana S, Melis V, Cossu G, Fadda P, Fratta W (2007) An endocannabinoid mechanism in relapse to drug seeking: a review of animal studies and clinical perspectives. Brain Res Rev 53:1–16
González S, Cascio MG, Fernández-Ruiz J, Fezza F, Di Marzo V, Ramos JA (2002) Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res 954:73–81
Ferrer B, Bermúdez-Silva FJ, Bilbao A, Alvarez-Jaimes L, Sanchez-Vera I, Giuffrida A, Serrano A, Baixeras E, Khaturia S, Navarro M, Parsons LH, Piomelli D, Rodriguez de Fonseca F (2007) Regulation of brain anandamide by acute administration of ethanol. Biochem J 404:97–104
Beardsley PM, Thomas BF (2005) Current evidence supporting a role of cannabinoid CB1 receptor (CB1R) antagonists as potential pharmacotherapies for drug abuse disorders. Behav Pharmacol 16:275–296
Tucci SA, Halford JC, Harrold JA, Kirkham TC (2006) Therapeutic potential of targeting the endocannabinoids: implications for the treatment of obesity, metabolic syndrome, drug abuse and smoking cessation. Curr Med Chem 13:2669–2680
Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF (2002) A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci USA 99:8394–8399
Parrish JC, Nichols DE (2006) Serotonin 5-HT(2A) receptor activation induces 2-arachidonoylglycerol release through a phospholipase c-dependent mechanism. J Neurochem 99:1164–1175
Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D (2005) Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA 102(51):18620–18625, Erratum in: Proc Natl Acad Sci USA 103:2465, 2006
Vandevoorde S, Lambert DM (2005) Focus on the three key enzymes hydrolysing endocannabinoids as new drug targets. Curr Pharm Des 11:2647–2668
Bari M, Battista N, Fezza F, Gasperi V, Maccarrone M (2006) New insights into endocannabinoid degradation and its therapeutic potential. Mini Rev Med Chem 6:257–268
Acknowledgments
This study has been partially supported by the Lega Italiana Cefalalgici. The authors express their gratitude to John A. Toomey for editing the English and Marisa M. Morson for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossi, C., Pini, L.A., Cupini, M.L. et al. Endocannabinoids in platelets of chronic migraine patients and medication-overuse headache patients: relation with serotonin levels. Eur J Clin Pharmacol 64, 1–8 (2008). https://doi.org/10.1007/s00228-007-0391-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-007-0391-4