Abstract
Objective
Aripiprazole is an atypical antipsychotic drug which is metabolized by the polymorphic enzyme cytochrome P450 2D6 (CYP2D6). The aim of the present study was to investigate the impact of the CYP2D6 genotype on serum concentrations of aripiprazole (ARI) and to determine the sum of ARI and the active metabolite dehydroaripiprazole (DARI) in psychiatric patients.
Methods
Data on steady-state serum concentrations and the CYP2D6 genotypes of patients treated with ARI were extracted from a routine therapeutic drug monitoring database. The 62 patients included in the analysis were stratified into the following subgroups according to CYP2D6 genotype: *1/*1 (homozygous extensive metabolizers, EMs; n = 37), *1/*3–6 (heterozygous extensive metabolizers, HEMs; n = 17) and *3–6/*3–6 (poor metabolizers, PMs; n = 8). Dose-adjusted serum concentrations (C/D ratios) of ARI and ARI + DARI were compared between the subgroups.
Results
The median serum concentration of ARI was 1.7-fold higher in PMs than in EMs (45.5 vs. 26.3 nM/mg, p < 0.01). The observed serum concentration of the active sum of ARI + DARI was 1.5-fold higher in PMs than in EMs (53.9 vs. 37.0 nM/mg, p < 0.05). Numerical differences in serum concentrations between HEMs and EMs were less pronounced, but statistically significant for both ARI (p < 0.05) and ARI + DARI (p < 0.05).
Conclusion
The present study demonstrates that serum concentrations of both ARI and the active sum of ARI + DARI in psychiatric patients were significantly affected by CYP2D6 genotype. The observed differences in median C/D ratios indicate that PMs typically need 30–40% lower doses to achieve a similar steady-state serum concentration as EMs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The metabolism of many psychotropic drugs is influenced by the activity of the polymorphic enzyme cytochrome P450 2D6 (CYP2D6) [1–3]. The CYP2D6 gene displays a marked allelic heterogeneity, with approximately 90 variants described to date (http://www.cypalleles.ki.se). Many alleles have been associated with altered phenotype, ranging from the loss of catalytic activity in patients with two defective alleles (poor metabolizers, PMs) to increased enzyme activity in patients with gene multiplication (ultra-rapid metabolizers, UMs). The most frequent alleles encoding defective enzyme activity include CYP2D6*3–*6, while multiplicated alleles, such as CYP2D6*2XN, are reported to encode increased enzyme activity. The prevalence of phenotypes differs between ethnic groups, with PMs being most frequent among Caucasians (5–10%) and UMs being most frequent among black Africans (up to 30%) [2].
Aripiprazole (ARI) is a novel atypical antipsychotic drug exerting a partial agonist activity at dopamine-2 (D2) receptors and serotonin-1A (5-HT1A) receptors and antagonistic activity at 5-HT2A receptors [4]. According to data on file, CYP2D6 is involved in the formation of the major metabolite dehydroaripiprazole (DARI), which displays similar D2 receptor activity as ARI [5]. Systemic exposure of DARI is approximately 40% of that of the parent drug [5], and this metabolite might therefore be of clinical relevance for the response of aripiprazole treatment. The aim of the present study was to evaluate the impact of the CYP2D6 genotype on steady-state serum concentrations of both ARI and the active moiety ARI + DARI in psychiatric patients.
Materials and methods
Materials and patients
This retrospective study was conducted at the Department of Psychopharmacology, Diakonhjemmet Hospital, Norway. A therapeutic drug monitoring (TDM) database was screened for all CYP2D6-genotyped patients receiving aripiprazole as part of their clinical treatment in the period January 2004 to December 2006. Sampling time had to be specified on the requisition form (max 30 h since the last drug intake). Confirmation of steady-state conditions was performed by contacting the prescribing physicians or reviewing the TDM history. The requisition forms were reviewed for concurrently prescribed drugs. Patients using the CYP2D6 inhibitors fluoxetine and paroxetine, the CYP3A4 inhibitors diltiazem, verapamil, fluconazole, itraconazole, ketoconazole, clarithromycin, erythromycin, nelfinavir and ritonavir or the CYP3A4 inducers carbamazepine, phenytoin and phenobarbital, were excluded. Only one patient was excluded due to concurrent use of an interacting drug (fluoxetine). In total, serum concentrations from 62 patients fulfilled the inclusion criteria (Table 1). Information on the CYP2D6 genotype, serum concentration of ARI and DARI, serum sampling time, drug dosage, gender and age was recorded. When multiple serum concentrations were available for the same patient, the sample nearest in time to the CYP2D6 genotyping was selected. The study was approved by the Regional Committee for Medical Research Ethics.
Statistics
All serum concentrations of ARI, DARI and ARI + DARI were dose-adjusted (C/D ratios; nM/mg per day). The population was separated into the following CYP2D6 genotype subgroups according to the number of functional alleles: zero functional alleles (poor metabolizer; PM), one functional allele (heterozygous extensive metabolizer; HEM) and two functional alleles (homozygous extensive metabolizer; EM). Data were analysed by Kruskal-Wallis non-parametric rank analysis of variance to establish an overall difference between the CYP2D6 genotype groups, and Dunn’s post-test was applied for multiple comparisons. GraphPad Prism ver. 4 was used as software for graphics and statistical analyses. Statistical significance was considered as p < 0.05.
Analysis of parent drug and metabolite
All serum samples were analysed by a liquid chromatography-mass spectrometry method [6]. Briefly, serum samples were purified by protein precipitation, and ARI and DARI serum levels were determined by liquid chromatographic (LC) separation and tandem mass spectrometric (MS/MS) detection. Calibration curves for ARI and DARI were linear in the ranges of 40–1600 nM and 20–600 nM, respectively, with imprecision and deviation of ≤6%. All serum concentrations were within these ranges. The reference material of ARI was kindly provided by Bristol-Myers Squibb (Oslo, Norway), while DARI was synthesized by Synthetica (Oslo, Norway).
CYP2D6 genotyping
During the time-span of the sample collection the laboratory changed its method for CYP2D6 genotyping. The former method, which has been described in detail elsewhere [7], was based on PCR analysis with allele-specific primers that specifically determined CYP2D6*3, *4, *5, *6, *7 and *8 and CYP2D6*2XN. The novel real-time PCR method detected CYP2D6*3, *4, *6, *7 and *8 using mutation-specific TaqMan probes (Assay Reagents Allelic Discrimination Biosystems, Foster City, CA) and determined the presence of CYP2D6 gene deletion or multiplication by copy number analyses (method described in [8]). Prior to PCR, genomic DNA was extracted from leukocytes using E.Z.N.A Blood DNA kit (VWR International, Oslo, Norway). Cross-validation of the two methods, based on genotyping data from patients, showed 100% agreement. The absence of mutated alleles was interpreted as presence of the functional wild-type allele (CYP2D6*1).
Results
Characteristics of the three subgroups are summarized in Table 1. There were no statistical differences in age, gender, dosage or sampling time between the CYP2D6 subgroups. Eight patients carried zero functional CYP2D6 alleles (PM subgroup), 17 patients carried one functional CYP2D6 allele (HEM subgroup) and 37 patients carried two functional CYP2D6 alleles (EM subgroup). No subjects were carriers of a multiplicated CYP2D6 allele. Population frequencies of defective alleles were 22% for CYP2D6*4, 4% for CYP2D6*5 and 0.8% for CYP2D6*6 (CYP2D6*3, *7 and *8 not detected).
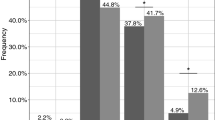
Across the CYP2D6 genotype subgroups, the range in serum concentrations of ARI and ARI+DARI between the individual with the lowest and the individual with highest serum concentration was 4.3-fold and 4.2-fold, respectively (Table 1). The median serum concentration of ARI was 1.7-fold higher in PMs than in EMs (p < 0.01) (Fig. 1a). No significant differences in the serum concentration of DARI were observed between the subgroups (Fig. 1b), but the median serum concentration of ARI + DARI was 1.5-fold higher in PMs than in EMs (p < 0.05) (Fig. 1c). Numerical differences in serum concentrations between HEMs and EMs was less pronounced, but statistically significant for both ARI (p < 0.05) and ARI + DARI (p < 0.05).
Individual concentration/dose (C/D) ratios of: a aripiprazole (ARI) – statistically significant difference between homozygous extensive metabolizers (EM) and poor metabolizers (PM) (p < 0.01), and between EMs and heterozygous extensive metabolizers (HEM) (p < 0.05), b dehydroaripiprazole (DARI) – no statistically significant differences between the CYP2D6 subgroups, c the active sum of ARI and DARI (ARI + DARI) – statistically significant difference between EMs and PMs (p < 0.05), and between EMs and HEM (p < 0.05). Medians are indicated in the three CYP2D6-genotype groups
Discussion
We found that the CYP2D6 genotype significantly affected steady-state serum concentrations of ARI and ARI+DARI in psychiatric patients. Median ARI and ARI + DARI values in PMs were 1.7-fold and 1.5-fold higher, respectively, than those in EMs. These findings indicate that PMs would typically need 30–40% lower dosages to achieve similar serum concentrations as EMs. There are currently no specific recommendations of dosage adjustment based on CYP2D6 metabolism, genotype or phenotype. However, the manufacturer’s prescribing information recommend a 50% dose reduction of aripiprazole during concomitant use of CYP2D6 inhibitors [5].
ARI exerts partial agonist activity at D2 receptors and 5-HT1A receptors, and antagonistic activity at 5-HT2A receptors [4]. Due to this complex mechanism of action, the clinical consequence of higher serum concentrations of ARI in PMs might be difficult to predict. Several clinical trials have established a favourable adverse event profile for aripiprazole [9, 10]. The most frequent adverse event reported leading to discontinuation was psychosis [11], and four case reports of exacerbation of psychosis related to initiation of aripiprazole therapy have been described [12]. Moreover, in a recent casuistic report, an episode with progressive symptoms of lethargy and memory loss was described when the aripiprazole dose was increased from 15 to 30 mg in a CYP2D6 PM patient [13]. Thus, it might be that the increased concentration in PMs actually trigger psychotic symptoms rather than induce non-psychotic adverse reactions.
The difference in median serum concentrations between EMs and PMs was statistically significant for both ARI + DARI and ARI, but less pronounced for the sum of ARI + DARI than compared to ARI. In therapeutic drug monitoring (TDM), it is common practice to summarize the parent drug and active metabolites when these are considered to have similar pharmacological activities. Due to dissimilar physio-chemical properties of the parent drug and active metabolite and thus potential differences in parameters such as brain distribution, a higher serum concentration of ARI may theoretically alter the clinical outcome to a greater extent than reflected by the sum of the active compounds [14]. For risperidone, another antipsychotic drug, it has been reported that the CYP2D6 PM phenotype is associated with an approximately three-fold higher risk of moderate-to-marked adverse drug reactions and discontinuation of therapy [15, 16], despite only a minor difference in the plasma concentration of the sum of risperidone and 9-hydroxyrisperidone between CYP2D6 PMs and EMs [17]. This supports that a drug and metabolite with similar pharmacological activities might differ in terms of in vivo action. Future studies should seek to compare the relative clinical importance of ARI and DARI.
There was a 4.3-fold range in serum ARI concentrations between EMs and PMs. Thus, the 1.7-fold difference in median serum concentration between EMs and PMs indicate that CYP2D6 is a substantial contributor to the inter-individual pharmacokinetic variability of ARI. In addition, ARI is metabolized by CYP3A4, and the extensive individual differences reported for this catalytic pathway are likely to be another important contributor to the variability in ARI serum concentrations [6]. A previous study by Kubo et al. has demonstrated that CYP2D6 and CYP3A4 are approximately equally responsible for the metabolism of ARI in CYP2D6 EMs [18]. Altogether, it is therefore likely to believe that CYP2D6 and CYP3A4 are the major enzymes involved in the metabolism of ARI.
According to data on file, the formation of DARI is mediated by CYP2D6 [5]. However, in the study reported here, the increased level of ARI in PMs relative to EMs was not accompanied by a corresponding decrease in DARI. This result indicates that CYP2D6 genetic variation is of minor importance for the serum concentration of DARI. The difference in impact of genetic variations in CYP2D6 on ARI and DARI may imply a possible involvement of CYP2D6 in alternative metabolic pathways of ARI. However, these results are in contrast to those of a previous study of Kubo et al., where a 1.6-fold higher exposure of ARI was accompanied by a 30–35% lower exposure of DARI in homozygous CYP2D6*10 carriers (intermediate metabolizers) compared to homozygous wild-type carriers [18]. While the present study was performed in psychiatric patients at steady-state, the study of Kubo et al. was carried out in healthy individuals following the administration of a single dose of aripiprazole. Due to the long elimination half lives (3–4 days [18]) of ARI and DARI, the steady-state concentrations are much higher than the concentrations obtained after a single dose. Thus, theoretically, the different outcomes of these two studies with respect to DARI might reflect differences in the formation or elimination kinetics of DARI.
In conclusion, we have demonstrated that the CYP2D6 genotype has a significant impact on steady-state serum concentrations of ARI and the sum ARI + DARI in psychiatric patients. Whether this implies a higher risk of increased psychotic symptoms and non-psychotic side-effects in PMs is uncertain as ARI and DARI display partial agonism at D2 receptors. Nevertheless, the observed differences in median C/D ratios indicate that PMs typically need 30–40% lower doses to achieve similar steady-state serum concentrations as EMs.
References
Kirchheiner J, Nickchen K, Bauer M, Wong ML, Licinio J, Roots I, Brockmoller J (2004) Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry 9:442–473
Bertilsson L, Dahl ML, Dalen P, Al-Shurbaji A (2002) Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol 53:111–122
Zanger UM, Raimundo S, Eichelbaum M (2004) Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol 369:23–37
Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R (2003) Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 28:1400–1411
Product information (2006) Abilify (Aripiprazole). Otsuka Pharmaceutical Europe, London
Molden E, Lunde H, Lunder N, Refsum H (2006) Pharmacokinetic variability of aripiprazole and the active metabolite dehydroaripiprazole in psychiatric patients. Ther Drug Monit 28:744–749
Rudberg I, Hendset M, Uthus LH, Molden E, Refsum H (2006) Heterozygous mutation in CYP2C19 significantly increases the concentration/dose ratio of racemic citalopram and escitalopram (S-citalopram). Ther Drug Monit 28:102–105
Schaeffeler E, Schwab M, Eichelbaum M, Zanger UM (2003) CYP2D6 genotyping strategy based on gene copy number determination by TaqMan real-time PCR. Hum Mutat 22:476–485
Kane JM, Carson WH, Saha AR, McQuade RD, Ingenito GG, Zimbroff DL, Ali MW (2002) Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 63:763–771
Potkin SG, Saha AR, Kujawa MJ, Carson WH, Ali M, Stock E, Stringfellow J, Ingenito G, Marder SR (2003) Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 60:681–690
Marder SR, McQuade RD, Stock E, Kaplita S, Marcus R, Safferman AZ, Saha A, Ali M, Iwamoto T (2003) Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res 61:123–136
Ramaswamy S, Vijay D, William M, Sattar SP, Praveen F, Petty F (2004) Aripiprazole possibly worsens psychosis. Int Clin Psychopharmacol 19:45–48
Oosterhuis M, Van De KG, Tenback D (2007) Safety of aripiprazole: high serum levels in a CYP2D6 mutated patient. Am J Psychiatry 164:175
Hendset M, Haslemo T, Rudberg I, Refsum H, Molden E (2006) The complexity of active metabolites in therapeutic drug monitoring of psychotropic drugs. Pharmacopsychiatry 39:121–127
de Leon J, Susce MT, Pan RM, Fairchild M, Koch WH, Wedlund PJ (2005) The CYP2D6 poor metabolizer phenotype may be associated with risperidone adverse drug reactions and discontinuation. J Clin Psychiatry 66:15–27
Kohnke MD, Griese EU, Stosser D, Gaertner I, Barth G (2002) Cytochrome P450 2D6 deficiency and its clinical relevance in a patient treated with risperidone. Pharmacopsychiatry 35:116–118
Huang ML, Van PA, Woestenborghs R, De CR, Heykants J, Jansen AA, Zylicz Z, Visscher HW, Jonkman JH (1993) Pharmacokinetics of the novel antipsychotic agent risperi1done and the prolactin response in healthy subjects. Clin Pharmacol Ther 54:257–268
Kubo M, Koue T, Inaba A, Takeda H, Maune H, Fukuda T, Azuma J (2005) Influence of itraconazole co-administration and CYP2D6 genotype on the pharmacokinetics of the new antipsychotic ARIPIPRAZOLE. Drug Metab Pharmacokinet 20:55–64
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hendset, M., Hermann, M., Lunde, H. et al. Impact of the CYP2D6 genotype on steady-state serum concentrations of aripiprazole and dehydroaripiprazole. Eur J Clin Pharmacol 63, 1147–1151 (2007). https://doi.org/10.1007/s00228-007-0373-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-007-0373-6