Abstract
Objective
To investigate the impact of age and co-treatment with other drugs on the serum concentrations of lamotrigine in children and adolescents.
Methods
A review of routine serum concentration measurements of lamotrigine performed in our laboratory yielded a total of 744 serum samples from 296 subjects (110 males, 186 females, age: 2–19 years) suitable for statistical analysis. The primary outcome variable was the dose-corrected lamotrigine serum concentration, expressed as the lamotrigine concentration/dose (C/D) ratio. A linear mixed model that allowed multiple observations from the same patient was used to identify and quantify the effect of factors influencing the lamotrigine C/D ratio.
Results
According to the model, the lamotrigine C/D ratio decreases by 6% per year of age. Valproate and levetiracetam were found to raise the lamotrigine C/D ratio, whereas the following co-medications reduced it: carbamazepine, clobazam, fluoxetine, clonazepam and ethinyl estradiol. The effect of carbamazepine decreased with increasing age. No gender difference was detected.
Conclusions
Age is an important factor with respect to the pharmacokinetics and the extent of drug interactions of lamotrigine in children and adolescents. In this population, older individuals will need higher doses than younger ones in order to achieve the same serum concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lamotrigine is an anticonvulsant drug with efficacy in various kinds of epileptic disorders in adults and in children. It has become a first-choice drug for a variety of seizure types and epileptic syndromes in childhood [1] and is also licensed for the treatment of bipolar disorder [2]. Children tolerate lamotrigine well, although the incidence of rash is higher than in adults [3, 4].
In humans, lamotrigine is not biotransformed by the hepatic cytochrome P-450 (CYP) system. Instead, it is metabolised almost exclusively via glucuronidation by uridindiphosphate glucuronosyltransferase (UGT), mainly UGT1A4. Only 10% of a given dose of lamotrigine is excreted unchanged [5]. Although these properties prevent lamotrigine from drug interactions involving the CYP system, several drugs, including combined oral contraceptives, may alter lamotrigine serum concentrations considerably, often necessitating dose adjustment [6–9].
Several studies on the pharmacokinetics of lamotrigine in pediatric populations have been published [10–14]. However, infants, children and adolescents do not form a homogenous group since it is well documented that drug metabolism is subject to developmental changes within childhood [15]. This applies particularly to the glucuronidation capacity, which may take from months to years post-partum to reach adult values [16, 17]. Recently, there has been growing interest for such developmental aspects of drug disposition [18]. While the pharmacological concept of clearance is central in this context, serum concentration and dose are more familiar terms for most clinicians [19]. The ratio formed by serum concentration divided by dose (C/D ratio) is essentially equivalent to 1/apparent clearance and thus equally suited to describe the relation between serum concentration and dose.
The aim of this study was to investigate the impact of age and drug interactions on the C/D ratio of lamotrigine, based on a large number of serum samples sent to our routine therapeutic drug monitoring service.
Materials and methods
Collection and analysis of samples
We reviewed all serum samples of patients aged 0–19 years which were analysed for lamotrigine in our laboratory over a period of 40 months. Our request form requires, among other information, statements of exact time for intake of the last dose and time of blood sampling, daily lamotrigine dose, number of daily intakes, diagnosis, co-medication and body weight. Age and gender were obtained from the population registry number on the request form. Unfortunately, body weight was not stated in the vast majority of samples. Samples without information on dose and/or time interval between last dose and sampling were excluded from the analysis. Moreover, samples taken less than 10 h or more than 24 h after intake as well as samples with a serum concentration below the limit of quantification of the analytical method were also excluded. Following these procedures, we obtained a total of 744 samples from 296 subjects aged 2.4 to 19.9 (median: 13.8) years that were suitable for statistical analysis.
All samples were analysed with a liquid chromatography-mass spectrometry (LC-MS) method as described previously [8]. In brief, the calibrated range of this method was 0.5–100 μmol/L. Six quality control samples covering the range from 2 to 45 μmol/L were analysed with every batch of unknown samples. The between-day coefficient of variation calculated from quality control samples was better than 9% at 2 μmol/L and 5% at 35 μmol/L. The lower limit of quantification was 0.2 μmol/L.
Statistical analysis
Basic descriptive statistic analysis of the raw data was performed with Microsoft Excel 2000 (Microsoft Corp, Redmond, Wash.), and with SPSS ver. 12 for Windows (SPSS Chicago, Ill.). Data are presented as means with standard deviations (SD) or 95% confidence interval (95% CI), or as medians with inter-quartile ranges (IQR) and/or total ranges, as appropriate. The lamotrigine C/D ratio was calculated by dividing the serum concentration of lamotrigine (expressed as μmol/L) by the total daily dose (in mg), and thus expresses the serum concentration per milligram lamotrigine given.
In terms of the elimination phase, we assumed a simple exponential model [5]. The distribution of the lamotrigine C/D ratio was found to be heavily right-skewed, and to achieve near normality, the natural logarithm of lamotrigine C/D ratio [i.e. log e (lamotrigine C/D ratio)] was employed as the outcome variable in the analysis.
Multiple samples were often available in the same patient. In order to utilise these data, we employed a linear mixed model that allows correlation between repeated observations [20]. This model assumes that each individual patient possesses a random intercept, i.e. an individual “offset”, in addition to being affected by the fixed factors. Model parameters, including variance components, were estimated using the method of restricted maximum likelihood (REML) with the software programme R ver. 2.4.0 [21, 22].
Model estimation proceeded backwards, starting with all potential explanatory variables (gender, age, and co-medications) in the model. At each successive step, the least significant factor was removed, and the model was re-fitted until only statistically significant factors, defined as p < 0.05, were present in the model. The generalised coefficient of determination, analogous to r 2 in multiple linear regression, which can take values between 0 and 1, was calculated from the residual variance under the null and full model, respectively [23]. According to the model, the expected lamotrigine C/D ratio can be calculated by the following equation:
where β0 represents the overall intercept and β1 to βi represent the coefficients of additional fixed factors.
The following co-medication was included in the model: valproate (n = 164), carbamazepine (n = 95), topiramate (n = 30), oxcarbazepine (n = 18), nitrazepam (n = 14), clobazam (n = 13), clonazepam (n = 12), combined oral contraceptives (n = 12), diazepam (n = 11), levetiracetam (n = 9), fluoxetine (n = 9) and phenobarbital/primidone (n = 8).
Results
Basic demographic and clinical data are given in Table 1. The distributions of dose, serum concentration and lamotrigine C/D ratio were all right-skewed, and values are therefore given as median with the IQR range in parentheses (n = 744): dose, 125 (75–200) mg/day; serum concentration, 13.1 (7.8–19.4) μmol/L; lamotrigine C/D ratio, 0.087 (0.055–0.148) (μmol/L)/(mg/day).
Table 2 gives the values for β0 (which represents the overall intercept) and for β1 to βi (representing the coefficients of additional fixed factors). No effect of gender was detected, but a significant age effect of −0.062 per year of age was estimated (note the logarithmic scale). The minus sign implies that the lamotrigine C/D ratio decreases with increasing age. We present here an example of how the β-coefficients are used: the intercept (β0) has a value of −1.694. The expected lamotrigine C/D ratio in a 4-year-old child (regardless of gender, and without co-medication) is therefore 0.14 (μmol/L)/(mg/day), as given by
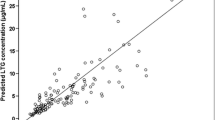
since the value for β1 is −0.062 (per year of age). In other words, each 1 mg lamotrigine given to a 4-year-old would be expected to raise the serum concentration by 0.14 μmol/L, and a daily dose of 100 mg would yield an expected concentration of 14 μmol/L. In contrast, a 12-year-old child would have a lamotrigine C/D ratio of \( {\text{e}}^{{{\text{ - 1}}{\text{.694 + 12}} \times {\left( {{\text{ - 0}}{\text{.062}}} \right)}{\text{ = 0}}{\text{.09}}{{\left( {\mu {\text{mol/L}}} \right)}} \mathord{\left/ {\vphantom {{{\left( {\mu {\text{mol/L}}} \right)}} {{\left( {{{\text{mg}}} \mathord{\left/ {\vphantom {{{\text{mg}}} {{\text{day}}}}} \right. \kern-\nulldelimiterspace} {{\text{day}}}} \right)}}}} \right. \kern-\nulldelimiterspace} {{\left( {{{\text{mg}}} \mathord{\left/ {\vphantom {{{\text{mg}}} {{\text{day}}}}} \right. \kern-\nulldelimiterspace} {{\text{day}}}} \right)}}}} \), and a daily dose of 100 mg would therefore yield a concentration of only 9 μmol/L. This is illustrated in Fig. 1.
If one or more of the other fixed factors were present, the expected C/D ratio would be altered accordingly (Table 2). If, for example, the above-mentioned 4-year-old child also was taking valproate, the expected lamotrigine C/D ratio would be \( {\text{e}}^{{{\text{ - 1}}{\text{.694 + 4}} \times {\left( {{\text{ - 0}}{\text{.062}}} \right)}{\text{ + 1}}{\text{.013 = 0}}{\text{.40}}{{\left( {{\text{ $ \mu $ mol/L}}} \right)}} \mathord{\left/ {\vphantom {{{\left( {{\text{ $ \mu $ mol/L}}} \right)}} {{\left( {{{\text{mg}}} \mathord{\left/ {\vphantom {{{\text{mg}}} {{\text{day}}}}} \right. \kern-\nulldelimiterspace} {{\text{day}}}} \right)}}}} \right. \kern-\nulldelimiterspace} {{\left( {{{\text{mg}}} \mathord{\left/ {\vphantom {{{\text{mg}}} {{\text{day}}}}} \right. \kern-\nulldelimiterspace} {{\text{day}}}} \right)}}}} \), with the profound consequence that a daily dose of 100 mg is expected to yield a serum concentration of 40 μmol/L.
Among the 13 drugs included in the initial model, we found not only the classical interacting agents valproate and carbamazepine to have a significant impact on lamotrigine C/D ratio, but also combined oral contraceptives, clobazam, clonazepam, levetiracetam, and fluoxetine (Table 2). Compared to valproate and carbamazepine, the effects of these drugs on the lamotrigine C/D ratio were smaller, and the relatively wide confidence intervals for these effects should be noticed. Oxcarbazepine (18 samples) and phenobarbital/primidone (eight samples) did not have statistically significant effects in our model.
The effect of carbamazepine on the lamotrigine C/D ratio was strongly age-dependent, as can be seen from the significant age and carbamazepine interaction term. The positive sign means that the effect of carbamazepine decreases with increasing age. As a final example of the calculation, consider again the 4-year old child, who this time is taking carbamazepine in addition to lamotrigine. The expected lamotrigine C/D ratio now equals \( {\text{e}}^{{{\text{ - 1}}{\text{.694 + 4}} \times {\left( {{\text{ - 0}}{\text{.062}}} \right)}{\text{ - 1}}{\text{.138 + 4}} \times {\text{0}}{\text{.039 = 0}}{\text{.05 }}{{\left( {{{\text{ $ \mu $ mol}}} \mathord{\left/ {\vphantom {{{\text{ $ \mu $ mol}}} {\text{L}}}} \right. \kern-\nulldelimiterspace} {\text{L}}} \right)}} \mathord{\left/ {\vphantom {{{\left( {{{\text{ $ \mu $ mol}}} \mathord{\left/ {\vphantom {{{\text{ $ \mu $ mol}}} {\text{L}}}} \right. \kern-\nulldelimiterspace} {\text{L}}} \right)}} {{\left( {{{\text{mg}}} \mathord{\left/ {\vphantom {{{\text{mg}}} {{\text{day}}}}} \right. \kern-\nulldelimiterspace} {{\text{day}}}} \right)}}}} \right. \kern-\nulldelimiterspace} {{\left( {{{\text{mg}}} \mathord{\left/ {\vphantom {{{\text{mg}}} {{\text{day}}}}} \right. \kern-\nulldelimiterspace} {{\text{day}}}} \right)}}}} \), and the expected serum concentration at a daily dose of 100 mg would be 5 μmol/L. This means a reduction in C/D ratio by 65%, compared to lamotrigine monotherapy (C/D ratio: 0.14; expected serum concentration at 100 mg/day: 14 μmol/l). Accordingly, a 12-year-old with the same medication would have a C/D ratio of \( {\text{e}}^{{{\text{ - 1}}{\text{.694 + 12}} \times {\left( {{\text{ - 0}}{\text{.062}}} \right)}{\text{ - 1}}{\text{.138 + 12}} \times {\text{0}}{\text{.039 = 0}}{\text{.045}}}} \), which means a lower reduction of the lamotrigine C/D ratio by carbamazepine of only 50%, (compared to the C/D ratio in monotherapy: 0.09). No interaction between age and valproate was detected.
For practical reasons, it may be desirable to know the dose necessary to achieve an intended target serum concentration. Using the above equation, and since the C/D ratio equals the serum concentration divided by dose, the required dose D can be found by the equation:
which is equivalent to:
where D is the daily dose in milligrams, and C is the target serum concentration in μmol/L (note the sign change of the exponent after transformation). For example, to achieve a target concentration of 20 μmol/L, an 8-year old individual not taking any of the drugs listed in Table 2 will need a dose of 20 × \( {\text{e }}^{{{\text{ - }}{\left( {{\text{ - 1}}{\text{.69 + 8}} \times {\left( {{\text{ - 0}}{\text{.062}}} \right)}} \right)}}} {\text{ = }}{{\text{178 mg}}} \mathord{\left/ {\vphantom {{{\text{178 mg}}} {\text{d}}}} \right. \kern-\nulldelimiterspace} {\text{d}} \). If this patient was also taking clonazepam, a dose increase towards 250 mg would be necessary to achieve the same serum concentration, according to the model.
On the log scale, the inter-individual (i.e. between patients) and intra-individual (i.e. within patients) variance components were estimated to be 0.412 and 0.352, respectively, yielding an intra-class correlation coefficient of 0.58, or 58%. This figure may be interpreted as the percentage of residual variation that can be attributed to individual patients [20].
The generalized coefficient of determination was found to be 0.35; thus, age and co-medication in Table 2 may be interpreted as explaining approximately 35% of the variation in the C/D ratio. Graphical residual analysis revealed no important deviations from the model assumptions of conditional normality or homoscedasticity (not shown).
Discussion
In accordance with established knowledge, we found that valproate was associated with an increased lamotrigine C/D ratio and that carbamazepine and combined oral contraceptives were associated with a decreased lamotrigine C/D ratio. These effects were statistically highly significant. The extent of these interactions were very similar to what has been reported earlier [8, 9, 14, 24], which supports the validity of our present model.
In addition, fluoxetine was identified as a factor reducing the lamotrigine C/D ratio. This result appears to be unexpected, but a study in adult patients [8] found the same effect, and of a similar magnitude. Fluoxetine is generally known as a potent enzyme inhibitor [25]. However, the enzymes known to be inhibited by fluoxetine all belong to the CYP system. Lamotrigine is not metabolized by CYP enzymes, but by UGT. As such, it can not be ruled out that fluoxetine exerts a dual effect on drug-metabolizing enzyme systems such as, for example, ethinyl estradiol. Ethinyl estradiol inhibits the CYP enzymes but induces UGT enzymes [26, 27].
We also found that the benzodiazepines clobazam and clonazepam reduced the lamotrigine C/D ratio, although this effect was comparatively weak. Clonazepam has previously been reported to reduce the serum levels of phenytoin to a small degree [28, 29]. However, other studies have failed to reproduce these findings [30–32], and it should be noted that the confidence intervals of our results were rather wide (Table 2). One may therefore speculate whether some of these findings may have arisen by chance. The same applies for levetiracetam. Levetiracetam has not previously been reported to alter the metabolism of other drugs, and its pharmacokinetic properties provide no good reason for the slightly increased lamotrigine C/D ratio.
Surprisingly, the effects of the enzyme-inducing drugs phenobarbital/primidone and oxcarbazepine did not reach statistical significance (p = 0.89; 95% CI: −0.394, 0.454 and p = 0.11; 95% CI: −0.404, 0.039, respectively). However, these drugs were used by a few patients serving with multiple samples, and many of these patients also used valproate. It is well known that the enzyme−inhibiting effect of valproate generally overrides the effects of concomitantly used enzyme-inducers [9, 22, 31]. Thus, these findings are most likely less reliable than the other observations in the present study.
In general, it should be noted that the results of this study do not enable firm conclusions to be drawn on possible causal relationships in terms of drug interactions. Routine data from a TDM service unit have its inherent limitations, mainly from the uncertain reliability and incompleteness of the clinical information accompanying the blood samples. Nevertheless, it is reasonable to believe that the large number of observations to some degree counterbalance these weaknesses.
In the recent years, there has been a growing interest in the ontogenic aspects of pharmacokinetics, and age is, of course, a crucial factor in this respect [18]. It has been proposed – based on population pharmacokinetic modeling – that lamotrigine pharmacokinetics in children may not be related to age but to body weight [33]. Unfortunately, information on body weight was generally not available in our material. In children, nevertheless, body weight is not an independent variable since it is mainly determined by age [34]. Moreover, age does not determine only body weight, but also organ weight, enzymatic function and regional blood flows [15–18, 35, 36]. Thus, it appears logical to focus on age as a determinant of drug disposition, and we found that age indeed has a highly significant effect on the lamotrigine C/D ratio, resulting in lower dose-normalized lamotrigine serum concentrations in older children.
On the basis of the data presented in Fig. 1, it seems that the variation in the lamotrigine C/D ratio decreases with increasing age. This may be due to a larger variation in the metabolic capacity among younger children compared to older ones as a result of differences in the degree of the maturation of their glucuronidating capacity [37]. The interaction of age with the effects of carbamazepine on the lamotrigine C/D ratio fits very well with this concept. We found that the impact of carbamazepine was most pronounced in younger children and decreased with increasing age. It has long been known that carbamazepine induces the glucuronidation of lamotrigine [9, 11, 14, 24]. Thus, it appears logical that the enzyme-inducing effect of carbamazepine is greater at a younger age, when the baseline glucuronidation capacity is low.
In summary, our results suggest that age influences the C/D ratio of lamotrigine in children and adolescents. This is supported by the finding that the impact of carbamazepine on lamotrigine serum concentration is also age-dependent. In accordance with previous studies, we found that valproate, carbamazepine and combined oral contraceptives had a significant impact on the lamotrigine serum levels in children. Clobazam, clonazepam, levetiracetam, and fluoxetine may also alter the serum levels of lamotrigine, but only to a small degree. Whether these latter findings are real or caused by method artifacts remains to be confirmed.
References
Wheless JW, Clarke DF, Carpenter D (2005) Treatment of pediatric epilepsy: expert opinion, 2005. J Child Neurol 20[Suppl 1]:S1–56
GlaxoSmithKline. Lamictal Prescribing Information. Available at: http://us.gsk.com/products/assets/us_lamictal.pdf. Accessed December 19, 2006
Hirsch LJ, Weintraub DB, Buchsbaum R, Spencer HT, Straka T, Hager M, Resor Jr SR (2006) Predictors of Lamotrigine-associated rash. Epilepsia 47:318–322
Culy CR, Goa KL (2000) Lamotrigine. A review of its use in childhood epilepsy. Paediatr Drugs 2:299–330
Dickens M, Chen C (2002) Lamotrigine. Chemistry, biotransformation and pharmacokinetics. In: Levy RH, Mattson R, Meldrum B, Perucca E (eds) Antiepileptic drugs. Lippincott Williams and Wilkins, Philadelphia, pp 370–379
Sabers A, Buchholt JM, Uldall P, Hansen EL (2001) Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res 47:151–154
Sabers A, Öhman I, Christensen J, Tomson T (2003) Oral contraceptives reduce lamotrigine plasma levels. Neurology 61:570–571
Reimers A, Skogvoll E, Sund JK, Spigset O (2005) Drug interactions between lamotrigine and psychoactive drugs: evidence from a therapeutic drug monitoring service. J Clin Psychopharmacol 25:342–348
May TW, Rambeck B, Jürgens U (1996) Serum concentrations of lamotrigine in epileptic patients: the influence of dose and comedication. Ther Drug Monit 18:523–531
Battino D, Croci D, Granata T, Mamoli D, Messina S, Perucca E (2001) Single-dose pharmacokinetics of lamotrigine in children: influence of age and antiepileptic comedication. Ther Drug Monit 23:217–222
Battino D, Croci D, Granata T, Estienne M, Pisani F, Avanzini G (1997) Lamotrigine plasma concentrations in children and adults: influence of age and associated therapy. Ther Drug Monit 19:620–627
Mikati MA, Fayad M, Koleilat M, Mounla N, Hussein R, Kazma A, Yunis K (2002) Efficacy, tolerability, and kinetics of lamotrigine in infants. J Pediatr 141:31–35
Chen C, Casale EJ, Duncan B, Culverhouse EH, Gilman J (1999) Pharmacokinetics of lamotrigine in children in the absence of other antiepileptic drugs. Pharmacotherapy 19:437–441
Armijo JA, Bravo J, Cuadrado A, Herranz JL (1999) Lamotrigine serum concentration-to-dose ratio: influence of age and concomitant antiepileptic drugs and dosage implications. Ther Drug Monit 21:182-190
Stewart CF, Hampton EM (1987) Effect of maturation on drug disposition in pediatric patients. Clin Pharm 6:548–564
de Wildt SN, Kearns GL, Leeder JS, van den Anker JN (1999) Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clin Pharmacokinet 36:439–452
Strolin Benedetti M, Baltes EL (2003) Drug metabolism and disposition in children. Fundam Clin Pharmacol 17:281–299
Edginton AN, Schmitt W, Voith B, Willmann S (2006) A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet 45:683–704
Kanner AM (2004) When thinking of lamotrigine and valproic acid, think “pharmacokinetically”! Epilepsy Curr 4:206–207
Everitt B, Rabe-Hesketh S (2001) Linear mixed models. Analyzing medical data using S-PLUS. Springer, New York, pp 243–268
RDevelopment Core Team (2004) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Pinheiro J, Bates D, DebRoy S et al (2004) nlme, linear and nonlinear mixed effects models. In: R-package. R Foundation for Statistical Computing, Vienna
Xu R (2003) Measuring explained variation in linear mixed effects models. Stat Med 22:3527–3541
Böttiger Y, Svensson JO, Ståhle L (1999) Lamotrigine drug interactions in a TDM material. Ther Drug Monit 21:171–174
Preskorn SH (1997) Clinically relevant pharmacology of selective serotonin reuptake inhibitors. An overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet 32[Suppl 1]:1–21
Shenfield GM (1993) Oral contraceptives. Are drug interactions of clinical significance? Drug Saf 9:21–37
Reimers A (2004) Oral contraceptives can affect the metabolism of other drugs. Tidsskr Nor Laegeforen 124:1785–1787
Edwards VE, Eadie MJ (1973) Clonazepam-a clinical study of its effectiveness as an anticonvulsant. Proc Aust Assoc Neurol 10:61-66
Saavedra IN, Aguilera LI, Faure E, Galdames DG (1985) Phenytoin/clonazepam interaction. Ther Drug Monit 7:481–484
Eeg–Olofsson O (1973) Experiences with Rivotril in treatment of epilepsy-particularly minor motor epilepsy-in mentally retarded children. Acta Neurol Scand Suppl 53:29–31
Huang CY, McLeod JG, Sampson D, Hensley WJ (1974) Clonazepam in the treatment of epilepsy. Med J Aust 2:5–8
Windorfer A, Jr., Sauer W (1977) Drug interactions during anticonvulsant therapy in childhood: diphenylhydantoin, primidone, phenobarbitone, clonazepam, nitrazepam, carbamazepin and dipropylacetate. Neuropadiatrie 8:29–41
Chen C (2000) Validation of a population pharmacokinetic model for adjunctive lamotrigine therapy in children. Br J Clin Pharmacol 50:135–145
WHO Multicentre Growth Reference Study Group (2006) WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl 450:76–85
Strassburg CP, Strassburg A, Kneip S, Barut A, Tukey RH, Rodeck B, Manns MP (2002) Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut 50:259–265
Bjorkman S (2005) Prediction of drug disposition in infants and children by means of physiologically based pharmacokinetic (PBPK) modelling: theophylline and midazolam as model drugs. Br J Clin Pharmacol 59:691–704
Liston HL, Markowitz JS, DeVane CL (2001) Drug glucuronidation in clinical psychopharmacology. J Clin Psychopharmacol 21:500–515
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reimers, A., Skogvoll, E., Sund, J.K. et al. Lamotrigine in children and adolescents: the impact of age on its serum concentrations and on the extent of drug interactions. Eur J Clin Pharmacol 63, 687–692 (2007). https://doi.org/10.1007/s00228-007-0308-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-007-0308-2