Abstract
Aims
The standard treatment of neonatal group B Streptococcus infection is intravenous amoxicillin for 10 days. We investigated whether effective serum amoxicillin concentrations could be reached by switching to oral amoxicillin after 48 h of intravenous administration in full-term neonates with group B Streptococcus infection.
Methods
Over 2 years, we included 222 full-term neonates who had early onset group B streptococcal disease responsive to 48 h of intravenous amoxicillin, at which point they were asymptomatic and fed orally. They were switched to oral amoxicillin (300 or 200 mg/kg per day in four divided doses). Steady-state serum amoxicillin concentrations were determined 48 h later by high-performance liquid chromatography; values ≥5 mg/l were considered effective.
Results
Mean gestational age was 39.32 ± 1.5 weeks ,and mean birth weight was 3,422 ± 533 g; 29 newborns were bacteremic. Median serum amoxicillin concentration on oral therapy was 31,.15 (range 11–118) and 25.80 (range 5–84.8) with 300 and 200 mg/kg per day, respectively. None of the infants had a concentration <5 mg/l (p < 0.001). Gastrointestinal tolerance was satisfactory; 216 patients were discharged at 5 days of age, and none was readmitted within the 3-month follow-up.
Conclusion
Early switching to the oral route in asymptomatic full-term newborns with early onset group B streptococcal disease maintained serum amoxicillin concentrations within our predefined therapeutic range (error risk<0.001). This strategy may hold potential for reducing treatment invasiveness and shortening hospital length of stay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Suspected infection transmitted from the mother is a common reason for admission of neonates. Antibiotics are started after microbiology sample collection. Antibiotic selection relies on a probabilistic approach based on epidemiological evidence that Streptococcus agalactiae causes two thirds of cases and Escherichia coli the remaining third [1], even if there are conflicting reports concerning the impact of intrapartum antibiotic prophylaxis on early onset sepsis caused by organisms other than group B Streptococcus [2].
Amoxicillin, together with penicillin G, is among the antibiotics of choice for group B streptococcal (GBS) infection [3]. Antibiotics are given intravenously, as swift attainment of therapeutic serum levels is vital to prevent the development of severe infection in these vulnerable patients. During the first few postnatal hours, oral absorption of antibiotics is erratic and can be affected by haemodynamic abnormalities [4]; therefore, such administration is not reliable.
In adults, however, early switching from the intravenous to the oral route is now widely used, even in patients with severe infections [5], as recommended in guidelines [6]. In full-term neonates, the bioavailability of oral amoxicillin is high, about 80% [7], and preliminary data [8, 9] from neonates with bacterial colonisation treated over the first 4 postnatal days showed similar serum amoxicillin concentrations with the intravenous and oral routes, except during the first 30 min, when levels were lower with oral administration. Because a rapid use of oral the route is nowadays prescribed by many paediatricians without any available pharmacological data about such practices, we therefore investigated early oral antibiotic therapy in neonates first treated intravenously for 48 h.
The objective of the present study was to test the hypothesis that oral amoxicillin therapy given to full-term neonates with GBS infection after only 48 h of intravenous administration provided effective serum amoxicillin levels and was well tolerated.
Materials and methods
Patients
The research project was approved by the Institutional Review Board of the Nantes Teaching Hospital, where the study was conducted. All neonates meeting the inclusion criteria listed below were entered into the study between 1 July 2003 and 31 December 2005. The study was conducted in a 60-bed neonatal intensive care unit that admits a large proportion of out-born infants transferred from maternity hospitals in the region. In the region served by the neonatal intensive care unit, a screening-based GBS infection prophylaxis was observed routinely in late pregnancy, as well as in neonates with established risk factors [3, 10]. A complete blood count, white blood cell count differential, C-reactive protein (CRP) and blood culture were obtained if the newborn was considered at risk for infection. A lumbar puncture was not performed in well-appearing infants [10]. Definite GBS infection was defined as a medical history or symptoms suggestive of infection (tachycardia, bradycardia, respiratory distress, apnoea episodes, jaundice deterioration, hepatosple nomegaly, poor perfusion, hypotension, lethargy, irritability, poor feeding, seizures and fever) plus GBS recovery from blood or cerebrospinal fluid cultures. Possible GBS infection was defined as a medical history or symptoms suggestive of infection plus GBS recovery from peripheral sites, such as gastric aspirates or placental specimens associated with a biological abnormality [leukopenia, leukocytosis, ratio of immature to total neutrophil forms on the leukocyte count <0.2, thrombocytopenia, thrombocytosis, cerebral spinal fluid (CSF) pleocytosis, or CRP >10 mg/l at 12–60 h after the first blood sample was taken [11]. In both situations, amoxicillin was given for 10 days [3].
Inclusion criteria
We included full-term infants (gestation age >36 weeks) with definite or possible GBS infection treated for 48 h with intravenous amoxicillin in a daily dosage of 100 mg/kg. To be eligible for the study, the infants had to be asymptomatic and fed enterally at completion of the 48-h intravenous treatment period.
Exclusion criteria
We excluded infants with any of the following: birth before 36 weeks’ gestation, neonatal infection without positive microbiology tests for GBS, central nervous system infection, and persistence after 48 h on intravenous amoxicillin of haemodynamic abnormalities (tachycardia >180/mn and/or mean arterial pressure <39 mmHg and/or urinary output <1 ml/kg/h, and/or serum lactate >3 mmol/l), or of gastrointestinal symptoms (vomiting or inadequate oral milk intake). Factors indicating a likelihood of poor treatment compliance by the family also led to patient exclusion.
Amoxicillin treatment protocol
Dosage
In patients with suspected neonatal infection, microbiological specimens were collected and amoxicillin started immediately. Amoxicillin was given intravenously in a dosage of 100 mg/kg per day for 48 h. Then, oral amoxicillin was given in a dosage of 300 mg/kg per day in four divided doses before feedings for 8 days. During a second period, regarding serum amoxicillin levels observed in the first patients, we reduced the dosage to 200 mg/kg per day in four divided doses. The first oral dose was given 12 h after the last intravenous injection, i.e. 48 h after amoxicillin initiation for both dosages.
Discharge
Oral treatment was given for 8 days. Infants could be discharged after 48 h of oral treatment after the parents received oral and written information about what to do in case of vomiting, refusal of feeds, or fever. Infants were discharged only if their serum amoxicillin level was within the therapeutic range.
Serum amoxicillin assays
Serum amoxicillin was measured by reverse-phase high-performance liquid chromatography according to a procedure derived from that of Jehl and colleagues [12]. After deproteinization of 100 μl of serum and extraction with dichloromethane, the extract was analysed in isocratic mode with a 250 mm LiChrospher 100RP-18 column (Merck, Whitehouse Station, NJ, USA). The mobile phase was 0.1 M ammonium acetate buffer, pH 3, with acetonitrile (200/5). Detection was done by ultraviolet radiation at 230 nm. Retention time was about 10 min. The detection limit was 1 mg/l with a coefficient of variation of 5%.
Trough serum amoxicillin levels were assayed after 48 h of oral treatment, i.e. after 5.5 amoxicillin half-lives, which corresponds to the steady state. The assays were done both to determine whether levels were effective and to look for evidence of accumulation. Blood was drawn by venipuncture 1 h after application of a topical anaesthetic (EMLA).
Protocol for adjusting oral amoxicillin levels
When the serum amoxicillin level was outside the therapeutic range, the patient was kept in the hospital. According to the study protocol, serum levels >100 mg/l led to amoxicillin discontinuation for 8 h followed by treatment in a reduced dosage of 30% of the initial dosage, and the serum level was assayed 24 h later. In patients with serum levels <5 mg/l, the amoxicillin dosage had to be increased (+10% of initial dosage) after verification that doses were given as ordered and that gastrointestinal tolerance was good; serum amoxicillin was assayed 24 h later. Patients who did not achieve therapeutic amoxicillin levels despite these adjustments, and those with recurrent vomiting, were to be switched back to the intravenous route.
Patient follow-up
Infants were re-evaluated routinely at the hospital outpatient clinic on day 8 of oral amoxicillin treatment. A physical examination was performed, and the parents were interviewed. Then, 3 months after discharge, the parents were contacted by telephone and asked whether the child had been readmitted for infection; when this was the case, the name of the infectious agent was recorded.
Statistical analysis
Evaluation criteria
The primary evaluation criterion was the steady-state trough serum amoxicillin level 48 h after the switch to the oral route. The criterion was considered met when the level was greater than 5 mg/l but lower than the neurotoxicity threshold [13]. We chose 5 mg/l as the lower end of our target range because we wanted a rapid and strong bactericidal effect; 5 mg/l is 50 times the usual minimal inhibitory concentration for GBS (i.e. 0.01 mg/l) and is yet considered as sufficiently high [14]. Secondary evaluation criteria were absence of potentially amoxicillin-related gastrointestinal symptoms and clinical recovery, defined as absence of recurrent infection with the same agent before 3 months of age.
Statistical tests
Median serum amoxicillin levels were compared using the nonparametric Mann–Whitney U test. The lower (5 mg/l) and upper (100 mg/l) bounds of the target range were tested with the alpha risk set at 0.025. Rates of occurrence were computed for gastrointestinal symptoms, clinical recovery, and recurrent GBS infection within 3 months. All statistical tests were run on Splus 6 software (University of North California, Chapel Hill, NC, USA).
Results
Patients
During the 2-year recruitment period, 222 neonates met our inclusion criteria: 133 (59%) boys and 89 (40%) girls. Mean gestational age was 39.32 ± 1.5 weeks, and mean birth weight was 3,422 ± 533 g Ninety-five patients (43%) were breast-fed. Definite GBS infection was diagnosed in 29 (13%) patients; all 29 patients had positive blood cultures for GBS, but none had meningitis. Of these 29 patients, 16 experienced early respiratory distress, four had transient evidence of shock (tachycardia and oliguria) and nine were asymptomatic. Five patients had a fever (38.4°C) during the first hours of life. All 29 patients were asymptomatic after 48 h of intravenous amoxicillin. Possible GBS infection was diagnosed in 193 infants. Among them, 112 had no symptoms but were evaluated for neonatal infection based on their perinatal medical history. Among the 81 remaining infants, 62 had respiratory distress, nine had transient haemodynamic abnormalities (including one who required dopamine therapy), three had early jaundice and seven had a fever (central temperature >38°C).
Serum amoxicillin levels
Among the 64 patients receiving 300 mg/kg per day of amoxicillin, the median steady-state serum amoxicillin level was 31.15 mg/l, with a range of 10–118 mg/l (Table 1). Thus, none of the infants had trough levels below our target threshold. In addition, the median value was far greater than the usual minimum inhibitory concentration (MIC) for GBS and was located within our target range (5–100 mg/l), with a p value < 0.0001. Median values were not significantly different between neonates with definite versus possible infection (29.45 and 33.12 mg/l, respectively). A single patient had a level >100 mg/l. No explanations to this high level were identified, and amoxicillin was stopped for 8 h then restarted at 200 mg/kg per day, as required by the study protocol; the follow-up assay showed a level of 40 mg/l and the patient had no evidence of toxicity.
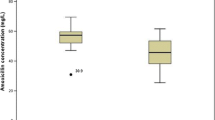
Among the 158 patients receiving 200 mg/kg per day of amoxicillin, the median steady-state serum amoxicillin level was 25.8 mg/l, with a range of 5–84.8 mg/l. Median values were not different between neonates with definite versus possible infection (23.25 and 26 mg/l, respectively), and none of the infants had trough levels below our target threshold. However, median values were significantly higher in the 300 mg/kg per day group than in the 200 mg/kg per day group (p < 0.001) (Fig. 1).
Amoxicillin values (median ± SD) in full-term neonates with early onset group B streptococcal disease 48 h after initiation of oral treatment (steady state) with a dosage of 200 or 300 mg/kg per day in four divided doses. This oral treatment was relaying a 48-h intravenous treatment of amoxicillin (100 mg/kg/j)
Outcomes
The gastrointestinal safety profile was consistently favourable in both 300 and 200 mg/kg per day groups. All patients readily accepted the oral medication. No instances of diarrhoea or vomiting were reported. No patient required premature treatment discontinuation. All but six patients were discharged at 5 days of age, and in most of cases, the mother was discharged at the same time as her infant. The median reduced length of stay in the hospital was 5.12 days. The outpatient clinic evaluation at 10 days of age showed a diaper rash in six patients, thrush in two patients and umbilical-cord inflammation in seven patients. Treatment compliance was assessed by parent interview and considered satisfactory. During the first 3 months of life, three patients experienced febrile illnesses and two required hospital admission in relation with bronchiolitis and gastroenteritis. Thus, all patients met our definition of recovery.
Discussion
In 222 neonates who were asymptomatic after a 48-hintravenous amoxicillin treatment, oral amoxicillin therapy consistently produced bactericidal serum amoxicillin levels. The 200 mg/kg per day dosage appears as efficient as 300 mg/kg per day without any neonate out of the therapeutic range. A level of 5 mg/l is highly bactericidal for GBS, as it is nearly 50 times the usual minimum inhibitory concentration in blood and five times that in cerebrospinal fluid. Thuisman-de Boer et al. considered that in infected newborns, these serum amoxicillin levels are sufficiently high and can be taking as a reference [14]. There was a less than 0.0001 probability that the mean serum amoxicillin level was <5 mg/l, indicating that our results can be extrapolated to all neonates meeting our inclusion criteria, with a very low risk of error. Tolerance was satisfactory. All patients accepted the drug readily, a fact that facilitated treatment by the parents.
Drug pharmacokinetics in neonates is influenced by a host of physiological and disease-related factors. Gastrointestinal absorption of orally administered drugs is influenced by gastric pH, gastric emptying time, intestinal absorption, bacterial colonisation of the gut and diet composition [15]. Studies of serum amoxicillin levels during oral therapy showed considerable interindividual variability. We minimised this obstacle by using a high dosage and by selecting a population of full-term newborns who were asymptomatic after 48 h of intravenous amoxicillin therapy. Use of a high dosage suggested a potential risk of toxicity; however, no clinical toxicity occurred in our patients, and a single patient had a serum amoxicillin level above the target range. In addition, there is little published evidence on amoxicillin toxicity in neonates [13].
The effectiveness of oral amoxicillin therapy in terms of morbidity and mortality was not assessed in our study. We did not seek to establish equivalence of oral versus intravenous amoxicillin therapy. Nevertheless, none of the patients experienced evidence of GBS infection within 3 months after discharge. Candida infections, which are typical complications of systemic antibiotic therapy via any route [16], occurred in 3.6% of our patients. Umbilical cord inflammation was noted in seven patients at the follow-up clinic visit; conceivably, this might have been related to antibiotic-induced changes in the periumbilical microbial flora involved in cord separation [17]. Oral antibiotics have a major impact on the gastrointestinal flora in neonates [18, 19], but this effect is probably not very different from that of intravenous antibiotics. No systemic infections potentially related to amoxicillin were recorded in our study. An investigation comparing the gut flora in two populations of neonates given oral and intravenous antibiotics, respectively, would be of interest to clarify this point.
Although our results hold promise for reducing intravenous infusion and hospital-stay durations, the limitations of oral amoxicillin therapy should be borne in mind. First, oral therapy should not be given from birth. Gastrointestinal intolerance and haemodynamic disturbances related to neonatal infection may reduce intestinal absorption of the drug, precluding prompt achievement of bactericidal serum levels [20]. The intravenous route should be used for the first 48 h. Second, our results indicate that early switching to oral therapy is effective in a selected population, i.e. full-term neonates with GBS infection and no symptoms after 48 h of intravenous amoxicillin. These results cannot be extrapolated to other populations. Third, the ease of use of oral antibiotic therapy should not lead to overuse of antibiotics in the neonatal period. Oral antibiotics should not be given to neonates in whom antibiotic therapy would be deemed unnecessary if the oral route were unavailable. The risk of selecting resistant micro-organisms is just as great with oral as with parenteral antibiotic therapy.
The reduced length of stay in the hospital, estimated at about 5 days on average in our study, is a major advantage of oral therapy that can be expected to promote parent–infant bonding and diminish health care costs. In addition, the shorter hospital stay and briefer use of an intravenous line probably decrease the risk of nosocomial infections [21].
Neonates in our study achieved therapeutic serum amoxicillin levels when switched from intravenous to oral amoxicillin after 48 h. This concept, which may prove extremely useful in the management of early onset GBS infections , has not been evaluated previously in a similar series of neonates. This suggests that more widespread use of oral antibiotic therapy in neonates after initial intravenous therapy might be successful. However, it seems important to underline the specific population studied here (asymptomatic full-term newborns with an early onset group B streptococcal disease). Any extrapolation to different neonates is not possible in this context. A bioequivalence study would be needed to confirm this possibility.
References
Klein JO, Remington JS (2001) Current concepts of infections of the fetus and newborn infant. In: Remington JS, Klein JO (eds) Textbook of infectious diseases of the fetus and newborn infant, 5th edn. W.B. Saunders Company, Philadelphia, pp 1–23
Alarcon A, Pena P, Salas S, Sancha M, Omenaca F (2004) Neonatal early onset Escherichia coli sepsis: trends in incidence and antimicrobial resistance in the era of intrapartum antimicrobial prophylaxis. Pediatr Infect Dis 23(4):295–299
American Academy of Pediatrics (2006) Group B streptococcal infections. In: Pickering LK, Baker CJ, Long SS, Mc Millan JA (eds). Red Book: 2006 report of the Committee of Infectious Diseases, 27th ed. American Academy of Pediatrics, Elk Grove Village, pp 620–627
Heimann G (1980) Enteral absorption and bioavailability in children in relation to age. Eur J Clin Pharmacol 18:43–50
Sevinc F, Prins JM, Koopmans RP (1999) Early switch from intravenous to oral antibiotics: guidelines and implementation in a large teaching hospital. J Antimicrob Chemother 43:601–606
Kern WV, Cometta A, De Brok R (1999) For the international antimicrobial cooperative group of the european organization for research and treatment of cancer. Oral versus intravenous empirical antimicrobial therapy for fever in patient with granulocytopenia who are receiving cancer chemotherapy. N Engl J Med 341:312–318
Cohen MD, Raeburn JA, Devine J, Kirkwood J, Elliott B, Cockburn F, Forfar JO (1975) Pharmacology of some oral penicillins in the newborn infant. Arch Dis Child 50(3):230–234
Autret E, Laugier J, Marimbu J, Vaillant MC, Furet Y, Breteau M (1988) Comparaison des concentrations plasmatiques d’amoxicilline par voie orale et intraveineuse dans les colonisations bactériennes néonatales. Arch Fr Pediatr 45(9):679–682
Giustardi A, Coppola G (1992) Comparison of plasma concentrations of amoxicillin administered by oral and venous routes in neonatal bacterial colonizations. Pediatr Med Chir 114:447–449
Puopolo KM, Madoff LC, Eichenwald EC (2005) Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics 115(5):1240–1246
Haque KN (2005) Definitions of bloodstream infection in the newborn. Pediatr Crit Care Med 6(3 Suppl):S45–S49
Jehl F, Birckel P, Monteil H (1987) Hospital routine analysis of penicillins, third-generation cephalosporins and aztreonam by conventional and high-speed high-performance liquid chromatography. J Chromatogr 23(413):109–119
Shaffer Cl, Davey Am, Ransom Jl (1998) Ampicillin-induced neurotoxicity in very-low-birth-weight neonates. Ann Pharmacother 32:482–484
Huisman-de Boer JJ, van den Anker JN, Vogel M, Goessens WH, Schoemaker RC, de Groot R (1995) Amoxicillin pharmacokinetics in preterm infants with gestational ages of less than 32 weeks. Antimicrob Agents Chemother 39(2):431–434
Heimann G (1980) Enteral absorption and bioavailability in children in relation to age. Eur J Clin Pharmacol 18:43–50
Seelig MS (1966) The role of antibiotics in the pathogenesis of Candida infections. Am J Med 40:887–917
Oudesluys-Murphy AM, de Groot CJ, Eilers GA (1986) Time of umbilical cord separation. J Pediatr 108(2):334
Nord C, Kager L, Heimdahl P (1984) Impact of antimicrobial agents on the gastrointestinal microflora and the risk of infection. Am J Med 23:99–106
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE (2006) Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118(2):511–521
McCracken GH (1983) Comparative evaluation of the aminopenicillins for oral use. Pediatr Infect Dis 2:317–320
Needleman J, Buerhaus P, Mattke S, Stewart M, Zelevinsky K (2002) Nurse-staffing levels and the quality of care in hospitals. N Engl J Med 30(346):1715–1722
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gras-Le Guen, C., Boscher, C., Godon, N. et al. Therapeutic amoxicillin levels achieved with oral administration in term neonates. Eur J Clin Pharmacol 63, 657–662 (2007). https://doi.org/10.1007/s00228-007-0307-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-007-0307-3