Abstract
Objective
The activity of the human cytochrome P450 and P-glycoprotein (P-gp) changes according to gender. The present study evaluated the effect of gender on the influence of carvedilol on serum digoxin levels in patients with heart failure.
Methods
Twenty-four patients (12 female and 12 male) with New York Heart Association class II-III heart failure were included in the study. Patients were taking oral digoxin (0.0625–0.25 mg, once a day) and were administered oral carvedilol (6.25 mg, two times daily) for 7 days.
Results
In the male group, carvedilol led to statistically significant increases in the area under the concentration time curve to 16 h (AUC0–16h) and the peak concentration (Cmax) for digoxin, with no change in time to peak (tmax)(AUC0–16h= 24.1±9.2 ng.h/ml vs. 15.4±5.8 ng.h/ml, p<0.001, Cmax=2.2±1.0 ng/ml vs. 1.6±0.6 ng/ml, p<0.01, tmax=2.4±2.2 h vs. 2.1±1.0 h, p>0.05). In the female group, carvedilol administration did not cause statistically significant change in the AUC0–16h, Cmax, or tmax for digoxin (p>0.05). In the male group, carvedilol resulted in a significant increase in the AUC0–16h and Cmax for digoxin compared with the female group (AUC0–16h=24.1± 9.2ng.h/ml vs. 17.0±6.8 ng.h/ml, Cmax=2.2±1.0 ng/ml vs. 1.5±0.6 ng/ml, p<0.05, respectively).
Conclusion
Men seem to have a higher activity relative to women for the drug efflux transporter P-gp. Our results suggest that carvedilol will cause drug interaction with digoxin following the inhibition of P-gp-mediated transcellular transport of digoxin in males.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effects of digoxin on survival in patients with heart failure were tested by the Digitalis Investigation Group (DIG) trial [16]. Subgroup analysis from the DIG trial showed a relationship between serum digoxin concentration and mortality. In this empirical observation, the risk of death increased significantly if the digoxin level exceeded 1 ng/ml [6].
Serum digoxin concentration is increased by various therapeutic compounds, including quinidine, verapamil, spironolactone, nitroprusside, alprazolam, and captopril [3, 5, 8, 10, 19]. Pharmacokinetic interaction between carvedilol and digoxin has been previously reported; carvedilol increased serum digoxin levels when carvedilol and digoxin were orally administered [4, 20]. Both carvedilol and digoxin are substrates of P-glycoprotein (P-gp). Carvedilol is metabolized by the cytochrome P450 (CYP) isoenzymes CYP2D6 and CYP2D9 [1, 12]. It has been known that gender differences affect the pharmacokinetics of some drugs, such as verapamil, beta blockers, and selective serotonin reuptake inhibitors. Gender-related pharmacokinetic differences include the generally lower body weight and organ size, higher percentage of body fat, lower glomerular filtration rate (GFR), different gastric motility, and different activity of drug transporters and drug-metabolizing enzymes in women compared with men [12]. The aim of this study was to evaluate the effect of gender on the influence of carvedilol on serum digoxin levels in patients with heart failure.
Methods
The protocol was approved by the Dokuz Eylul University School of Medicine and the National Ethics Committee of Drug Investigations, and informed consent was obtained from each patient included in the study. Twenty-four patients (12 male and 12 female) with New York Heart Association class II–III heart failure were enrolled in the study. All patients were nonsmokers. The mean patient age was 67.8±8.2 years. Renal clearance was calculated with the Cockcroft-Gault formula [(140−age in years) × body weight in kg × K/serum Cr (μmol/l), where K is a constant: 1.23 for males and 1.04 for females] for all patients [17]. Individuals with GFR ≤30 ml/min or other systemic chronic disease were excluded from the study.
All patients were taking oral digoxin (0.0625–0.25 mg, once a day) and were administered oral carvedilol (6.25 mg, two times daily) for 7 days. On days 0 (the day before carvedilol administration) and 7 (the day of the end of the carvedilol administration), venous blood samples were collected at 0, 1, 2, 4, 8, and 16 h after digoxin administration to measure serum digoxin concentrations. On days 1–6, serum digoxin levels were measured only in the morning before the daily digoxin dose was given. Serum digoxin concentrations were measured by enzyme-multiplied immunoassay technique (CEDIA Digoxin II Assay, Hitachi 912 analyzer, Germany). The minimum detectable concentration of the assay is 0.15 ng/ml (0.19 μmol/l). Other concomitant medications included furosemide, acetylsalicylic acid, isosorbide mononitrate, lisinopril, enoxaparin, perindopril, sertraline, gliclazide, and acarbose.
The pharmacokinetics of digoxin were evaluated using the area under the 16-h digoxin concentration time curve (AUC0–16 h), the peak concentration (Cmax), and the time to peak (tmax). The area under the curve of serum digoxin concentration versus time was measured by trapezoidal methods [10].
Statistical analysis of the pharmacokinetic parameters between groups was done with Student’s t-test. Comparison of patient characteristics was assessed by analysis of variance followed by Tukey-Kramer multiple comparison tests for among-group comparison (GraphPad InStat, 1990–1994, GraphPad Software). All results are expressed as mean±SD. A p-value <0.05 was accepted as the level of statistical significance.
Results
Patient characteristics are presented in Table 1. Both groups were comparable for age and weight (p>0.05). There were no significant differences (p>0.05) between the groups in blood values (hemoglobin, hematocrit), liver function tests (alanine amino transferase, aspartate amino transferase), or renal function tests (blood urea nitrogen, creatinine). Clinical signs or symptoms of digoxin toxicity were not observed in any patient during the study.
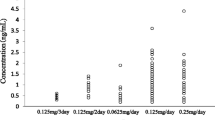
The mean plasma concentration time curve for digoxin at the end of the carvedilol treatment period is shown in Fig. 1. There were no significant differences between groups in the AUC0–16 h, Cmax, or tmax values before carvedilol was added to the treatment (Table 2). In the male group, carvedilol led to statistically significant increases in the AUC0–16 h and Cmax for digoxin, with no change in tmax at the 7th day. In the female group, carvedilol administration did not cause statistically significant change in the AUC0–16 h, Cmax, or tmax for digoxin (Table 2). In the male group, carvedilol resulted in a significant increase in the AUC0–16 h and Cmax for digoxin compared with the female group (AUC0–16 h=24.1±9.2 ng.h/ml vs. 17.0±6.7 ng.h/ml, p<0.05, 95% CI 0.27–13.95; Cmax=2.2±1.0 ng/ml vs. Cmax=1.5±0.6 ng/ml, p<0.05, 95% CI 0.01–1.35, Table 2). Trough digoxin concentrations during the intervening 6 days of digoxin and carvedilol treatment did not differ significantly in either group (male group: range 0.9±0.5–1.3±0.8 ng/ml; female group: range 0.9±0.5–1.1±0.6 ng/ml).
Discussion
In this study we observed that gender differences exist regarding the influence of carvedilol on serum digoxin levels in patients with heart failure. There were statistically significant differences between the male and female groups in the area under the curve (AUC0–16 h) and the peak concentration of digoxin (Cmax) after 1 week of carvedilol administration. Carvedilol caused a statistically significant increase in the AUC0–16 h and Cmax for digoxin in the male group, whereas it produced no significant effect on these parameters in the female group. The time to peak (tmax) of digoxin showed no difference after carvedilol administration in either group.
Previous reports in pediatric and adult patients have documented interaction between digoxin and carvedilol [4, 13, 20]. De Mey et al. investigated the interaction by using both oral and intravenous preparations of digoxin with carvedilol. They observed that carvedilol increased serum concentrations of digoxin only after oral administration, not after intravenous administration. Carvedilol did not alter the plasma or urinary kinetics of intravenously administered digoxin, indicating that in adults this interaction is caused mainly by enhancement of the extent of digoxin absorption [4]. Wermeling et al. evaluated the effects of multiple oral doses of carvedilol on steady-state plasma digoxin pharmacokinetics in patients with mild to moderate hypertension. They found that carvedilol increased AUC and Cmax for digoxin, with no change in tmax [20]. But Grunden et al. reported that carvedilol administration was associated with a 26% increase in steady-state serum digoxin concentration. This small increase in serum digoxin concentration was not statistically significant versus placebo [7]. In a study of children, carvedilol increased serum concentrations of digoxin. These investigators suggested that its dose may need to be reduced to avoid toxicity [13].
Carvedilol is metabolized by the cytochrome P450 (CYP) isoenzymes CYP2D6 and CYP2C9. CYP2D6 is the second most important CYP isoenzyme for drug metabolism. CYP2D6 activity is highly variable and is independent of gender and age [2, 11]. Hagg et al. demonstrated that women of fertile age have slightly higher CYP2D6 activity compared with men [9]. CYP2C9 activity does not seem to be gender-dependent, at least not to a clinically significant extent [12]. In our study, the carvedilol and digoxin combination did not induce any significant change in the pharmacokinetic parameters of digoxin in females.
The multidrug transporter MDR1 (P-glycoprotein, P-gp) appears to play a significant role in drug absorption and disposition. Digoxin and carvedilol are Pg-p substrates. The tubular secretion of digoxin has been found to be mediated via P-gp [15, 18]. The potential role of carvedilol in inhibiting the renal tubular secretion of digoxin was studied by Takara et al. in porcine kidney epithelial cells transfected with MDR1 complementary DNA. In this model, carvedilol inhibited basal-to-apical transport of H-digoxin [14]. Aiba et al. demonstrated that the intestinal absorption of digoxin is primarily dominated by the efflux process on the luminal side of the intestine and that carvedilol inhibits the transporters and suppresses digoxin efflux on the apical membrane [1]. Men seem to have a higher activity relative to women for the drug efflux transporter P-gp [12]. Therefore, it is likely that P-gp inhibition by carvedilol increased the absorption of digoxin (by inhibiting its efflux) and decreased the renal secretion of digoxin (by inhibiting efflux into the urine) in the male group.
Finally, these findings indicate that gender affected the influence of carvedilol on the serum digoxin level in patients with heart failure. Carvedilol increased the area under the curve and peak concentration of digoxin in men but not in women. When these agents are combined, it is recommended that serum digoxin levels be monitored.
Study limitation
The power of our study is not so high because the number of our population was relatively low. A larger study might be necessary.
References
Aiba T, Ishida K, Yoshinaga M, Okuno M, Hashimoto Y (2005) Pharmacokinetic characterization of transcellular transport and drug interaction of digoxin in Caco-2 cell monolayers. Biol Pharm Bull 28:114–119
Bebia Z, Buch SC, Wilson JW, Frye FR, Romkes M, Ceccheti A, Chaves-Gnecco D, Branch RA (2004) Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin Pharmacol Ther 76:618–627
Cogan JJ, Humphreys MH, Carlson CJ, Benowitz NL, Rapaport E (1981) Acute vasodilator therapy increases renal clearance of digoxin in patients with congestive heart failure. Circulation 64:973–976
De Mey C, Brendel E, Enterling D (1990) Carvedilol increases the systemic bioavailability of oral digoxin. Br J Clin Pharmacol 29:486–490
Doering W (1983) Effect of coadministration of verapamil and quinidine on serum digoxin concentration. Eur J Clin Pharmacol 25:517–521
Gheorghiade M, Pitt B (1997) Digitalis Investigation Group (DIG) trial: a stimulus for further research. Am Heart J 134:3–12
Grunden JW, Gilbert EM, Munger MA (1994) Augmented digoxin concentrations with carvedilol dosing in mild-moderate heart failure. Am J Ther 1:157–161
Guven H, Tuncok Y, Guneri S, Cavdar C, Fowler J (1993) Age-related digoxin-alprazolam interaction. Clin Pharmacol Ther 54:42–44
Hagg S, Spigset O, Dahlqvist R (2001) Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers. Br J Clin Pharmacol 51:169–173
Kirimli O, Kalkan S, Güneri S, Tuncok Y, Akdeniz M, Ozdamar M, Guven H (2001) The effects of captopril on serum digoxin levels in patients with severe congestive heart failure. Int J Clin Pharmacol Ther 39:311–314
Labbe L, Sirois C, Pilote S, Arseneault M, Robitaille NM, Turgeon J, Hamelin BA (2000) Effect of gender, sex hormones, time variables and physiological urinary pH on apparent CYP2D6 activity as assessed by metabolic ratios of marker substrates. Pharmacogenetics 10:425–438
Meibohm B, Bierle I, Derendorf H (2002) How important are gender differences in pharmacokinetics? Clin Pharmacokinet 41:329–342
Ratnapalan S, Griffiths K, Costei AM, Benson L, Koren G (2003) Digoxin-carvedilol interactions in children. J Pediatr 142:572–574
Takara K, Kakumoto M, Tanigawara Y, Funakoshi J, Sakaeda T, Okumura K (2002) Interaction of digoxin with antihypertensive drugs via MDR1. Life Sci 70:1491–1500
Tanigawara Y, Okamura N, Hirai M, Yasuhara M, Ueda K, Kioka N, Komano T, Hori R (1992) Transport of digoxin by human P-glycoprotein expressed in a porcine kidney epithelial cell line (LLC-PK1). J Pharmacol Exp Ther 263:840–845
The Digitalis Investigation Group (1997) The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 336:525–533
Toto RD (1995) Conventional measurement of renal function utilizing serum creatinine, creatinine clearance, inulin and para-aminohippuric acid clearance. Curr Opin Nephrol Hypertens 4:505–509
Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, Komano T, Hori R (1992) Human p-glycoprotein transports cortisol, aldosterone and dexamethasone, but not progesterone. J Biol Chem 267:24248–24252
Waldorff S, Andersen JD, Heeboll-Nielsen N et al (1978) Spironolactone-induced changes in digoxin kinetics. Clin Pharmacol Ther 24:162–167
Wermeling DP, Field CJ, Smith DA, Chandler MH, Clifton GD, Boyle DA (1994) Effects of long-term oral carvedilol on the steady-state pharmacokinetics of oral digoxin in patients with mild to moderate hypertension. Pharmacotherapy 14:600–606
Author information
Authors and Affiliations
Corresponding author
Additional information
The first two authors contributed equally.
Rights and permissions
About this article
Cite this article
Baris, N., Kalkan, S., Güneri, S. et al. Influence of carvedilol on serum digoxin levels in heart failure: is there any gender difference?. Eur J Clin Pharmacol 62, 535–538 (2006). https://doi.org/10.1007/s00228-006-0138-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0138-7