Abstract
Objectives
To characterise the population of Alzheimer’s disease patients treated with acetylcholinesterase inhibitors, to analyse effectiveness and drug safety in the clinical practice, and to identify variables that may predict the response to therapy.
Methods
From September 2000 to December 2001, a total of 5,462 patients diagnosed with mild to moderate Alzheimer’s disease were enrolled at the time of their first prescription of the study drugs and followed up for an average of 10.5 months. Responders were defined as patients with a mini-mental state examination (MMSE) score improvement of 2 or more points from baseline after 9 months of therapy.
Results
At 9 months, 2,853 patients (52.2%) completed the study. The mean change from baseline in MMSE scores was an improvement of 0.5 points (±3.0). The proportion of responders to the therapy was 15.7% at 9 months. A greater probability of response at 9 months was observed among patients without concomitant diseases at baseline [odds ratio (OR)=2.1, 95% confidence interval (CI) 1.5–2.9] and among those with a response at 3 months (OR=20.6, 95% CI 17.2–24.6). During the study period, 285 patients (5.2%) discontinued the treatment because of an adverse drug reaction.
Conclusions
Effectiveness of acetylcholinesterase inhibitors on cognitive symptoms of patients with mild to moderate Alzheimer’s disease is modest. At 9 months, improvement was evident only in a subgroup of patients without concomitant diseases and who had demonstrated a response at 3 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One proposed current standard of care for mild to moderate Alzheimer’s disease includes treatment with second-generation acetylcholinesterase inhibitors (donepezil, rivastigmine and galantamine) [1]. The efficacy of these drugs is modest and essentially symptomatic [2]. In the pivotal phase-III randomised clinical trials, an average change of −1.3 and 2.2 points on the cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog) was reported among treated and placebo patients over a 6-month period. The ADAS-cog score ranges from 0 to 70 [3–5], where decreasing score indicates an improvement, and the clinical importance of a 3.5-point improvement is not obvious. A panel of experts convened by the US Food and Drug Administration has defined as clinically significant an improvement of at least 4 points in ADAS-cog score [6]. Following this definition, 24–36% of patients treated in the trials can be considered as “responders” at 6 months versus 15–21% in the placebo groups [3–5]. The European Medicines Evaluation Agency, in the approved summary of product characteristics of acetylcholinesterase inhibitors, has defined as clinically significant a 4-point or greater improvement on the ADAS-cog, no worsening based on clinician’s assessment (clinician interview based impression of change scale-CIBIC plus) and no worsening on activities of daily living scale. With these criteria, 10–21% of treated patients are reported as responders to treatment versus 6–10% for the placebo groups [7–9].
Given the limited amount of information on the efficacy and safety of the treatment when applied in the general population of Alzheimer’s disease patients, the Italian Ministry of Health and the National Institute of Health implemented the Cronos study [10]. Its main objectives were to characterise the population of Alzheimer’s disease patients treated with acetylcholinesterase inhibitors; monitor effectiveness and drug safety in the field practice; identify variables that may predict the response to therapy; and inform physicians and caregivers about the correct use of these drugs.
The present cohort study was conducted on a sample of 5,462 patients treated with acetylcholinesterase inhibitors at 118 Italian Alzheimer’s disease units.
Patients and methods
The Italian National Health Service provided donepezil and rivastigmine (from September 2000) and galantamine (from September 2001) free of charge to all patients with mild to moderate Alzheimer’s disease cared for by the specialists (neurologists, geriatricians and psychiatrists) of the 503 Alzheimer’s disease units located throughout the country. Physicians were free to choose any of the acetylcholinesterase inhibitors. For each treated patient, a standard form was to be filled in, at each visit, following the Cronos study protocol [10].
A newsletter was distributed during the study period to the healthcare professionals involved in the study. A dedicated web site was also created (http://www.alzheimer-cronos.it).
Patients and data collection
A random sample, stratified by region, of 118 Alzheimer’s disease units was selected, and information was collected for all patients enrolled in these units from 1 September 2000 to 31 December 2001. A total of 7,395 patients were treated during the study period in the sampled Alzheimer’s disease units; 5,462 patients were treated with donepezil, rivastigmine or galantamine for the first time (new users) [11] and included in the study cohort (Fig. 1). Patients were followed up until occurrence of the first of the following events: discontinuation of therapy for any reason, a Mini Mental State Examination (MMSE) score less than 10, admission to hospital or nursing home, death or 31 December 2002. Patients who switched therapy from one acetylcholinesterase inhibitor to another contributed to the cohort until the discontinuation of the first drug.
Patients were diagnosed with: probable Alzheimer’s disease according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; a mild to moderate dementia defined as a MMSE score of 14–26; and the presence of cognitive deficits for more than 6 months [10, 12, 13].
Evaluations were performed at baseline, at 1, 3 and 9 months and, then, every 6 months. At each visit, a standard form was completed for each treated patient. The following information was collected: type and dosage of cholinesterase inhibitors; MMSE score; activities of daily living (ADL) and instrumental activities of daily living (IADL) scores; pharmacological treatment; and concomitant diseases [asthma, central nervous system (CNS) disturbances, diabetes, disturbances of cardiac rhythm, gastroduodenal ulcer, hepatic failure, hypertension, obstructive pulmonary disease and renal failure] [10, 14, 15]. Physicians were also asked to report adverse drug reactions (ADRs), which they believed were possibly correlated with the treatment, regardless of their severity. The ADRs were coded according to the World Health Organization-Adverse Reaction Terminology System (WHO-ART).
The main outcome measure was the proportion of responders at 9 months of therapy. “Responders” were defined as patients who completed the therapy, with a MMSE score improvement from baseline of 2 points or more. All other patients were considered “non-responders”. The 2-point threshold in MMSE was chosen as it corresponds to approximately 5 points in the ADAS-cog scale, thus allowing for a comparison with clinical trial results [16].
Statistical analysis
“Responders” to the therapy at 9 months were compared with “non-responders” using the logistic regression model. The covariates used in the models were: age, sex, MMSE score at baseline, concomitant diseases, use of drugs that act on the CNS, cholinesterase inhibitor (donepezil, rivastigmine, and galantamine), dosage of the cholinesterase inhibitor and response at 3 months.
To make the dosage of the three study drugs comparable, the following dose definitions have been adopted: minimal effective dose=donepezil 5 mg/day, rivastigmine 6 mg/day, galantamine 16 mg/day; “low”=donepezil 5 mg/day, rivastigmine ≤6 mg/day, galantamine ≤16 mg/day; “high”=donepezil 10 mg, rivastigmine >6 mg, galantamine >16 mg; “modified” if the dosage was changed (from low to high and vice versa) one or more times during the study period.
All patients included in the cohort were evaluated for safety. The proportion of patients experiencing an ADR was calculated for each WHO-ART category. A logistic regression model was used to compare patients who experienced at least one ADR to those without ADRs. In this analysis, for each patient, the first ADR was considered. The drug dosage used is that recorded before the occurrence of the ADR; for patients without ADRs the dosage was that before the last visit.
Comparisons are presented as adjusted odds ratios (ORs), as an estimate of the relative risk, and 95% confidence intervals (CIs) calculated using Wald tests. The software used was SPSS (Version 11.0).
Results
The demographic and clinical characteristics of the 5,462 patients included in the cohort are shown in Table 1. The mean age was 76 years, with 2.3% of patients younger than 60 years and 33% of patients older than 79 years. About two-thirds of the patients were females. Physicians did not always comply with the inclusion criteria and 542 patients (9.9%) had MMSE scores of 10–13. All patients were included in the analysis.
Patients treated with the three study drugs showed some differences. In particular, patients receiving galantamine were in poorer health than those treated with donepezil or rivastigmine: the mean MMSE score was 17.7; concomitant diseases were present in 28.4% of patients, and 12.3% took other CNS drugs.
Physicians did not always use the dosage regimen recommended by the summary of product characteristics: 10.1% of patients on rivastigmine and 8.1% of those on galantamine were treated, during the entire study period, with dosages lower than the minimal effective dose. Moreover, 13.2% of patients received at the first prescription a dosage higher than the starting dose suggested for each study drug.
A total of 2609 patients (47.8%) discontinued treatment (Fig. 1). Since the physicians were not requested to actively trace the patients, the specific reasons for withdrawal are not always known. The majority of patients (n = 1672, 64.1%) failed to return at the scheduled visit. These patients who were lost to follow-up were observed for a median time of 84 days. They had different characteristics at baseline than those followed up for the entire study period. In particular, they were older (>79 years: 36% versus 30%), more likely to have concomitant diseases (35.5% versus 5.3%), more frequently treated with other CNS drugs (15.3% versus 3.0%) and had a worse initial cognitive status (MMSE 10–15: 27.6% versus 24.3%). The 282 patients who switched drugs included 81 patients for whom the reason was the occurrence of an ADR.
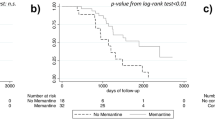
At 9 months, 2,853 patients (52.2%) had completed the study. The mean change from baseline in MMSE scores was an improvement of 0.5 points (±3.0); scores on ADL were substantially unchanged (ADL, −0.1±0.9; IADL, −0.2±1.5). The proportion of responders to the therapy was 17.8% at 3 months and 15.7% at 9 months. Among the 972 responders at 3 months, 67.1% were still responders at 9 months.
Patients without concomitant diseases (OR, 2.1; 95% CI, 1.5–2.9) and those who had a response to therapy at 3 months (OR, 20.6; 95% CI, 17.2–24.6) had the higher probability of responding to treatment at 9 months (Table 2). Response to treatment did not vary among groups with different MMSE scores at baseline. Modifications of the dosage were associated with a lower probability of having a response. The results were similar when the analysis was conducted excluding the patients (n=192) treated with a dosage lower than the minimal effective dose.
Data on CIBIC plus scale were not available in our study, thus we could not adopt the EMEA definition of responders. Nevertheless, the proportion of patients with a MMSE score improvement of 2 or more points from baseline and no worsening on ADL or IADL scores was estimated. With these criteria, 14.6% of patients were responders to the therapy at 9 months.
If cognitive stability was considered, the proportion of responders (MMSE score change from baseline ≥0 points) was 50.9% at 3 months and 32.9% at 9 months. The last one decrease to 30.3% when the criteria of no worsening on ADL or IADL scores was added.
During the 9-month observation period, 783 (14.3%) patients experienced at least one ADR (for a total of 1237 events), and 285 patients (5.2%) discontinued the treatment or switched the therapy because of an ADR (Table 3). Of every 1000 patients, 3 had hallucinations, 2 presyncope episodes, 2 atrio-ventricular blocks, 1 stroke, 1 epileptic seizure and 1 urinary incontinence. The incidence of myocardial infarction and syncope was 0.5 per 1000 patients.
Half of the ADRs occurred within the first 3 months of therapy. Patients experiencing cardiovascular ADRs were more likely to discontinue the treatment because of the ADR. Pre existing conditions, potentially associated with the occurrence of the ADR, were present among 1% of patients with gastrointestinal events and 9% of those with cardiovascular events. Among patients experiencing psychiatric symptoms and disturbances of the nervous system, 10% and 14%, respectively, were receiving other CNS drugs.
A higher proportion of patients receiving rivastigmine and galantamine (19% and 24%) compared with donepezil (13%) experienced an ADR. The difference was mainly due to a higher incidence of gastrointestinal events, which occurred in 6% of those taking donepezil, 14% of those taking rivastigmine and 24% of those on galantamine.
During the study, 24 patients (mean age 80 years) died, of whom 71% had concomitant diseases. None of these deaths was reported to be related to treatment. The cause of death is known for 12 patients: six had a stroke, four others died of cardiovascular disease, one of breast cancer and one a non-defined infection.
Old age, concomitant diseases, use of other CNS drugs and high dosage of acetylcholinesterase inhibitors were associated with likelihood of developing ADRs (Table 4). The probability of occurrence of ADRs was higher when rivastigmine or galantamine were used.
Discussion
Our study shows a modest effectiveness of cholinesterase inhibitors on cognitive symptoms of patients with mild to moderate Alzheimer’s disease. At 9 months, 2,853 patients (52.2%) had completed the study with a mean improvement from baseline of 0.5 points (±3.0) in MMSE scores. The conditions of one-third of patients were judged not to have deteriorated at 9 months, while a subgroup of 857 patients (15.7%) had an improvement from baseline of at least 2 points on MMSE [17, 18].
The treatment effect did not vary with the drug dose, and the strongest predictive variable for the response was an early improvement at 3 months.
In our study we used as primary outcome the “proportion of responders” defining a MMSE equal to 2 as a threshold for a clinically significant effect. The 2-point threshold in MMSE was chosen because it corresponds to approximately 5 points in ADAS-cog scale, which is the threshold adopted by the FDA. The EMEA suggested an even narrower definition of response: a 4-point or greater improvement on the ADAS-cog, no worsening on CIBIC plus scale, and no worsening on ADL scale.
If we use this definition (excluding the CIBIC plus criteria), 14.6% of patients were responders to the therapy at 9 months.
In a recent meta-analysis on efficacy of cholinesterase inhibitors, [2] the pooled mean proportion of cognitive responders (defined as an improvement of 4 or more points on the ADAS-cog) in excess of that for placebo treatment was 10% (95% CI, 4–17%).
Our results are not directly comparable with those obtained from RCTs, because of the lack of a comparison non-treated cohort. Nevertheless, the results on our treated patients may be compared with data on the treatment arms in randomised clinical trials where, at 6 months, 24–36% of patients can be considered as responders [3–5]. The difference is probably attributable to our longer study period (9 months versus 6 months) and to the characteristics of the patient population. The patients treated in our study differ from those usually included in clinical trials on acetylcholinesterase inhibitors: they were older (mean age 76 years versus 72–73 years); they were more likely to have used other drugs acting on the CNS and to have had concomitant diseases. Patients included in the CRONOS cohort are, however, representative of the general population of Alzheimer’s disease patients treated in the clinical practice.
The patients were enrolled from a random sample of Alzheimer’s disease units representative of the total Italian ADUs as for setting (territorial, hospital and university), health personnel employed, examinations offered (computed tomography and magnetic resonance imaging scans and laboratory tests), neuropsychological assessment, counselling activities and relationship with caregiver associations.
Data on mean change from baseline in MMSE score are difficult to compare with trial results as well. Our data were derived from the proportion of patients who completed the study, while trials reported data using the last observation carried forward (LOCF) approach. The LOCF approach entails restrictive assumptions, e.g. that subjects’ responses would have been constant from the last observed value to the endpoint of the trial, and that the mechanism of missingness is missing completely at random (MCAR). These assumptions may not always be valid, and violations may overestimate the effect of treatments in clinical situations, such as AD, where the patient’s condition is expected to deteriorate over time. Moreover, to express the treatment effect through the average change in test scores could not be easily translated into clinically significant outcomes.
All these issues emphasize the problem of generalisability of trial results, and the complementary role of observational studies in providing data for everyday clinical practice. Nevertheless, we attempted to compare our data with the results from a population-based trial (AD 2000) [18]. At 9 months, the mean change from baseline in MMSE score was slightly above zero in the treated patients, and about −1 in the placebo group [18].
Within the Cronos study, physicians were asked to report ADRs that they believed were possibly correlated with the treatment; the proportion of patients experiencing at least one ADR (14.3%) was lower than reported by trials (70–90%) in which all adverse events are recorded. The proportion of patients discontinuing the treatment because of an ADR was comparable with those of the trials: 5% versus 6–20% [3–5, 19]. The most frequent ADRs were gastrointestinal and psychiatric disorders, probably related to the mechanism of action of the drugs and to the natural history of the disease. As expected, more adverse events were reported with higher doses. This result, along with the small proportion of patients treated with high doses, may explain the low incidence of ADRs found in our study. In the large Cronos population some rare and clinically relevant events, which were unlikely to be detected by clinical trials, have been observed (e.g. atrio-ventricular blocks, stroke, epileptic seizure, urinary incontinence, myocardial infarction and syncope, etc.). Even if physicians were asked to report only ADRs correlated with treatment, we cannot conclude, in the absence of an untreated comparison cohort, that these reactions are certainly related to the treatment.
A higher incidence of adverse events was found for galantamine and rivastigmine, which may be a consequence of different pharmacokinetic and pharmacodynamic parameters among the acetylcholinesterase inhibitors [20].
A relatively large number of patients (31%) were lost to follow up after a median time of treatment of about 3 months. Since acetylcholinesterase inhibitors could be obtained free of charge only within the Alzheimer’s disease units it is likely that these patients discontinued the treatment. These patients were older than those followed up for the entire study period, had a higher proportion of concomitant diseases and had a worse initial cognitive status. One limitation of our study is that the specific reasons for discontinuing the treatment are unknown for these patients, precluding a full description of the safety of the study drugs.
During all the study period, 10% of patients on rivastigmine and 8% of those on galantamine were treated with dosages lower than the minimal effective dose. This probably reflects the difficulty to follow the titration scheme as reported on the summary of product characteristics [7–9].
In this cohort study, approximately 2 of 10 patients showed a response at 3 months and only 1 maintained the response at 9 months. Approximately 1 of every 7 treated patients developed an ADR, and 36% of these patients discontinued the treatment. At 9 months, improvement was evident only in a subgroup of patients without concomitant diseases and who had demonstrated a response at 3 months.
Based on our results, and according to NICE criteria [21], physicians should be suggested to accurately re-evaluate patients, after 3 months of therapy, in order to decide whether the risk-benefit profile is still favourable.
References
Scarpini E, Scheltens P, Feldman H (2003) Treatment of Alzheimer’s disease: current status and new perspectives. Lancet Neurol 2:539–547
Lanctôt KL, Herrmann N, Yau KK et al (2003) Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 169:557–564
Rogers SL, Fralow MR, Doody RS, Mohs R, Friedhoff LT, Donepezil Study Group (1998) A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 50:136–145
Rosler M, Anand R, Cicin-Sain A et al (1999) Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trail. BMJ 318:633–640
Wilcock GK, Lilienfeld S, Gaens E on behalf on the Galantamine International-1 Study Group (2000) Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. BMJ 321:1–7
Peripheral and Central Nervous System Drugs Advisory Committee Meeting, July 7, 1989. Department of Health and Human Services, Public Health Service, Food and Drug Administration, Rockville, MD, p 227
Aricept® (donepezil hydrochloride) (1997) Summary of product characteristics. Pfizer, Italy
Exelon® (rivastigmine) (1998) Summary of product characteristics. Novartis, Italy
Reminyl® (galantamine) (2001) Summary of product characteristics. Janssen, Italy
Government of Italy. Protocollo di monitoraggio dei piani di trattamento farmacologico per la malattia di Alzheimer. D M July 20, 2000. Gazzetta Ufficiale della Repubblica Italiana n. 204, 1 Settembre 2000
Ray WA (2003) Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158:915–920
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzhemeir’s disease. Neurology 34:939–944
Folstein M, Folstein S, Mc Hugh PR (1975) Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA 185:914–919
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9:179–186
Doraiswamy PM, Bieber F, Kaiser L, Krishnan KR, Reuning-Scherer J, Gulanski B (1997) Patterns and predictors of baseline cognitive performance in multicenter Alzheimer’s disease trials. Neurology 48:1511–1517
Winblad B, Brodaty H, Gauthier S et al (2001) Pharmacotherapy of Alzheimer’s disease: is there a need to redefine treatment success? Int J Geriatr Psychiatry 16:653–666
AD2000 Collaborative Group (2004) Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomized double blind trial. Lancet 363:2105–2115
Winblad B, Engedal K, Soininen H et al (2001) A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 57:489–495
Imbimbo BP (2001). Pharmacodynamic-Tolerability relationships of cholinesterase inhibitors for Alzheimer’s disease. CNS Drugs 15:375–390
National Institute for Clinical Excellence. Guidance on the Use of Donepezil, Rivastigmine and Galantamine for the Treatment of Alzheimer’s disease. Technology Appraisal Guidance—No. 19. Available at: http://www.nice.org.uk (Accessed October 4, 2004)
Acknowledgements
We thank Umberto Senin (Perugia University), Ovidio Brignoli (General Practitioner), and Teresa Di Fiandra (Ministry of Health) for supervising the study; Francesca Ravaioli (Ministry of Health) for coordinating data collection; Monica Bolli and Paola Ruggeri (Istituto Superiore di Sanità) for data collection; Clara Bianchi, Roberto Da Cas, Francesca Menniti Ippolito, Stefania Spila Alegiani, and Giuseppe Traversa (Istituto Superiore di Sanità) who discussed the study findings. Expenses were covered by National Health Service funds.
Author information
Authors and Affiliations
Corresponding author
Additional information
The list of Alzheimer’s disease unit investigators is reported in the Appendix
Appendices
Appendix
Alzheimer’s disease unit investigators
Abruzzo: Gabriele A. Servizio Neurologia, Sulmona (AQ)—Lechiara MC. Presidio Ospedaliero “S. Rinaldi”, Pescina (AQ). Basilicata: Paciello M. UVA Ospedale San Carlo, Potenza. Calabria: Ambrosio L. ASL4 Ospedale Annunziata, Cosenza; Buccomino D. Centro Salute Mentale, Roggiano Gravina (CS)—Carabetta V. ASL9 Locri (RC)—Filastro F. Centro Alzheimer ASL7, Girifalco (CZ)—Lamenza F. Centro Demenze ASL3, Rossano (CS)—Bruzzese T. Centro Salute Mentale ASL9, Locri (RC). Campania: Caterino L. Coordinamento UVA ASL CE/2, Aversa (CE)—Cerqua G. Distretto 40, Castelvolturno (CE)—D’amore A. Distretto 37, Casal di Principe (CE) e Distretto 35—Carinaro (CE)—De Martino G. ASL NA/5, Castellammare di Stabia (NA)—Di Fusco A. Distretto 38, S. Maria Capua Vetere (CE)—Di Sato G. Dipartimento Medicina Interna e Specialistica ASL BN/1, Cerreto Sannita (BN)—Feleppa MAO. Rummo, Benevento—Ianniello P. Distretto 39, Capua (CE)—Maiello M. Distretto 43, Sessa Aurunca (CE)—Iazeolla M. Distretto 17, Benevento, Distretto 18, Cautano (BN), Distretto 22, Morcone (BN) e UOAA Distretto 19, Montesarchio (BN)—Maio G. UOAA Distretto 23, S. Bartolomeo in Galdo (BN)—Marino MP. Distretto 20, S. Agata dei Goti (BN) e Distretto 24, S. Giorgio del Sannio (BN)—Nuzzo P. Distretto 42, Mondragone (CE)—Femina G. Centro Alzheimer ASL AV2, Atripalda (AV)—Roma M. Distretto 36, Frignano (CE)—Santagata P. Distretto 21, Telese Terme (BN)—Schipani G. UOAA Distretto 102, Battipaglia (SA) e Distretto 97, Salerno—Scognamiglio P. Distretto 41, Pignataro Maggiore (CE).
Emilia Romagna: Alberti M. Centro Demenze, Guastalla (RE)—Arena C. Poliambulatorio, San Lazzario di Savena (BO)—Boiardi R. Centro Distrettuale Demenze, Castelnovo Ne’ Monti (RE)—Ferrari P. Ospedale, Scandiano (RE)—Ghidoni E. UVA Arcispedale Santa Maria Nuova, Reggio Emilia e AUSL Centro Distrettuale Disturbi Cognitivi, Reggio Emilia—Lucchetti L. Consultorio Aziendale per i Disturbi Cognitivi, AUSL Piacenza—Miconi G. Ambulatorio Consulenza Geriatria AUSL Bologna Sud, Porretta Terme (BO)—Pellati M. Centro Distrettuale Demenze, AUSL Reggio Emilia, Correggio (RE)—Roberti R. Centro Demenze, Montecchio (RE)—Friuli Venezia Giulia: Prati P. ASL4 Ospedale Gervasutta, Udine—Lazio: Alfonsi S. ASL Frosinone Distretto C Dipartimento Salute Mentale, Sora (FR)—Giramma F. IPFD Venditti, Centro Provinciale UVA, Ospedale S. Maria Goretti, Latina—Di Cioccio L. UOC di Geriatria e Centro UVA Servizio Geriatrico, Aquino (FR)—Lancetti USL Ospedale Bel Colle, Viterbo. Liguria: Carniglia De Carli R. ASL4 Pres.San. Territoriale, Chiavari (GE)—Colameo A. Unità Geriatrica di Cura per le Demenze ASL5 Spezzino, Sarzana—Montanari GP. Neurologia Geriatria USL5 Spezzino, La Spezia. Lombardia: Alberoni M. UVA IRCCS S. Maria Nascente, Fondazione Don Gnocchi, Milano—Bargnani C. Clinica S. Rocco di Franciacorta, Ome (BS)—Boffelli S. UO Geriatria, Ospedale Poliambulanza, Brescia—Bosio, Clinica S. Anna, Neurologia, Brescia—Bugiani, Ospedale Besta, Milano—Casale R. ISP Maugeri, Clinica del Lavoro, Montescano (PV)—Chia F. UVA AO Mellino Mellini, Chiari (BS)—Clerici F. UVA Clinica Neurologica Ospedale L. Sacco, Milano—Cuzzoni G. IPAB Istituto S. Margherita, S. Margherita (PV)—Farina PM. IPAB Pii Istituti Unificati, Belgioioso (PV)—Franzoni S. Ospedale “Richiedei”, Gussago (BS)—Gerini Niguarda CA’ Granda, Milano—Magnani G. Divisione di Neurologia Ospedale San Raffaele, Milano—Magrotti E. Neurologia ASL Pavia, Pavia—Padovani A. Spedali Civili, Brescia—Ranzenigo A. UO Geriatria Ospedale S. Orsola Fatebenefratelli, Brescia—Sacilotto G. Istituti Clinici Perfezionamento, Milano – Sinforiani, Istituto Neurologico Mondino, Pavia—Spinnler H. Clinica Neurologica III Ospedale S. Paolo, Milano—Viti N. CDC Istituto Palazzolo, Fondazione Don Carlo Gnocchi, Milano—Zanetti O. UO Alzheimer IRCCS Centro S. Orsola Fatebenefratelli, Brescia. Marche: Livini L. Distretto Centro UVA, ASL 11, Fermo—Masotti, AUSL6, Fabriano (AN)—Molise: Cuccaro, ASL3 “Centro Molise”, Campobasso. Piemonte: Diarassouba A. Az. Regionale ASL7, Chivasso (TO)—Francesconi M. ASL19, Asti—Guala A. ASL12, Osp. Infermi, Divisione Geriatria, Biella—Infantino C. Az. Regionale USL5, Collegno, Rivoli (TO)—Seliak D. ASL17 UOA Neurologia, Savigliano (CN). Provincia autonoma di Bolzano: Gasperi A. Servizio Neurologia Azienda Sanitaria, Brunico (BZ). Puglia: De Matteis C. ASL LE/1 Geriatria P O S.Giuseppe da Copertino, Copertino (LE)—Elia A. ASL LE/1, Lecce—Fulgido ASL LE/1 UVA Galatina, Galatina (LE)—Totaro G. Ospedale S. Michele ASL Cerignola, Monte S. Angelo (FG). Sardegna: Capelli P. UVA, Geriatria Ospedale San Francesco ASL3, Nuoro—Cosseddu G. ASL2 Ospedale S. Giovanni di Dio, Olbia (SS)—Minnai G. ASL6 Ospedale S. Martino, Oristano. Sicilia: Arena MG. Clinica Neurologica, Policlinico Universitario, Messina—Di Pasquale MR. Neurologia Ospedale Piemonte, Messina—Emilici A. Dipartimento Salute Mentale, Patti (ME)—Lalicata L. ASL AG/1 Centro Salute Mentale, Canicattì (AG)—Nastasi G. Ospedale Papardo Neurologia, Messina—Scifo E. USL1, Agrigento—Xerra AM. ASL ME/5 Centro di Salute Mentale, Milazzo (ME). Toscana: Cipriani G. Neurologia Ospedale “Versilia”, Viareggio (LU). Umbria: Mecocci P. Geriatria Policlinico Monteluce, Perugia—Parnetti L. Neurologia Policlinico Monteluce, Perugia—Pollioni F. ASL2 S.M. Angeli, Assisi—Ricci S. ASL2 Ambulatorio Neurologico di Bastia Umbra (PG), Ellera (PG), Castiglione del Lago (PG), Città della Pieve (PG), Ponte San Giovanni (PG) e Serv. Mal. Cerebrovasc. e Neurol, Perugia. Valle d’Aosta: Bottacchi E. UB Neurologia e Neurofisiopatologia, Ospedale Regionale della Valle d’Aosta, Aosta. Veneto: Cester A. Geriatria Ospedale, Dolo (VE) e Geriatria Ospedale, Noale Mirano (VE)—Garonna F. ASL3 Psichiatria Ospedale, Bassano del Grappa (VI)—Zanetti L. UO Geriatria Ospedale di Conegliano, Pieve di Soligo (TV).
Rights and permissions
About this article
Cite this article
Raschetti, R., Maggini, M., Sorrentino, G.C. et al. A cohort study of effectiveness of acetylcholinesterase inhibitors in Alzheimer’s disease. Eur J Clin Pharmacol 61, 361–368 (2005). https://doi.org/10.1007/s00228-005-0946-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0946-1