Abstract
Common conditions predisposing to atherosclerosis, such as dyslipidemia, hypertension, diabetes, and smoking, are associated with increased vascular production of reactive oxygen species (ROS). One of the major consequences of increased vascular ROS production is a reduction of endothelial nitric oxide (NO) bioavailability. Importantly, endothelial NO not only produces endothelium-dependent vasodilation but also has potent antiatherogenic properties, including inhibition of platelet aggregation and adhesion molecule expression. This concept has been supported by several recent clinical studies, suggesting a close association of impaired endothelium-dependent vasomotion and clinical cardiovascular events. Increased vascular ROS production reduces endothelial NO not only by direct inactivation, but also as a consequence of increased oxidation of tetrahydrobiopterin and inhibition of dimethylarginine dimethylaminohydrolase. This may in part explain the profound impact of increased endothelial oxidant stress on endothelial NO bioactivity. An increased activity of NAD(P)H oxidase, a major vascular oxidant enzyme system, has been observed in both experimental atherosclerosis and human coronary disease and likely represents an important source of ROS. Moreover, NAD(P)H oxidase has been shown to cause endothelial NO synthase “uncoupling” and to promote xanthine oxidase-dependent superoxide production. In addition, in human coronary disease a reduced vascular activity of the antioxidant enzyme extracellular superoxide dismutase has been observed. Notably, cardiovascular treatment strategies such as statin, angiotensin-converting enzyme (ACE) inhibitor, and angiotensin I receptor blocker treatment may exert potent “antioxidant” effects by reducing NAD(P)H oxidase and increasing extracellular superoxide dismutase activity, leading to improved endothelial function. Restoring endothelial function has become an attractive therapeutic target, given accumulating observations supporting a prognostic role of endothelial dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
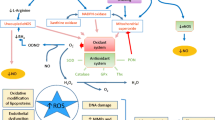
Over the last 2 decades it has become evident that the endothelium is not an inert single-cell lining covering the internal surface of blood vessels, but plays a crucial role in vascular homeostasis, i.e., by regulating vascular tone and structure. Furthermore, a healthy endothelium inhibits platelet and leukocyte adhesion to the vascular surface and keeps a balance between profibrinolytic and prothrombotic activity (Fig. 1) [1]. Common conditions predisposing to atherosclerosis, such as hypercholesterolemia, hypertension, diabetes, and smoking, are associated with endothelial dysfunction leading to a proinflammatory and prothrombotic phenotype of the endothelium. Endothelial dysfunction may therefore play a pivotal role in the development and progression of atherosclerosis and its clinical complications [1–3].
The healthy endothelium not only mediates endothelium-dependent vasodilation, but also actively suppresses thrombosis, vascular inflammation, and hypertrophy. Nitric oxide (NO) is a particularly important mediator of endothelium-dependent vasodilation as well as anti-inflammatory and antithrombotic effects of the endothelium. Endothelium-dependent vasomotion is therefore thought to represent a surrogate marker of other important functions of the endothelium
Endothelial function has mostly been assessed as endothelium-dependent vasomotion, largely based on the assumption that impaired endothelium-dependent vasomotion also reflects alterations of other functions of the endothelium. An important rationale for this approach has been the observation that endothelium-derived nitric oxide (NO), synthesized by the endothelial NO synthase (eNOS) from the precursor L-arginine, not only mediates endothelium-dependent vasodilation, but is also critically involved in the regulation of other protective properties of the healthy endothelium [4–7]. This may, at least in part, underlie the consistent observation of recent clinical studies, demonstrating that the degree of impairment of endothelial function (assessed as endothelium-dependent vasomotion) represents a strong and independent predictor of cardiovascular events in patients with coronary disease and its risk factors [8–11].
Vascular oxidant stress: a major cause of reduced endothelial NO availability
The impairment of endothelium-dependent, NO-mediated vasodilation in experimental models of cardiovascular disease, such as atherosclerosis and hypertension, is reversed after administration of superoxide dismutase (SOD) or other superoxide scavengers, suggesting a pivotal role of increased vascular superoxide production for endothelial dysfunction [12–15].
Numerous clinical studies have demonstrated that the acute administration of structurally unrelated antioxidants (i.e., high local dose of ascorbic acid or N-acetylcysteine) improves endothelium-dependent vasodilation in patients with coronary disease or cardiovascular risk factors, supporting the concept that increased vascular reactive oxygen species (ROS) formation plays an important role for endothelial dysfunction in patients at risk for cardiovascular events [10, 16–20]. An example from our laboratory is shown in Fig. 2a [18], indicating that administration of a high local dose of the antioxidant vitamin C reverses impaired NO-mediated vasodilation in patients with coronary disease. Importantly, Heitzer et al. [10] have recently demonstrated that the beneficial effect of vitamin C (high local dose) on endothelial dysfunction has profound and independent prognostic implications in patients with coronary disease (Fig. 2b). In a study on 179 patients with coronary disease it was shown that patients with a substantial improvement of endothelium-dependent vasodilation after vitamin C had a markedly higher cardiovascular event rate as compared to patients with limited effect of vitamin C on endothelial function [10], suggesting that increased vascular oxidant stress is associated with an impaired prognosis. These findings have further stimulated an interest in understanding the mechanisms causing increased vascular superoxide production and consequently a reduced endothelial NO bioavailability.
a Effect of the antioxidant vitamin C on endothelium-dependent, NO-mediated vasodilation in patients with coronary artery disease (CAD) and healthy control subjects. A high local dose of the antioxidant vitamin C (250 mg i.a. in 10 min) was administered. Adapted and modified from [18]. b Proportion of patients with CAD without cardiovascular events dependent on their initial response to vitamin C on endothelium-dependent vasodilation. Adapted and modified from [10]
Mechanisms of vascular oxidant stress in cardiovascular disease
Antioxidant enzyme systems
Vascular cells are equipped with three isoenzymes of SOD that dismute superoxide anions to oxygen and hydrogen peroxide. These isozymes have different cellular localizations: the cytosolic Cu,Zn-SOD, the mitochondrial Mn-SOD, and the extracellular SOD (ecSOD), which is bound to the cellular surface [21]. Inhibition of vascular SOD causes a profound impairment of NO-mediated vasodilation, suggesting that vascular SOD activity is critical for endothelial NO availability [22–24]. We therefore analyzed SOD isoenzyme activity in coronary arteries from patients with coronary disease as compared to nonatherosclerotic arteries. In coronary arteries from patients with coronary disease there was no change of the activity of the intracellular SOD isoenzymes (Cu,Zn-SOD, and Mn-SOD); however, there was a marked reduction of the extracellular SOD activity in atherosclerotic coronary arteries [18]. Extracellular SOD, which is expressed at approximately 100-fold higher levels in the vascular wall as compared to other tissues such as muscle or fat tissue, likely has a special function in the vessel [25, 26]. Observations from mice lacking ecSOD have shown that ecSOD deficiency is associated with an impaired endothelium-dependent, NO-mediated vasomotion and a markedly augmented endothelial dysfunction in response to hypertension [27]. In order to determine whether reduced ecSOD activity in patients with coronary disease is related to endothelium-dependent, NO-mediated vasodilation we analyzed endothelium-bound ecSOD activity (released from the endothelium to plasma by heparin bolus injection) in patients with coronary disease in vivo [18]. ecSOD is bound to heparan sulfate on the endothelial cell surface and can therefore be rapidly released to plasma by heparin bolus injection [28]. In line with the observations in coronary arteries, endothelium-bound ecSOD activity was markedly reduced in patients with coronary disease as compared to healthy subjects and was closely related to endothelium-dependent, NO-mediated vasodilation, suggesting that reduced ecSOD activity contributes to reduced vascular NO availability (Fig. 3; [18]). A mutation of the ecSOD heparin-binding domain, which impairs binding of the enzyme to the endothelial surface, has recently been identified. Carriers of this ecSOD mutation had a significantly higher risk of developing coronary disease, as was shown in the Copenhagen City Heart Study by analyzing more than 9,000 subjects, suggesting a protective function of vascular-bound ecSOD [29].
a Endothelium-bound extracellular SOD (ecSOD) activity (released from endothelium to plasma by heparin bolus injection) in patients with coronary artery disease (CAD) and healthy control subjects, suggesting a marked reduction of endothelium-bound ecSOD activity in patients with CAD. b Relation of ecSOD activity and endothelium-dependent, NO-mediated vasodilation in patients with CAD. Adapted and modified from [18]
Oxidant enzyme systems
Although there are numerous potential enzymatic sources of ROS in vascular cells, including the mitochondrial electron transport chain, the arachidonic acid metabolizing enzymes lipoxygenase and cyclooxygenase, the cytochrome P450s, NADPH oxidase, xanthine oxidase, and “uncoupled” NO synthase, the last three enzymes have been studied rather extensively over the last several years and likely play a predominant role in cardiovascular disease [30].
The NADPH oxidase, a superoxide producing enzyme, has been found to be expressed in all vascular cells, i.e., endothelial, vascular smooth muscle cells, fibroblasts. This enzyme consists of two membrane-integrated subunits (nox and p22phox) and three cytosolic subunits (p47phox, p67phox, and the small GTPase Rac) (Fig. 4a). There are several Nox isoforms that vary in their tissue distribution, cellular localization, and requirement for cytosolic factors. In vascular homogenates of hypercholesterolemic or hypertensive animals NADPH oxidase activity is increased [12, 31]. By using a p22phox-antisense or vascular smooth muscle and endothelial cells genetically deficient in p47phox it was shown that superoxide production in response to important stimuli such as angiotensin II depends on the activation of the NADPH oxidase [32–34]. Furthermore, mice that are genetically deficient in the cytosolic subunit p47phox had no increase in vascular oxidant stress in response to angiotensin II (in contrast to wild-type mice), suggesting that activation of the NADPH oxidase is critically important for the development of vascular oxidant stress in vivo [35, 36]. Importantly, the blood pressure response to angiotensin II or deoxycorticosterone acetate (DOCA)-salt hypertension is significantly blunted in p47phox-deficient mice [35, 37], suggesting an important role of NADPH oxidase activation for the development and progression of hypertension, likely at least partly related to a reduced endothelial NO availability. In this respect, Li et al. [38] have shown that p47phox and the vascular NADPH oxidase are critically involved in the reduction of endothelial NO availability in response to angiotensin II.
a Structure of the NADPH oxidase enzyme system, which is composed of two membranous subunits (nox and p22phox) and three cytosolic subunits (p47phox, p67phox, and the small GTPase Rac) that translocate to the membrane upon activation of the enzyme. For the membranous component nox several homologues have now been identified in vascular cells with different cellular localization (i.e., nox1, 2, and 4). b NADPH oxidase activity as determined by electron spin resonance (ESR) spectroscopy in human atherosclerotic and nonatherosclerotic coronary arteries, suggesting activation of the enzyme in human coronary disease. A representative ESR scan of NADPH-stimulated superoxide production from an atherosclerotic artery (CAD) and nonatherosclerotic artery (Control) is shown. Adapted and modified from [40]
In patients with coronary disease the vascular expression of the NADPH oxidase subunit p22phox is increased in all vascular cells [39]. By using electron spin resonance spectroscopy we have recently demonstrated that the activity of the NADPH oxidase is markedly increased in coronary arteries from patients with coronary disease as compared to nonatherosclerotic coronary arteries, suggesting that this enzyme is activated in human coronary disease (Fig. 4b; [40]).
Another important source of superoxide in the endothelium that has received increasing attention is the uncoupled eNOS. When eNOS is uncoupled, the enzyme produces superoxide upon activation in addition to NO [41, 42]. In particular, a deficiency of the cofactor tetrahydrobiopterin promotes “uncoupling” of the enzyme [41, 42]. In experimental hypertension we have observed a markedly increased superoxide production derived from eNOS [37]. Importantly, vascular superoxide production was lower in eNOS-deficient mice as compared to wild-type mice with DOCA-salt hypertension. eNOS uncoupling was not observed, however, in p47phox-deficient mice, suggesting a redox-sensitive process underlying eNOS uncoupling in vivo [37]. Of note, vascular levels of tetrahydrobiopterin were markedly reduced in hypertensive wild-type mice, but not in hypertensive p47phox-deficient mice, suggesting that at least one mechanism whereby eNOS becomes uncoupled in vivo relates to NADPH oxidase-dependent oxidation of tetrahydrobiopterin. This implies that activation of the NADPH oxidase can further promote vascular oxidant stress by causing eNOS uncoupling that increases superoxide production and reduces NO formation by eNOS in the endothelium. These observations may partly explain why Barry-Lane et al. [43] have observed a reduction of atherosclerotic lesion formation in the aorta of apoE-deficient mice lacking p47phox. Several studies in patients with coronary disease or conditions predisposing to atherosclerosis have now demonstrated a beneficial effect of tetrahydrobiopterin administration on endothelium-dependent vasomotion, suggesting that eNOS uncoupling is importantly involved in the impairment of endothelial NO availability in human cardiovascular disease [42, 44]. Of note, overexpression of eNOS has augmented atherosclerotic lesion formation in apoE-deficient mice, an effect that could be corrected by tetrahydrobiopterin administration, strongly suggesting that eNOS uncoupling has the potential to promote atherosclerotic lesion formation [45].
Finally, xanthine oxidase has been suggested as an important oxidant enzyme system contributing to vascular oxidant stress in cardiovascular disease [46, 47]. In experimental atherosclerosis xanthine oxidase-derived superoxide has been found to be a major cause of endothelial dysfunction [48, 49]. In patients with coronary disease, coronary and endothelium-bound xanthine oxidase activity are increased and closely related to endothelium-dependent, NO-mediated vasodilation, suggesting that increased xanthine oxidase activity contributes to endothelial dysfunction in human cardiovascular disease (Fig. 5) [50]. Recent experimental data have suggested that endothelial expression of xanthine oxidase is regulated in a redox-sensitive way, dependent on the endothelial NADPH oxidase [50].
Potential mechanisms leading to increased vascular superoxide production in atherosclerosis. Increased vascular superoxide production likely relates to activation of the vascular NADPH oxidase enzyme system that can promote further superoxide production from uncoupled endothelial NO synthase (eNOS) and xanthine oxidase. In advanced atherosclerosis vascular extracellular SOD (ecSOD) activity is reduced, which may further enhance oxidant stress and thereby contribute to reduced vascular NO bioavailability
Summary and conclusion
Accumulating evidence suggests that increased vascular oxidant stress represents a major cause of reduced endothelial NO bioavailability in experimental and clinical cardiovascular disease. The mechanisms leading to increased vascular production of ROS include activation of the vascular NADPH oxidase, and likely as a consequence increased superoxide formation from uncoupled eNOS and endothelium-bound xanthine oxidase (Fig. 5). In addition, in patients with advanced atherosclerosis there is a significant reduction of the vascular extracellular SOD activity that may further augment endothelial dysfunction. Increased oxidant stress, in particular superoxide production, may reduce endothelial NO availability not only by direct inactivation of endothelium-derived NO, but also by oxidation of tetrahydrobiopterin leading to eNOS uncoupling [37], and by inhibition of dimethylarginine dimethylaminodydrolase (DDAH), the enzyme that hydrolyzes the endogenous NOS inhibitor asymmetric dimethylarginine (ADMA) (Fig. 6) [51].
Mechanisms by means of which increased vascular reactive oxygen species (ROS) production may reduce the bioavailability of endothelium-derived NO. ROS may reduce endothelial NO availability at least via three pathways: (1) Direct inactivation of NO• by superoxide (O2 •−) resulting in loss of NO bioactivity. (2) Reduced eNOS activity due to increased levels of asymmetric dimethylarginine (ADMA), an endogenous eNOS inhibitor, resulting from redox-sensitive inhibition of dimethylarginine dimethylaminohydrolase (DDAH), the ADMA-metabolizing enzyme. (3) eNOS uncoupling at least in part due to increased oxidation of tetrahydrobiopterin (H4B), a critical cofactor of eNOS, resulting in reduced NO production and increased superoxide production from eNOS. These mechanisms may explain why changes of the endothelial redox state have a profound impact on endothelial NO availability
Of note, whereas short-term administration of a high local dose of the antioxidant vitamin C improved endothelial function in numerous clinical studies, long-term oral administration of vitamin E/C appears not to be effective in improving coronary or peripheral endothelium-dependent vasomotion [52]. This observation may partly explain why administration of vitamin E in recent large-scale clinical trials had no beneficial effect in primary or secondary prevention [3].
In contrast, recent studies suggest that common treatment strategies that improve prognosis in patients with cardiovascular disease, in particular angiotensin-converting enzyme (ACE) inhibition and statin treatment, may have potent “antioxidant” effects by preventing activation of vascular oxidases, i.e., NADPH oxidase [31, 53–55], or by restoring antioxidant enzyme capacity, i.e., ecSOD [53, 56], that may importantly contribute to their therapeutic effect. A better understanding of the regulation and the pathophysiological importance of alterations of oxidant/antioxidant enzyme systems in cardiovascular disease may provide important novel insights into the pathophysiology of cardiovascular disease with a potential to improve prognosis, and may lead to novel therapeutic targets.
References
Ross R (1999) Atherosclerosis-an inflammatory disease. N Engl J Med 340:115–126
Vita JA, Keaney JF Jr (2002) Endothelial function: a barometer for cardiovascular risk? Circulation 106:640–642
Landmesser U, Harrison DG (2001) Oxidant stress as a marker for cardiovascular events: Ox marks the spot. Circulation 104:2638–2640
Tsao PS, McEvoy LM, Drexler H, Butcher EC, Cooke JP (1994) Enhanced endothelial adhesiveness in hypercholesterolemia is attenuated by L-arginine. Circulation 89:2176–2182
Zeiher AM, Fisslthaler B, Schray-Utz B, Busse R (1995) Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res 76:980–986
Tomita H, Egashira K, Kubo-Inoue M, Usui M, Koyanagi M, Shimokawa H, Takeya M, Yoshimura T, Takeshita A (1998) Inhibition of NO synthesis induces inflammatory changes and monocyte chemoattractant protein-1 expression in rat hearts and vessels. Arterioscler Thromb Vasc Biol 18:1456–1464
Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL (2001) Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 104:448–454
Schachinger V, Britten MB, Zeiher AM (2000) Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101:1899–1906
Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A (2000) Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101:948–954
Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T (2001) Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104:2673–2678
Halcox JPJ, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KRA, Quyyumi AA (2002) Prognostic value of coronary vascular endothelial dysfunction. Circulation 106:653–658
Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG (1996) Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97:1916–1923
Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG (1997) Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 95:588–593
Mugge A, Elwell JH, Peterson TE, Hofmeyer TG, Heistad DD, Harrison DG (1991) Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res 69:1293–1300
Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG (2001) Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103:1282–1288
Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF Jr, Vita JA (1996) Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 93:1107–1113
Hornig B, Arakawa N, Kohler C, Drexler H (1998) Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation 97:363–368
Landmesser U, Merten R, Spiekermann S, Buttner K, Drexler H, Hornig B (2000) Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation 101:2264–2270
Andrews NP, Prasad A, Quyyumi AA (2001) N-acetylcysteine improves coronary and peripheral vascular function. J Am Coll Cardiol 37:117–123
Prasad A, Andrews NP, Padder FA, Husain M, Quyyumi AA (1999) Glutathione reverses endothelial dysfunction and improves nitric oxide bioavailability. J Am Coll Cardiol 34:507–514
Landmesser U, Drexler H (2002) Toward understanding of extracellular superoxide dismutase regulation in atherosclerosis: a novel role of uric acid? Arterioscler Thromb Vasc Biol 22:1367–1368
Mugge A, Elwell JH, Peterson TE, Harrison DG (1991) Release of intact endothelium-derived relaxing factor depends on endothelial superoxide dismutase activity. Am J Physiol 260:C219–C225
Omar HA, Cherry PD, Mortelliti MP, Burke-Wolin T, Wolin MS (1991) Inhibition of coronary artery superoxide dismutase attenuates endothelium-dependent and -independent nitrovasodilator relaxation. Circ Res 69:601–608
Lynch SM, Frei B, Morrow JD, Roberts LJ II, Xu A, Jackson T, Reyna R, Klevay LM, Vita JA, Keaney JF Jr (1997) Vascular superoxide dismutase deficiency impairs endothelial vasodilator function through direct inactivation of nitric oxide and increased lipid peroxidation. Arterioscler Thromb Vasc Biol 17:2975–2981
Stralin P, Karlsson K, Johansson BO, Marklund SL (1995) The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol 15:2032–2036
Fukai T, Folz RJ, Landmesser U, Harrison DG (2002) Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res 55:239–249
Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP (2003) Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res 93:622–629
Karlsson K, Marklund SL (1987) Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J 242:55–59
Juul K, Tybjaerg-Hansen A, Marklund S, Heegaard NH, Steffensen R, Sillesen H, Jensen G, Nordestgaard BG (2004) Genetically reduced antioxidative protection and increased ischemic heart disease risk: the Copenhagen City Heart Study. Circulation 109:59–65
Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844
Warnholtz A, Nickenig G, Schulz E, Macharzina R, Brasen JH, Skatchkov M, Heitzer T, Stasch JP, Griendling KK, Harrison DG, Bohm M, Meinertz T, Munzel T (1999) Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation 99:2027–2033
Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK (1996) p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem 271:23317–23321
Lavigne MC, Malech HL, Holland SM, Leto TL (2001) Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation 104:79–84
Li JM, Shah AM (2003) Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem 278:12094–12100
Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG (2002) Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40:511–515
Brandes RP, Miller FJ, Beer S, Haendeler J, Hoffmann J, Ha T, Holland SM, Gorlach A, Busse R (2002) The vascular NADPH oxidase subunit p47phox is involved in redox-mediated gene expression. Free Radic Biol Med 32:1116–1122
Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG (2003) Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial nitric oxide synthase in hypertension: role of the NAD(P)H oxidase. J Clin Invest 111:1201–1209
Li JM, Wheatcroft S, Fan LM, Kearney MT, Shah AM (2004) Opposing roles of p47phox in basal versus angiotensin II-stimulated alterations in vascular O2- production, vascular tone, and mitogen-activated protein kinase activation. Circulation 109:1307–1313
Azumi H, Inoue N, Takeshita S, Rikitake Y, Kawashima S, Hayashi Y, Itoh H, Yokoyama M (1999) Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation 100:1494–1498
Spiekermann S, Landmesser U, Dikalov S, Gamez G, Tatge H, Hornig B, Drexler H, Harrison DG (2003) Electron spin resonance characterization of vascular NAD(P)H- and xanthine-oxidase-activity in patients with coronary artery disease: relation to endothelium dependent vasodilation. Circulation 107:1383–1389
Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA Jr (1998) Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A 95:9220–9225
Alp NJ, Channon KM (2004) Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24:413–420
Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS (2001) p47phox is required for atherosclerotic lesion progression in ApoE(-/-) mice. J Clin Invest 108:1513–1522
Tiefenbacher CP, Bleeke T, Vahl C, Amann K, Vogt A, Kubler W (2000) Endothelial dysfunction of coronary resistance arteries is improved by tetrahydrobiopterin in atherosclerosis. Circulation 102:2172–2179
Ozaki M, Kawashima S, Yamashita T, Hirase T, Namiki M, Inoue N, Hirata K, Yasui H, Sakurai H, Yoshida Y, Masada M, Yokoyama M (2002) Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J Clin Invest 110:331–340
Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, Freeman BA (1999) Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem 274:4985–4994
Adachi T, Fukushima T, Usami Y, Hirano K (1993) Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial-cell surface. Biochem J 289:523–527
Ohara Y, Peterson TE, Harrison DG (1993) Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 91:2546–2551
White CR, Darley-Usmar V, Berrington WR, McAdams M, Gore JZ, Thompson JA, Parks DA, Tarpey MM, Freeman BA (1996) Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc Natl Acad Sci U S A 93:8745–8749
McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG (2003) Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285:H2290–H2297
Cooke JP (2000) Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol 20:2032–2037
Kinlay S, Behrendt D, Fang JC, Delagrange D, Morrow J, Witztum JL, Rifai N, Selwyn AP, Creager MA, Ganz P (2004) Long-term effect of combined vitamins E and C on coronary and peripheral endothelial function. J Am Coll Cardiol 43:629–634
Hornig B, Landmesser U, Kohler C, Ahlersmann D, Spiekermann S, Christoph A, Tatge H, Drexler H (2001) Comparative effect of ace inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease: role of superoxide dismutase. Circulation 103:799–805
Wassmann S, Laufs U, Baumer AT, Muller K, Konkol C, Sauer H, Bohm M, Nickenig G (2001) Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol 59:646–654
Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M (2000) Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol 20:61–69
Landmesser U, Bahlmann F, Mueller M, Spiekermann S, Kirchhoff N, Schulz S, Manes C, Fischer D, de Groot K, Fliser D, Fauler G, Marz W, Drexler H (2005) Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 111:2356–2363
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Landmesser, U., Harrison, D.G. & Drexler, H. Oxidant stress—a major cause of reduced endothelial nitric oxide availability in cardiovascular disease. Eur J Clin Pharmacol 62 (Suppl 1), 13–19 (2006). https://doi.org/10.1007/s00228-005-0012-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0012-z