Abstract
Objective
The purpose of this study was to elucidate the pharmacokinetics of each enantiomer of lansoprazole and 5-hydroxylansoprazole in three different CYP2C19 genotype groups of Japanese subjects.
Methods
Healthy subjects (n=18), of whom 6 were homozygous extensive metabolizers (homEMs), 6 were heterozygous extensive metabolizers (hetEMs) and 6 were poor metabolizers (PMs), participated in the study. After a single oral dose of 60 mg of racemic lansoprazole, the plasma concentrations of the lansoprazole enantiomers, 5-hydroxylansoprazole enantiomers and lansoprazole sulfone were measured for 24 h post-dose.
Results
The plasma concentrations of (R)-lansoprazole were remarkably higher in all three CYP2C19 genotype groups than those of the corresponding (S)-enantiomer. The mean maximum plasma concentration (C max) of (S)-lansoprazole differed significantly among the three groups, whereas there was no difference for the (R)-enantiomer. The relative area under the plasma concentration (AUC) ratios of (R)- and (S)-lansoprazole in the homEMs, hetEMs, and PMs were 1:1.5:4.0 and 1:1.8:7.4, respectively. Yet, the relative AUC ratios of 5-hydroxylansoprazole to lansoprazole for the (R)- and (S)-enantiomers in the homEMs, hetEMs, and PMs were almost the same (1:0.73:0.12 and 1:0.77:0.13, respectively). However, the AUC ratios of the (S)-enantiomer were 13-fold greater for the three CYP2C19 genotypes than those of the corresponding (R)-enantiomer.
Conclusions
The magnitude of the contribution of CYP2C19 to the 5-hydroxylation of (S)-lansoprazole was greater than that of the (R)-enantiomer. The R/S ratios for the AUC of lansoprazole for the homEMs, hetEMs and PMs were 12.7, 8.5 and 5.8, respectively, suggesting a significant effect of CYP2C19 polymorphisms on the stereoselective disposition of lansoprazole.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lansoprazole [2-{(3-methyl-4-(2,2,2-trifluoethoxy)-2-pyridyl)methyl}sulfinylbenzimidazole] (Fig. 1) is a proton-pump inhibitor that inhibits gastric acid secretion by interacting with (H+/K+)-ATPase in gastric parietal cells [1]. Although lansoprazole possesses asymmetric sulfur in the chemical structure, it has been commercially marketed as a racemic mixture. Both the R(+)- and S(−)-enantiomers of lansoprazole inhibit (H+/K+)-ATPase activity in isolated canine microsomes and acid formation stimulated by dibutyryl cyclic adenosine monophosphate (db-cAMP) in isolated canine parietal cells [2]. Until now, the pharmacological activities of each enantiomer as seen in an in vitro study were considered to be identical for two assay systems [2]. However, the clinical outcomes of the effects of each lansoprazole enantiomer have not been clarified.
Lansoprazole is extensively metabolized in the liver; major detectable metabolites in the plasma include 5-hydroxylansoprazole and lansoprazole sulfone [3–5]. 5-Hydroxylation of lansoprazole is mainly mediated by CYP2C19, whereas sulfoxidation is mediated by CYP3A4 [3, 5] (Fig. 1). In individuals with the CYP2C19 poor metabolizer (PM) phenotype, the area under the plasma concentration (AUC)–time curve of lansoprazole is markedly increased [3, 4, 6–8]. The AUC values of (R)- and (S)-lansoprazole in the PMs following an oral dose of 30 mg of racemic lansoprazole are also 4.6-fold and 5.8-fold greater, respectively, than in the extensive metabolizers (EMs) [9]. Furthermore, in the EMs and PMs of CYP2C19, the plasma concentrations of (R)-lansoprazole are higher at all times than those of the (S)-enantiomer; the AUC ratios of the R/S-enantiomer in EMs and PMs are 8.5 and 5.7, respectively [9]. Such differences among the pharmacokinetics of lansoprazole enantiomers are assumed to be influenced by enantioselective metabolism [9, 10].
The aim of this investigation was to elucidate the pharmacokinetics of each enantiomer of lansoprazole and 5-hydroxylansoprazole among three different CYP2C19 genotype groups (homozygous EMs, heterozygous EMs and PMs) in Japanese subjects.
Materials and methods
Subjects
The demographic characteristics of the subjects are listed in Table 1. Healthy Japanese subjects (n=18) [homozygous EM group (homEMs, n=6), heterozygous EM group (hetEMs, n=6) and PMs (n=6)] were selected to participate in this study. None of the subjects had a history of significant medical illness or hypersensitivity to any drug. All subjects were nonsmokers. The study protocol was approved by the ethics committee of Hirosaki University Hospital, and all subjects gave their written informed consent before participating.
Study protocols
All subjects received a single oral dose of 60 mg of lansoprazole (TAKEPRON, Takeda) with a glass of tap water at 0900 hours. Venous blood samples used to determine the plasma concentration of lansoprazole enantiomers and their metabolites were taken prior to and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12 and 24 h later. The samples were centrifuged at 3000 g immediately after collection and stored at −80° until they were analyzed. All subjects fasted for 10 h prior to the administration of lansoprazole and had a standard meal 4 h later. Beverages containing alcohol and caffeine were forbidden during the test period.
CYP2C19 genotyping
The genotyping procedures used to identify the CYP2C19 wild-type gene and its two mutant alleles, CYP2C19*2 in exon 5 and CYP2C19*3 in exon 4, were performed using a polymerase chain reaction-restriction fragment length polymorphism method [11]. CYP2C19 genotype analysis revealed five different patterns as follows: *1/*1 in 6, *1/*2 in 3, *1/*3 in 3, *2/*2 in 5 and *2/*3 in 1. Subjects with these genotype patterns were divided into three groups: the homEMs (*1/*1, n=6), the hetEMs (*1/*2 and *1/*3, n=6) and the PMs (*2/*2 and * 2/*3, n=6).
Reagents and chemicals
Lansoprazole enantiomers and their metabolites (5-hydroxylansoprazole and lansoprazole sulfone) were purchased from Takeda Pharmaceutical Co. Ltd., (Osaka, Japan). (S)-omeprazole was kindly donated by AstraZeneca (Mölndal, Sweden). An Oasis HLB extraction cartridge was purchased from Waters (Milford, Mass., USA). All solvents used were of high-performance liquid chromatography (HPLC) grade (Wako Pure Chemical Industries, Osaka, Japan), and all other reagents and chemicals were purchased from Wako Chemical Industries or Nacalai Tesque (Kyoto, Japan).
Analysis of lansoprazole enantiomers and their metabolites in plasma
The plasma concentrations of the lansoprazole enantiomers and their metabolites were determined according to the HPLC method of Miura et al. [12]. In brief, after (S)-omeprazole (20 ng) in methanol (10 μl) was added to the samples (100 μl) as an internal standard, the samples were diluted with water (1.0 ml), and the solutions were briefly mixed. Each mixture was applied to an Oasis HLB extraction cartridge that had been previously activated with methanol and water (1.0 ml each). The cartridges were then washed with 40% methanol in water (1.0 ml) and eluted with 80% methanol in water (1.0 ml). The eluates were evaporated to dryness in a vacuum at 60° by a rotary evaporator (Iwaki, Tokyo, Japan). The residues were dissolved in 50 μl of methanol and 50 μl of the mobile phase, and for each an aliquot (50 μl) was injected into the HPLC apparatus. The HPLC column used was a Chiral CD-Ph (250 mm×4.6 mm I.D., Shiseido Co., Ltd., Tokyo, Japan). The mobile phase consisted of 0.5 M NaClO4–acetonitrile–methanol (60:30:10, v/v), which was degassed in an ultrasonic bath prior to use. A flow rate of 0.5 ml/min was used at ambient temperature, and the wavelength was set at 285 nm. The lower limit of quantification for this assay was 10 ng/ml for each enantiomer of lansoprazole and 5-hydroxylansoprazole, whereas it was 5 ng/ml for lansoprazole sulfone. The coefficient of variation of inter- and intra-day assays was less than 8.0%, and the accuracy was within 8.4% for all analytes (concentration range of 10–4000 ng/ml).
Pharmacokinetic analysis
Pharmacokinetic analysis of the lansoprazole enantiomers and their metabolites was carried out by a standard noncompartmental method using WinNonlin (Pharsight Co., CA, USA version 4.0.1). The elimination half-life was obtained by log-linear regression of the terminal phase of the concentration–time data for the least points (elimination half-life=ln 2/ke; ke=elimination rate constant). The total AUC–time curve was calculated using the linear trapezoidal rule. Extrapolation of AUC from the last measurable concentration (C t ) to infinity (AUC t-∞) was performed by adding the value C t /ke (where C t = plasma concentration for t h after lansoprazole administration). The maximum plasma level (C max) and time required to reach the peak (t max) were directly obtained from the profile.
Statistical analysis
All results were expressed as mean values±SD. Statistical comparisons of the parameters were supplemented with the multiple comparison procedure of Fisher using the Stat View program (SAS Institute, Cary, N.C., USA version 5.0). A P value of less than 0.05 was considered to be statistically significant.
Results
Pharmacokinetics of lansoprazole enantiomers
The mean plasma concentrations of both lansoprazole enantiomers were highest in the PMs, intermediate in the hetEMs and lowest in the homEMs (Fig. 2, Table 2). The mean C max values of (S)-lansoprazole differed significantly among the three CYP2C19 genotype groups, whereas there were no significant differences for those of the (R)-enantiomer. The relative AUC ratios of (R)- and (S)-lansoprazole in the homEMs, hetEMs and PMs were 1:1.5:4.0 and 1:1.8:7.4, respectively. The elimination half-lives for (R)- and (S)-lansoprazole in the PMs were significantly longer than the homEMs and hetEMs (P<0.001 each).
The AUC0-∞ C max and elimination half-life of (R)-lansoprazole were significantly greater and longer, respectively, than those of the (S)-enantiomer for all three genotype groups.
Pharmacokinetics of 5-hydroxylansoprazole enantiomers
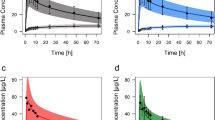
The mean AUC0-∞ and C max of (R)-5-hydroxylansoprazole in the PMs were significantly smaller than in the homEMs and hetEMs (Fig. 3a, b, Table 3). The mean C max value of (R)-5-hydroxylansoprazole in the homEMs was significantly higher than that of hetEMs (P<0.05). However, the mean elimination half-life was 2.4-fold longer for the hetEMs than with the homEMs. Consequently, there was no difference in the AUC0-∞ of the (R)-enantiomer between the homEMs and hetEMs. Furthermore, the ratio of the AUC of (R)-5-hydroxylansoprazole to that of (R)-lansoprazole differed significantly among the three groups, with the relative ratio in the homEMs, hetEMs and PMs being 1:0.73:0.12. However, the mean AUC0-∞ values of (S)-5-hydroxylansoprazole were also significantly smaller in the PMs than in the homEMs and hetEMs. The C max values of the (S)-enantiomer were comparable between the homEMs and hetEMs, but the mean elimination half-life was 1.5-fold longer for the hetEMs than the homEMs. Consequently, the mean AUC0-∞ value of the (S)-enantiomer was slightly greater in the hetEMs than in the homEMs. However, the ratios of the AUC of (S)-5-hydroxylansoprazole to that of (S)-lansoprazole differed significantly among the three groups similar to those of the (R)-enantiomer, with a relative ratio for the homEMs, hetEMs and PMs being 1:0.77:0.13.
Mean±SD plasma concentration–time profiles of a (R)-5-hydroxylansoprazole, b (S)-5-hydroxylansoprazole and c lansoprazole sulfone after a 60-mg oral dose of racemic lansoprazole for homozygous extensive metabolizers (EMs) (solid circles), heterozygous EMs (open circles) and poor metabolizers (PMs) (solid squares)
Pharmacokinetics of lansoprazole sulfone
The plasma concentration of lansoprazole sulfone at all time points was highest for the PMs, intermediate for the hetEMs and lowest for the homEMs (Fig. 3c, Table 4). The mean AUC0-∞ and C max values of lansoprazole sulfone also differed significantly among the three genotype groups. The elimination half-lives of lansoprazole sulfone were 12.5- and 11.5-fold longer for the PMs than with the homEMs and hetEMs, respectively (P<0.001 each).
Discussion
In the present study, we examined the pharmacokinetics of lansoprazole enantiomers and their metabolites in relation to CYP2C19 genotype status by administering 60 mg of racemic lansoprazole. Present knowledge indicates no statistically significant difference in the pharmacokinetics of lansoprazole between homEMs and hetEMs [7, 13]. Our results from this study showed no statistically significant differences in the pharmacokinetics of (R)-lansoprazole between the homEMs and hetEMs. However, our results showed that the pharmacokinetics of (S)-lansoprazole were more intensely affected by a CYP2C19 polymorphism than those of the (R)-enantiomer. The plasma concentrations of (R)-lansoprazole were remarkably higher than those of the corresponding (S)-enantiomer. Therefore, the pharmacokinetics of lansoprazole (racemate) reported previously must be similar to those of the (R)-enantiomer.
The AUC ratios of (S)-5-hydroxylansoprazole to (S)-lansoprazole were 13-fold greater for the three genotype groups than those of the corresponding (R)-enantiomer. This indicates that the magnitude of the contribution of CYP2C19 to the metabolism of (S)-lansoprazole is greater than that of the (R)-enantiomer. We were able to confirm the stereoselective property of CYP2C19 in humans. However, we could not determine the contribution of sulfone metabolite formation from each lansoprazole enantiomer, because the sulfone metabolite is achiral. In vitro experiments on human liver microsomes and cDNA-expressed CYP3A4 showed that the formation rate of the sulfone metabolite from (S)-lansoprazole is greater than that for the (R)-enantiomer [8, 14]. Therefore, significant differences in the AUC between (R)- and (S)-lansoprazole for the three genotype groups are due to their stereoselective conversion of (S)-lansoprazole into (S)-5-hydroxylansoprazole and lansoprazole sulfone by CYP2C19 and CYP3A4, respectively.
The AUC ratio of (R)-lansoprazole to (S)-lansoprazole for the PMs was 5.8, which is almost the same as the ratio previously reported by Kim et al. [9]. However, the R/S ratios of the AUC for the homEMs and hetEMs were 12.7 and 8.5, respectively. Consequently, the R/S ratio for the homEMs was 2.2-fold higher than for the PMs (P<0.05, data not shown). The mean AUC0-∞ values of (R)- and (S)-lansoprazole for the PMs were 4.0-fold and 7.4-fold greater, respectively, than those of the homEMs.
Until now, stereoselective differences in the pharmacological effects and safety for each lansoprazole enantiomer have not been well established. Similar to a previous in vitro study [2], if the pharmacological effects of the (R)- and (S)-enantiomers of lansoprazole for the inhibition of acid secretion are identical in the human body, there appears to be little clinical significance for (S)-lansoprazole, which is effectively metabolized to pharmacologically inactive 5-hydroxy and sulfone metabolites when the drug is administered as a racemate [15].
In conclusion, the present study indicates that the magnitude of the contribution of CYP2C19-mediated metabolism of (S)-lansoprazole is greater than that of the (R)-enantiomer. The R/S ratios for the AUCs of lansoprazole in the homEMs, hetEMs and PMs are 12.7, 8.5 and 5.8, respectively. The pharmacokinetic outcomes of lansoprazole enantiomers were significantly different among the three genotype groups. Based on the data from the present study, dependence of the ratios of the (R)- and (S)-enantiomers of lansoprazole on CYP2C19 genotypes status can be expected.
References
Nagaya H, Satoh H, Maki Y (1990) Possible mechanism for the inhibition of acid formation by the proton pump inhibitor AG-1749 in isolated canine parietal cells. J Pharmacol Exp Ther 252:1289–1295
Nagaya H, Inatomi N, Nohara A, Satoh H (1991) Effects of the enantiomers of lansoprazole (AG-1749) on (H++K+)-ATPase activity in canine gastric microsomes and acid formation in isolated canine parietal cells. Biochem Pharmacol 42:1875–1878
Pearce RE, Rodrigues AD, Goldstein JA, Parkinson A (1996) Identification of the human P450 enzymes involved in lansoprazole metabolism. J Pharmacol Exp Ther 277:805–816
Katsuki H, Nakamura C, Arimori K, Fujiyama S, Nakano M (1997) Genetic polymorphism of CYP2C19 and lansoprazole pharmacokinetics in Japanese subjects. Eur J Clin Pharmacol 52:391–396
Pichard L, Curi-Pedrosa R, Bonfils C, Jacqz-Aigrain E, Domerque J, Joyeux H, Cosme J, Guengerich FP (1995) Oxidative metabolism of lansoprazole by human liver cytochromes P450. Mol Pharmacol 47:410–418
Sohn DR, Kwon JT, Kim HK, Ishizaki T (1997) Metabolic disposition of lansoprazole in relation to the S-mephenytoin 4′-hydroxylation phenotype status. Clin Pharmacol Ther 61:574–582
Sakai T, Aoyama N, Kita T, Sakaeda T, Nishiguchi K, Nishitora Y, Hohda T, Sirasaka D, Tamura T, Tanigawara Y, Kasuga M, Okumura K (2001) CYP2C19 genotype and pharmacokinetics of three proton pump inhibitors in healthy subjects. Pharm Res 18:721–727
Furuta T, Shirai N, Xiao F, Ohashi K, Ishizaki T (2001) Effect of high-dose lansoprazole on intragastic pH in subjects who are homozygous extensive metabolizers of cytochrome P4502C19. Clin Pharmacol Ther 70:484–492
Kim K, Shon J, Park J, Yoon Y, Kim M, Yun D, Kim M, Cha I, Hyun M, Shin J (2002) Enantioselective disposition of lansoprazole in extensive and poor metabolizers of CYP2C19. Clin Pharmacol Ther 72:90–99
Katsuki H, Hamada A, Nakamura C, Arimori K, Nakano M (2001) Role of CYP3A4 and CYP2C19 in the stereoselective metabolism of lansoprazole by human liver microsomes. Eur J Clin Pharmacol 57:709–715
De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA (1994) Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol 46:594–598
Miura M, Tada H, Suzuki T (2004) Simultaneous determination of lansoprazole enantiomers and their metabolites in plasma by liquid chromatography with solid-phase extraction. J Chromatogr B 804:389–395
Ieiri I, Kishimoto Y, Okochi H, Momiyama K, Morita T, Kitano M, Morisawa T, Fukushima Y, Nakagawa K, Hasegawa J, Otsubo K, Ishizaki T (2001) Comparison of the kinetic disposition of and serum gastrin change by lansoprazole versus rabeprazole during an 8-day dosing scheme in relation to CYP2C19 polymorphism. Eur J Clin Pharmacol 57:485–492
Kim KA, Kim MJ, Park JY, Shon JH, Yoon YR, Lee SS, Liu KH, Chun JH, Hyun MH, Shin JG (2003) Stereoselective metabolism of lansoprazole by human liver cytochrome p450 enzymes. Drug Metab Dispos 31:1227–1234
Inatomi N, Nagaya H, Ishisaka Y, Satoh H (1991) Effects of AG-1749 (Lansoprazole) and its metabolites on acid secretion and experimental ulcers. Jpn Pharmacol Ther 19:477–486 (in Japanese)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miura, M., Tada, H., Yasui-Furukori, N. et al. Pharmacokinetic differences between the enantiomers of lansoprazole and its metabolite, 5-hydroxylansoprazole, in relation to CYP2C19 genotypes. Eur J Clin Pharmacol 60, 623–628 (2004). https://doi.org/10.1007/s00228-004-0809-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0809-1