Abstract
Objectives
To compare the systemic exposure for intranasal mometasone furoate (MF) and fluticasone propionate (FP) aqueous nasal sprays (ANS) in terms of serum and urinary cortisol parameters and plasma pharmacokinetics.
Methods
Twelve healthy subjects completed this three-way, cross-over study. They received FPANS (50 μg/spray), MFANS (50 μg/spray) or placebo ANS, eight sprays per nostril every 8 h for 4 days. Cortisol measurements were made at baseline and day 4. FP and MF plasma concentrations were also measured on day 4.
Results
MFANS produced similar mean plasma AUC (123 pmol/l h) to FPANS (112 pmol/l h). Despite the use of high doses, necessary to generate adequate pharmacokinetic data, only minor reductions in cortisol parameters were found, with no difference between FPANS and MFANS.
Conclusions
FP and MF have similar and very low systemic bioavailability when administered intranasally using a high-dose regimen. It is therefore unlikely that therapeutic doses of intranasal FP or MF will produce dissimilar or significant degrees of systemic exposure or systemic effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluticasone propionate (FP) and mometasone furoate (MF) are potent topical corticosteroids available as aqueous nasal sprays (ANS) and are extremely effective in reducing nasal symptoms associated with rhinitis [1, 2, 3, 4]. Although the efficacy and safety of topical corticosteroids for the management of rhinitis are well established, concern remains that these agents may reach the systemic circulation in sufficient concentrations to produce side effects. Intranasal FP has been shown to have negligible systemic bioavailability [5]. However, comparative data are not available for MF.
The normal daily dose regimen of FPANS and MFANS (200 µg/day) has been shown not to produce readily detectable plasma drug concentrations [6, 7]. Therefore, the two formulations were compared using a high-dose regimen, previously shown for FPANS to produce measurable plasma concentrations of FP [5]. This provided a means of comparing their systemic exposure in terms of pharmacokinetic and pharmacodynamic end points and, thereby, the relative potential for systemic side effects.
Methods
This was a randomised, single-blind, placebo-controlled, three-way cross-over study, with a 4-day treatment period and a 1-week washout period. To ensure subjects were blinded to treatment allocation, study drugs were re-labelled and spray bottle caps removed at the time of dosing. The study protocol was approved by the investigational centre’s ethics committee, and informed consent was obtained from each subject before enrolment in the study.
Fifteen healthy subjects (13 males and 2 females) with a mean age of 32.3±9.1 years and a body mass index of 25.85±2.26 kg/m2 were randomised to treatment. Based on data from a previous study with an intra-subject variability of 0.15, twelve subjects were required for 90% power to detect a 20% change in 24-h serum cortisol AUC (area under the plasma concentration–time curve). Twelve volunteers completed the study. The allocated treatments were administered for 4 days as eight sprays per nostril every 8 h: FPANS (Flonase, 50 µg per spray), MFANS (Nasonex, 50 µg per spray) and placebo ANS (matching Beconase AQ).
On the day before the first dose, baseline measurements were made for 24-h urinary cortisol excretion, and blood samples (2 ml) were drawn for measurement of serum cortisol concentrations at 0, 1, 2, 3, 4, 6, 8, 12, 16, 20 and 24 h. On days 1–4, medication was taken three times daily at 0800, 1600 and 2400 hours. Subjects were housed in the research unit for five days and six evenings during each treatment period. Following the 0800-hours dose on day 4, the 24-h urinary and serial serum cortisol measurements were repeated. Blood (5 ml) was also drawn to measure FP and MF plasma concentrations at pre-dose, 5 min, 15 min and 30 min, and 1, 1.5, 2, 3, 4, 6 and 8 h post the 0800-hours dose. Plasma samples were analysed for FP and MF using a liquid-chromatography tandem mass-spectrometry (LC-MS-MS) assay with a detection limit of 20 pg/ml [8]. Cortisol in both urine and serum was assayed using a competitive radioimmunoassay (Chiron ACS:180SE Automated Chemiluminescence System) with a detection limit of 6 nmol/l.
Urinary and serum cortisol parameters were log-transformed and analysed using an analysis of covariance with mean baseline values as covariate, allowing for effects due to subject, period and treatment. Pharmacokinetic parameters for FP and MF were estimated using WinNonlin Professional (version 1.5). These included area under the plasma concentration–time curve over the 8-h dose interval (AUCτ), maximum observed plasma concentration (Cmax) and time of Cmax (tmax). Values for tmax and log-transformed values of AUCτ and Cmax were analysed using the Wilcoxon signed-rank method. To provide an estimate of the absolute systemic bioavailability, previously reported intravenous pharmacokinetic parameters for FP and MF in healthy subjects were used [5, 9].
Results
Table 1 summarises AUCτ, Cmax and tmax after dosing with FPANS and MFANS. There were no significant differences between FP and MF in the rate or extent of systemic absorption. Of the twelve evaluable subjects, two and three subjects for FP and MF, respectively, had all plasma samples below the lower limit of quantification (<20 pg/ml). The estimated absolute intranasal bioavailabilities of FP and MF were very similar (0.42% and 0.46%, respectively) (Table 1).
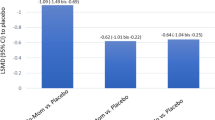
Neither FPANS nor MFANS produced significant decreases in 24-h serum cortisol concentrations (Fig. 1). Geometric mean post/pre-treatment ratios of serum cortisol AUC24 (95% confidence intervals) were 0.89 (0.79, 1.01), 0.94 (0.85, 1.04) and 1.03 (0.94, 1.14) for FPANS, MFANS and placebo, respectively (Fig. 1). The geometric mean post/pre-treatment ratios of serum cortisol Cmin (95% confidence intervals) were 0.85 (0.60, 1.20), 0.84 (0.67, 1.05) and 1.18 (0.81, 1.70) for FPANS, MFANS and placebo, respectively (Fig. 1). FPANS and MFANS did not significantly reduce 24-h urinary cortisol excretion (Fig. 1). The post/pre-treatment ratios (95% confidence intervals) were 0.83 (0.44, 1.56) for FP, 0.81 (0.59, 1.10) for MF and 1.21 (0.81, 1.82) for placebo.
Pharmacokinetic and pharmacodynamic data for 12 volunteers measured on day 4 following multiple 800-µg doses (eight sprays per nostril) three times daily for 4 days of fluticasone propionate aqueous nasal spray (50 µg/spray), mometasone furoate aqueous nasal spray (50 µg/spray) and placebo nasal spray. Results are expressed as a post/pre-treatment ratio with upper 95% confidence intervals
Discussion
This study showed that both FP and MF have similar and very low systemic bioavailability when administered intranasally using a high-dose regimen and a sensitive LC-MS-MS assay. Previous attempts to assess the bioavailability of MFANS and FPANS were not reliable as a relatively insensitive assay and/or low-dose regimens were used [6, 7, 10]. However, the estimates of absolute bioavailability for FPANS in the present study (0.46%) were in agreement with the value reported previously (0.51%) where a similar high-dose study design was used [5].
Our findings confirmed the technical difficulty in measuring the plasma concentrations of FP and MF following intranasal administration, as even the high doses used in this study produced mean peak plasma concentrations just above the assay limit of detection. In designing this study, consideration was given to the possibility that administering multiples of the recommended clinical dose may not be representative of the nasal deposition and absorption that occurs following the standard clinical dose. For this reason, the daily dose was divided into three administrations, and approximately 1 min was allowed to elapse between sprays to the same nostril to minimise run-off. In addition, studies with intranasal beclomethasone dipropionate (BDP) ANS (400, 800 and 1600 µg) [11] and triamcinolone acetonide (TAA) ANS (110, 220 and 440 µg) [12] showed approximately dose-proportional increases in plasma concentrations and therefore provide reassurance that our study design was reasonable. The nasal bioavailability of budesonide (BUD) ANS has also been estimated using doses above those used therapeutically (800 µg) [13]. Therefore, it is likely that the present study can be extrapolated to clinical dose regimen in adults. Furthermore, use of healthy subjects is justified in this cross-over study as a means of reducing variability, since it has also been shown that the systemic exposure to intranasal corticosteroids is not appreciably different in healthy subjects relative to subjects with rhinitis [14].
The cortisol endpoints were included in the study as an additional means of quantifying the systemic exposure to the two intranasal corticosteroids. Following administration of 12 times the clinical dose, serum and urinary cortisol concentrations following both FPANS and MFANS were lower than pre-dose values. However, mean differences were all within 20% of pre-treatment values and 95% confidence intervals included unity. These results are consistent with previous findings showing a lack of systemic effects at higher than recommended doses of FPANS or MFANS administered for longer periods than used in this study [15, 16, 17, 18].
The low intranasal bioavailability of FP and MF is most likely explained by their very poor aqueous solubilities (≈0.1 μg/ml) and high first-pass metabolism. Both formulations are administered as aqueous suspensions that need to dissolve before absorption can occur across the nasal mucosa. However, nasal ciliary clearance is likely to remove most of the drug prior to significant dissolution and absorption, resulting in the major part of the dose being swallowed. Subsequently, extensive first-pass metabolism of the swallowed drug results in negligible bioavailability [19]. In contrast intranasal corticosteroids with higher aqueous solubility and/or significant oral absorption have greater bioavailability via the nasal route: 44%, 31% and 46% for BDP, BUD and TAA, respectively [11, 13, 20].
References
Dolovich J, O’Connor M, Stepner N, Smith A, Sharma RK (1994) Double-blind comparison of intranasal fluticasone propionate, 200 micrograms, once daily with 200 micrograms twice daily in the treatment of patients with severe seasonal allergic rhinitis to ragweed. Ann Allergy 72:435–440
Pedersen B, Dahl R, Richards DH (1995) Once daily fluticasone propionate aqueous nasal spray controls symptoms of most patients with seasonal allergic rhinitis. Allergy 50:794–799
Hebert JR, Nolop K, Lutsky BN (1996) Once-daily mometasone furoate aqueous nasal spray (Nasonex) in seasonal allergic rhinitis: an active- and placebo-controlled study. Allergy 51:569–576
Drouin M, Yang WH, Bertrand B (1996) Once daily mometasone furoate aqueous nasal spray is as effective as twice daily beclomethasone dipropionate for treating perennial allergic rhinitis patients. Ann Allergy Asthma Immunol 77:153–160
Daley-Yates PT, Baker R (2001) Systemic bioavailability of fluticasone propionate administered as nasal drops and the aqueous nasal spray formulations. Br J Clin Pharmacol 51:103–105
McDowell JE, Mackie AE, Ventresca GP, Bye A (1997) Pharmacokinetics and bioavailability of intranasal fluticasone in humans. Clin Drug Invest 1:44–52
Schering-Plough Research Institute (1998) SCH 32088: single dose absolute bioavailability study of mometasone nasal spray in volunteers with evidence of allergic rhinitis. Study No. C93–196 (data on file)
Callejas S, Biddlecombe R, Jones A, Joyce K, Pereira A, Pleasance S (1998) Determination of the glucocorticoid fluticasone propionate in plasma by automated solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr 718:243–250
FDA Summary Basis of Approval (2000) NDA 20762, Nasonex Nasal Spray Medical Officer Review
Price AC, Daley-Yates PT, Wright AM, Callejas S (2000) Negligible absolute bioavailability and no HPA-axis effects after multiple 200 µg daily doses of Fluticasone propionate (FP) administered from the aqueous nasal spray (FPANS). Eur Resp J 16:279s
Daley-Yates PT, Price AC, Sisson JR, Pereira A, Dallow N (2001) Beclomethasone dipropionate: absolute bioavailability, pharmacokinetics and metabolism following intravenous, oral, intranasal and inhaled administration in man. Br J Clin Pharmacol 51:400–409
Jeal W, Faulds D (1997) Triamcinolone acetonide. A review of its pharmacological properties and therapeutic efficacy in the management of allergic rhinitis. Drugs 53:257–280
Thorsson L, Borga O, Edsbaker S (1999) Systemic availability of budesonide after nasal administration of three different formulations: pressurized aerosol, aqueous pump spray and powder. Br J Clin Pharmacol 47:619–624
Argenti D, Colligon I, Heald D (1994) Nasal mucosal inflammation has no effect on the absorption of intranasal triamcinolone acetonide. J Clin Pharmacol 34:854–858
Vargas R, Dockhorn RJ, Findlay SR, Korenblat PE, Field EA, Kral KM (1998) Effect of fluticasone propionate aqueous nasal spray versus oral prednisone on the hypothalamic-pituitary-adrenal axis. J Allergy Clin Immunol 102:191–197
Van As A, Bronsky E, Grossman J, Meltzer E, Ratner P, Reed C (1991) Dose tolerance study of fluticasone propionate aqueous nasal spray in patients with seasonal allergic rhinitis. Ann Allergy 67:156–162
Brannan MD, Seiberling M, Cutler DL (1996) Lack of systemic activity with intranasal mometasone furoate. J Allergy Clin Immunol 97:198
Brannan MD, Herron JM, Reidenberg P (1996) Lack of HPA axis suppression following 36 days of intranasal mometasone furoate. Ann Allergy Asthma Immunol 78:154
Crim C, Pierre LN, Daley-Yates PT (2001) A review of the pharmacology and pharmacokinetics of fluticasone propionate and mometosone furoate. Clin Therapeut 23:1339–1354
Daley-Yates PT, Richards DH (2001) Pharmacokinetic and pharmacodynamic relationships for intranasal corticosteroids (INCS). J Allergy Clin Immunol 107:S313
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daley-Yates, P.T., Kunka, R.L., Yin, Y. et al. Bioavailability of fluticasone propionate and mometasone furoate aqueous nasal sprays. Eur J Clin Pharmacol 60, 265–268 (2004). https://doi.org/10.1007/s00228-004-0763-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0763-y