Abstract
Fine-scale movement dynamics of adult invasive lionfish may inform the spatial scale of negative impacts to local food webs, the design and efficacy of ongoing removal efforts, and the speed at which lionfish may spread into new habitats, but have not previously been characterized. An acoustic Vemco positioning system (VPS) was used to track fine-scale (<10 m) movements of adult lionfish (288–395 mm total length; n = 20) tagged in situ at artificial reefs off Destin, Florida (USA). We estimated the spatial scale of movement, activity patterns, and individual home ranges, as well as whether these variables were affected by lionfish size or density. Lionfish were tracked up to 89 days and had 95% kernel utilization distribution (KUD) home ranges between 158 and 4051 m2. Daily distances moved (range 93–807 m) exceeded previous estimates, and 40% of tagged lionfish were tracked moving to reefs up to 2 km from initial tagging locations. Movement pattern and velocity data revealed two (10%) tagged lionfish were consumed by fast-moving predators, while another two emigrated outside the array. Acoustic detection of the remaining tagged fish ended prematurely following two hurricanes that passed over the array, which may implicate the storms in displacing tagged fish, causing tag loss, or contributing to lionfish mortality. Overall, results suggest invasive lionfish have larger home ranges and display greater movement than reported previously which has important implications for artificial reef management in Florida, and elsewhere, in response to the lionfish invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indo-Pacific lionfish, Pterois volitans/miles complex (Linnaeus 1758), have become well-established members of fish communities in the tropical and subtropical western Atlantic Ocean owing to an invasion that has spanned >30 years (Schofield 2010; Schofield et al. 2014). High planktonic dispersal potential coupled with broad environmental tolerances have led to rapid and widespread establishment of lionfish populations across a diversity of habitat types and locations (Ahrenholz and Morris 2010; Barbour et al. 2011; Lesser and Slattery 2011; Muñoz et al. 2011; Dahl and Patterson 2014). As generalist mesopredators, lionfish pose significant long-term ecological threats in areas they invade by reducing prey biomass and species richness (Green et al. 2012, Dahl et al. 2016, Ballew et al. 2016) to an extent that may alter community and trophic structure (Lesser and Slattery 2011; Dahl et al. 2016). Highly efficient foraging due to novel feeding behaviors (Albins and Lyons 2012) and prey naiveté (Black et al. 2014; Anton et al. 2016), coupled with an apparent lack of natural predators to limit lionfish populations (Hackerott et al. 2013), further exacerbate the negative impacts of lionfish predation on native prey populations.
Considerable investment and effort have been made in developing population management and control strategies for invasive lionfish aimed at reducing their numbers and predicting corresponding impacts (Barbour et al. 2011; Frazer et al. 2012; Green et al. 2014; Dahl et al. 2016; Chagaris et al. 2017). Targeted lionfish removals via spearfishing remain the most viable control option for reducing local lionfish densities, but other strategies, such as lionfish-specific traps, are being developed for use on larger spatial scales (Gittings et al. 2017). Such mitigation strategies may only be effective when accounting for the effects of immigration or recruitment from neighboring habitats via larval transport or post-settlement movement, where removal effort must be high enough to offset the recolonization of new individuals. Post-settlement movement is a central demographic process shaping fish populations, whereby animals may move in response to ontogeny, changing gradients of resource availability, intraspecific density, or environmental conditions (Winemiller and Jepsen 1998; Freiwald 2012). Movement is also an essential component of spatially explicit approaches to marine conservation and population management, given it defines how and at what scales animals interact with their environment and neighboring communities (Gallien et al. 2010).While current-driven larval dispersal has been documented to disperse lionfish larvae at scales of 100s of km (Kitchens et al. 2017), post-settlement movement of adult lionfish remains relatively undescribed, particularly at high resolution.
Invasive lionfish tagged in conventional mark–recapture studies have displayed relatively high site fidelity overall (Jud and Layman 2012; Akins et al. 2014), but site fidelity may decrease at higher densities (Tamburello and Côté 2015). In the northern Gulf of Mexico (nGOM), lionfish densities on artificial reefs have been among the highest (>30 fish per 100 m2) reported in the western Atlantic range since 2014, following initial observations of lionfish in 2010 (Dahl and Patterson 2014; Dahl et al. 2019). In that region, density effects have been documented on lionfish feeding ecology (Dahl and Patterson 2014; Dahl et al. 2018), and growth and body condition (Dahl et al. 2019), and adults quickly recolonized experimentally cleared reefs (Dahl et al. 2016). If movement is also density dependent, then intense intraspecific competition on nGOM artificial reefs may promote lionfish movement on greater scales than has been reported elsewhere in the invaded range.
Unlike conventional tagging techniques, acoustic telemetry allows for passive, continuous monitoring of movement via an acoustic transmitter (i.e., tag) which transmits uniquely coded acoustic pings to underwater acoustic receivers (Heupel et al. 2005; Hussey et al. 2015) with any accompanying sensor data (e.g., depth, temperature). Acoustic telemetry has been used to track lionfish movements in two published studies, but position estimates lacked fine-scale resolution (Bacheler et al. 2015; McCallister et al. 2018). Recently developed acoustic telemetry positioning systems enhance the precision and accuracy of aquatic species movement information via arrays of receivers with overlapping detection ranges used to triangulate real-time position at scales <10 m (Espinoza et al. 2011). Such positioning systems rely on a time-difference-of-arrival positioning algorithm and the speed of sound and time delay between detections of a common acoustic ping detected on at least three receivers (Smedbol et al. 2014). Over large (>10 km2) areas, Vemco VR2W Positioning Systems (VPS; Vemco Ltd., Bedford, Nova Scotia, Canada) can be used to estimate geoposition for multiple tagged fish simultaneously for continuous, long-term (months to years) tracking (Espinoza et al. 2011; Özgül et al. 2015), with coverage dependent on the number and spatial distribution of receivers deployed. Fine-scale movement data from VPS arrays may inform the design of population management and control strategies for lionfish, as well as the speed at which they may spread into new habitats (Crossin et al. 2017).
Here, we report results from a three-dimensional geopositioning acoustic telemetry study on lionfish tagged at nGOM artificial reefs. Receivers were deployed in a VPS array to characterize and quantify the movements of invasive lionfish around nGOM artificial reefs for up to 10 months, a method which has been used previously to estimate movement dynamics for a variety of marine fishes (Furey et al. 2013; Skerritt et al. 2015; Bohaboy et al. 2019), including around artificial reefs (Herbig and Szedlmayer 2016; Özgül et al. 2019; Bacheler et al. 2019a), but has not been reported for invasive lionfish. Specific objectives were to (1) track and quantify the extent of fine-scale movement of acoustically tagged lionfish, (2) estimate lionfish home ranges and activity patterns, (3) examine the effects of lionfish density, body size, time of day, and other factors on their movement dynamics, and (4) estimate the fate of tagged lionfish following temporary or permanent acoustic signal loss.
Materials and methods
Acoustic array and reference transmitters
An array of 25 Vemco VR2 acoustic receivers (Vemco Ltd., Bedford, Nova Scotia, Canada) was deployed in ~35 m of water approximately 27 km south of Destin, Florida, in the nGOM from June 2017 to April 2018 (Fig. 1a). The study array existed within the Okaloosa-C Large Area Artificial Reef System (LAARS) and encompassed several known artificial reefs (Fig. 1b) originally deployed on the seabed by the Florida Fish and Wildlife Conservation Commission in 2003. Reefs consist of single pyramid, paired tetrahedrons, paired cylinders with rounded tops, or a steel frame module. Habitats within the array consist of open sand bottom apart from relatively high-relief (1–2 m) artificial reef structures (Thompson et al. 1999).
Map of a the ~3.5 km2 area acoustic telemetry receiver array (square) located south of Destin, Florida (star), in the Gulf of Mexico within the Okaloosa-C Large Area Artificial Reef Site. Isobaths are 10, 20, 30, 50, 100, and 200 m depths. b Receivers (circles, 01–25) were spaced in an array 350 m apart centered around known reefs (triangles, A–J). Five additional receivers (R1–R5) were stationed off the northeast corner of the array to log reference tag detections
Acoustic receivers were deployed as a Vemco Positioning System (VPS) in a 5 × 5 grid pattern with 350 m spacing that enabled tags to be detected within a 5 km2 area and geoposition estimation in a 3.5 km2 area (Fig. 1b). A combination of Vemco VR2Tx (n = 15) and VR2W (n = 10) receivers were deployed in the array, with Tx receivers deployed with internal synchronization (sync) transmitters set to very high output (160 dB) and W receivers deployed with Vemco V16-5× sync tags suspended with a float 2 m above the receiver. Each receiver was attached to the top of a 2-m tall PVC support pipe that was set in a 36-kg cement base with grab lines for deployment and retrieval. Each receiver-base unit was lowered from the side of the boat to the sea floor on a hook and rope using a swinging J-frame boom and electric mooring winch.
The spacing between adjacent receivers was based on previous acoustic detection range estimates in the region (Patterson 2013); however, due to inherent variability in detection probability as a result of signal distance and environmental factors, a range test was simultaneously conducted (Kessel et al. 2014). Five receivers were deployed off the northeast corner of the array at distances of 278–434 m from reference transmitters (Fig. 1b). Vemco V8 reference transmitters (n = 5) were affixed to receiver-base 05 at varying heights (2 m, 1 m, and 5 cm) off the seabed, with or without antifouling paint (Pettit Transducer Paint, Flexdel Corp., NJ, USA), on 8 July 2017 and left for the duration of the array deployment.
Environmental data which might affect both the performance of the acoustic array or the behavior of tagged fish were collected throughout array deployment. Temperature (°C), ambient noise (mV), and tilt angle (°) data were logged every hour and collected from the VR2Tx receiver at the center of the array (receiver 13; Fig. 1b). Hourly mean windspeed (m s−1), and wave height (m) were acquired from the nearest National Data Buoy Center Station, 42012, which is approximately 90 km west of the center of our array (National Oceanic and Atmospheric Administration and National Weather Service 2017). However, no buoy data exist between 27 October 2017 and 15 March 2018 due to buoy malfunction.
In situ lionfish tagging and reef surveys
All applicable institutional or national guidelines for the care and use of animals were followed during the course of this study. A pre-tagging survey of array reefs was conducted to estimate lionfish densities with SCUBA divers prior to tagging in July 2017 (Table 1). Based on these surveys, lionfish density was manipulated on some reefs to exhibit a low lionfish density (<10 fish 100 m−2) condition, while others were left at higher (>10 fish 100 m−2) densities. Divers removed lionfish from three tagging reefs via spearfishing where fish were speared immediately posterior to the skull–spinal column juncture and then placed in a saltwater ice slurry to euthanize. Counts of remaining lionfish from paired diver surveys were divided by the area (176.7 m2) of a 15-m-wide cylinder to estimate fish density following methods for fish density estimation on small artificial reefs (Patterson et al. 2009).
Lionfish (n = 20) were acoustically tagged among five reef sites with Vemco V8 (n = 15) or V9P (n = 5) acoustic transmitter tags (Table 1; Fig. 2a) which transmitted a unique acoustic ID code approximately every 210 s (range 170–250 s). The estimated battery life of V8 and V9P tags was 358 and 220 days, respectively. V8 tags weighed 0.9 g in water, and the larger V9P tags weighed 2.9 g in water. One fish at each tagging reef was tagged with a V9P tag which had an integrated pressure sensor from which fish depth along with geoposition could be estimated. An additional three lionfish at each tagging reef were tagged with V8 tags.
Digital images of a Vemco V8 acoustic transmitter tag with fully assembled tag mount including Domeier umbrella darts (~15 mm) with Dacron fiber sleeve, and marine grade heat-shrink tubing over nylon coated stainless steel wire, b in situ capture of a lionfish made by hand net on SCUBA at tagging reef, c placement of lionfish in weighted cradle for measurement and tagging, and d a tagged lionfish leaving the cradle seconds after tagging
Tags were externally attached to lionfish in situ at depth (~35 m) to ensure a rapid and minimally intrusive tagging process (Jepsen et al. 2015). Tagging in situ at depth should result in a lowered risk of mortality, injury, stress, and behavioral changes experienced by the fish following tagging (Starr et al. 2000; Lindholm et al. 2005; Feeley et al. 2018), and has been performed on lionfish previously (Akins et al. 2014; Tamburello and Côté 2015; McCallister et al. 2018). Furthermore, external tagging was preferred to avoid additional stress associated with surgery and has been shown to result in higher detection probabilities compared to internally implanted tags (Dance et al. 2016). Another consideration in choosing external over internal tagging at depth was safety, given limited bottom time and risks to divers performing surgery at depth on fish that possess venomous spines.
Each acoustic tag was sprayed with antifouling paint (Pettit Transducer Paint, Flexdel Corp., NJ, USA) prior to deployment to inhibit biofouling, and was attached to a nylon Domeier umbrella dart (~15 mm) with nylon coated stainless steel leader (Domeier et al. 2005). Dart heads contained Dacron fiber sleeves designed to heal into the muscle tissue of tagged fish (Domeier et al. 2005), and marine grade heat shrink tubing was applied around the tag caps to reduce movement and friction against the side of the body (Yergey et al. 2012). The dart head of the tag assembly was inserted into the musculature, between the pterygiophores at the base of the spinous dorsal, placement which allowed tags to be shielded by the pectoral fins when swimming in and out of reef structure.
Twenty lionfish were tagged in situ at depth on five reefs between 8–11 July 2017 (Table 1). Lionfish were captured by divers with a hand net and then transferred to a weighted cradle on the seafloor (Fig. 2b). Fish were measured to the nearest mm total length (TL), and then an activated transmitter was externally attached to the fish using a Floy applicator needle (Fig. 2c). Neither sex nor maturity stage could be determined for tagged fish. After tagging, fish were left to recover in the cradle where post-tagging condition was observed (Fig. 2d). All tagged fish appeared to exhibit normal swimming ability after tagging, and swam back to the reef structure within 1–2 min post-tagging (Fig. 2d). Reefs were visited by divers twice after tagging (September 2017 and December 2017) to observe tagged lionfish and look for any signs of biofouling, tag loss, or emigration.
Data analyses
Fixed-position reference transmitters
The probability of detection of fixed-position reference transmitters was estimated across five receivers (R1, R2, R3, R4, and R5) between 10 July 2017 and 24 April 2018 using R (version 3.6.0, R Core Team 2016) (Fig. 1b). The effects of distance, diel period, month, height off bottom (5 cm, 1 m, or 2 m), presence of antifouling paint, and bottom temperature on reference transmitter detection rate were tested with a generalized linear mixed model (GLMM) in which the response variable was binomial (detected, not detected), and reference transmitter was a random effect (Zuur et al. 2009). Detailed methods on reference transmitters and model fitting can be found in the Electronic Supplementary Material.
Fixed-position reference transmitters provided estimates of measured horizontal error (HPEm) to compare to the unitless error sensitivity (HPE) values provided for animal tag positions (Smith 2013). Lionfish geoposition estimates with HPE values in the upper 5th percentile (≥15.9) were excluded from further analyses which eliminated position estimates that were highly uncertain.
Lionfish acoustic data and fate assignment
All receiver data were downloaded using VUE software (Vemco Ltd.) and sent to Vemco (Nova Scotia) for geoposition estimation with the Vemco Positioning System (VPS) analysis protocol (Smedbol et al. 2014). Both raw detection and VPS data were summarized over the study to estimate the presence versus absence within the array, as well as changes in detection of tagged fish over time. The Euclidean distance (m) traveled between consecutive VPS positions (i.e., step-length) was calculated in R as
where X and Y represent longitude and latitude. Maximum and mean cumulative daily distance moved was calculated for each lionfish, as well as total distance tracked over the study. Additionally, the rate of movement (ROM) between consecutive VPS positions was calculated in R by dividing the linear distance moved between consecutive VPS positions by the time (t) elapsed between them. Thus, the rate of movement (m s−1) was calculated as.
where X and Y represent longitude and latitude, and t represents time in minutes. Mean daily ROM between consecutive VPS positions was calculated and used in conjunction with geoposition data to infer the activity of tagged lionfish.
Acoustic data from receivers (i.e., raw detections) were used to calculate the center of activity (COA) position estimates to assist in fate determination when VPS-calculated (i.e., exact) positions ceased. For each tagged fish, COA estimates of position were calculated from the mean of receiver positions in an X- and Y-coordinate system weighted by the number of tag detections received on each over a specified time interval of 60 m following the methods of Simpfendorfer et al. (2002). These estimates represent the mean position of a given fish per hour, rather than an exact location at an exact time.
Geoposition data from both VPS and COA analyses were projected into ArcGIS (version 10.5.1) to map spatial movement of lionfish within the acoustic array. Lionfish VPS geoposition data were categorized into diel periods to investigate lionfish diel activity patterns using the getSunlightTimes function in the SunCalc package in R (Version 3.4.1) which calculates sunlight phases using date and location (Teets 2003; Agafonkin and Thieurmel 2017). The four diel periods used were dawn (nautical dawn to sunrise end), day (sunrise end to sunset start), dusk (sunset start to night) and night (night to nautical dawn). The nearest array reef to each VPS position and the distance (m) between each point and reef was calculated using the Near tool (Analysis toolkit) in ArcGIS. Height off bottom (m) was calculated for detections on V9P transmitters, accounting for differences in depths among reefs.
Following cessation of tag detection, tagged fish were assigned a fate based on VPS-calculated movement, changes in COA, rate of movement, and height off bottom (for V9P tags with depth sensor), with possible fates being emigration, predation, tag loss, death, and survival. Emigration occurred when fish moved toward the edge of the array and disappeared from detection, but exhibited a typical lionfish rate of movement (i.e., swimming speed). Predation was indicated by an abrupt change in tag movement and/or depth, where tags also displayed ROM faster than 0.5 m s−1 through the array and displayed no affinity for reef structure (Bohaboy et al. 2019). For tags that became stationary on the seafloor, it was not possible to differentiate between death and tag loss. Statistical analysis of movement only occurred with data collected up to the date when one of the above fates was assigned.
Lionfish movement and home range analyses
Lionfish activity was examined using distance from nearest reef and height off bottom derived from VPS geoposition data. GLMMs were computed to test the effects of diel period, lionfish density, lionfish body size, and environmental variables (bottom temperature, wind speed, and wave height) on lionfish distance from the nearest reef for all fish and height off bottom for fish tagged with V9P tags. Data were examined with quantile–quantile (QQ) plots for normally distributed errors with homogenous variance. GLMMs were fitted using a Gaussian distribution with individuals as a random factor to account for non-independence of detections from the same lionfish. Model selection was performed using Akaike information criterion (AIC; Akaike 1974) and maximum likelihood based on GLMM fitting protocols (Zuur et al. 2009; Bolker et al. 2009).
Home range metrics were estimated with ArcGIS (version 10.5.1) for tagged lionfish and reference transmitters with geoposition data obtained from VPS. Home range was estimated using kernel density estimation (KDE) which discriminates habitat use using a probability density map (Kie et al. 2010). A 95% kernel utilization distribution (KUD) indicated total lionfish home range (i.e., where an individual occurred 95% of the time), and a 50% KUD indicated core area use. KUDs were calculated with the kernel density function (Spatial Analyst toolbox) using a standardized search radius (i.e., bandwidth) of 6 m and cell size output of 0.1 m to ensure comparable home ranges between individuals and to avoid over-smoothing and inflation of area use (Walter et al. 2009; Kie et al. 2010; Skerritt et al. 2015). Home range was also estimated by minimum convex polygon (MCP), which is the space enveloping all animal positions across the study and is not subject to potential biases of KDE estimates (Hooge et al. 2001; Kie 2013). MCPs were calculated with the Minimum Bounding Geometry function (Data Management toolbox). GLMM models with individuals as a random factor were computed to test the effect of lionfish TL and density on home range (MCP, 50% and 95% KUD) size. The most parsimonious model was identified by comparing AIC values among models (Burnham and Anderson 2002), and pseudo r2 values were calculated to assess the extent to which the most parsimonious models explained deviance in the data (Nakagawa and Schielzeth 2013).
Results
Lionfish tagging
Results from pre-tagging surveys indicated moderate (~10 fish 100 m−2) to high (>15 fish 100 m−2) lionfish density on sites within the array, and 63 lionfish were removed from low-density (<10 fish 100 m−2) treatment reefs C, D, and H the day prior to tagging. Estimated densities on tagging reefs ranged from 5.7 to 39.8 fish 100 m−2 (Table 1).
Tagged fish ranged from 288 to 395 mm TL (mean 339 mm TL). Based on the weight–length relationship for lionfish in this region (Dahl et al. 2019), V8 tags and V9P tags were estimated to weigh less than 0.25% of the body mass of the smallest lionfish tagged with each transmitter type (V8: 288 mm TL, estimated lionfish mass 0.34 kg; V9P: 320 mm TL, estimated lionfish mass 0.48 kg).
Array conditions and performance
The acoustic array was deployed for 305 days, from 28 Jun 2017 to 29 Apr 2018, with receivers stationed between depths of 29.9 and 33.0 m. Maximum and minimum bottom temperature within the array were 28.5 and 15.1 °C, respectively (Fig. 3). Hurricane Irma, a major Category 4 hurricane, passed through the Gulf of Mexico (GOM) and impacted our array during 11–12 Sept 2017. The center of the hurricane tracked approximately 350 km east of the array, and tropical storm force winds extended over the study area (Cangialosi et al. 2018) which coincided with drastic declines in bottom temperature and increases in both tilt angle and ambient noise recorded on VR2Tx receivers (Fig. 3, Electronic Supplementary Fig. S1). Mean bottom temperature dropped >10 °C between 10 and 12 Sept 2017, nearly reaching the minimum bottom temperature recorded across the entire study period (16.2 °C). A second major meteorological event impacted the acoustic array during 7–8 Oct 2017 when the center of Category 1 Hurricane Nate passed approximately 250 km west of our array, and tropical storm force winds extended over the study area (Beven and Berg 2018). A significant decrease in reference tag (Electronic Supplementary Fig. S2), sync tag, and animal tag (Fig. 3) detections was experienced during both hurricane events, and buoy wind speed and wave height data further corroborate the timing of strong storms affecting our array (Fig. 3).
Environmental data and daily acoustic detections of tagged lionfish over time. Wind speed (solid line) and wave height (dashed line) data were obtained from NOAA buoy 42012 only through October 2017. Bottom temperature was obtained from the VR2Tx receiver stationed in the center of the array. Plotted lines are daily means from hourly readings collected at the NOAA buoy and moored receiver. Daily lionfish raw acoustic detections (gray fill) are plotted through 20 Feb 2018, though tag detections were recorded in low numbers until array retrieval in April 2018. Vertical reference lines indicate Hurricanes Irma and Nate which passed through the Gulf of Mexico (GOM) and near our array between 11–12 September 2017 and 7–8 October 2017, respectively
Across all reference transmitters, mean monthly detection probabilities generally declined over time and with increasing distance between transmitters and receivers (Electronic Supplementary Fig. S2). Transmitters moored higher in the water column (i.e., ≥1 m off the bottom) were detected more often than those near the seafloor (χ2 = 19.98, p < 0.001), a pattern that amplified with increasing distance between tag and receiver (Electronic Supplementary Table S1, S2). Detection probabilities were positively (effect = 0.120) influenced by temperature (χ2 = 571.22, p < 0.001), an effect that was likely tied to hurricanes that brought unseasonably cold water to the study array in summer 2017 (Fig. 3). Diel period had a significant effect on detection probability (χ2 = 1280.99, p < 0.001), with detection probability relative to dawn being higher during the day (effect = 0.063, p < 0.001), and reduced at night (effect = − 0.089, p < 0.001), but was not significantly different from dusk (p = 0.526).
In total, 490,473 raw detections were logged from 20 transmitters attached to lionfish over the course of array deployment (Table 1; Fig. 3). Of these, total raw detections among individual fish ranged from 1339 (ID 1861) to 78,298 (ID 1096), with two tags detected in the array up to the last day of study (IDs 1864, 1869) (Fig. 4). Tag transmissions that were detected simultaneously on three or more receivers resulted in 41,321 VPS geoposition estimates, which were obtained for all tagged fish starting on the day of tagging until the latest detection on 17 Feb 2018 (Table 1; Fig. 4). Positions with high (≥15.9) HPE were removed prior to analyses (n = 2025). Based on the HPEm of fixed-position reference transmitters, HPE for the 95th percentile of positions represented a positional error mean (± SE) of 2.5 (± 0.01 m) and a median of 1.9 m, indicating fish geoposition estimates used in spatial analyses were accurate and precise.
Abacus plot indicating continuous tracking of tagged lionfish (n = 20) by raw and VPS acoustic detections across the study period, from July 2017 to April 2018. Fish IDs 1092–1096 were tagged with V9P transmitters, and fish IDs 1857–1871 were tagged with V8 transmitters. Fates determined at the cessation of normal lionfish behavior are indicated by symbols for each record of VPS movement. Vertical reference lines indicate Hurricanes Irma and Nate which passed through the Gulf of Mexico and near our array between 11–12 September 2017 and 7–8 October 2017, respectively
Lionfish movements and fate
Lionfish VPS detections decreased steadily over the study (Fig. 3). Individual tags were tracked for a minimum of 3 days (ID 1858) and a maximum of 223 days (ID 1096) (Fig. 4). Depth data were recorded for all lionfish with V9P tags, with a minimum depth recorded of 27.4 m and a maximum of 35.2 m (Fig. 5). Lionfish exhibited a mean rate of movement (ROM) of 0.030 m s−1 (± 0.0003 SE) between consecutive detections across the study that rarely (<1%) exceeded 0.09 m s−1 (Fig. 6a, Electronic Supplementary Fig. S3). Fine-scale positions of lionfish showed regular (55% of 39,133 positions) short-range homing movements (<5 m) and regular (29%) foraging movements (5–10 m) in all directions of reef structure, but over 1000 (3%) movements >20 m from tagging reefs, including transiting movement to reefs that were up to 1.8 km away (Table 2; Fig. 6b, Electronic Supplementary Fig. S4). In addition to known reefs within the array, lionfish position estimates suggested homing behavior to other suspected but unknown reefs within the array (Table 2, Electronic Supplementary Figs. S5, S6). While 12 lionfish displayed patterns of consistently high site fidelity, with little movement away from tagging reefs detected beyond assumed foraging forays, the remaining 8 lionfish displayed lower site fidelity and visited more than one known reef, or visited a suspected reef (Table 2). As many as eight total reefs were visited by one individual (ID 1096) during 27 unique transits (Table 2, Electronic Supplementary Fig. S4). VPS positions for this fish were up to ~2 km apart, with area use >1 km2.
Mean total distance traveled per day, calculated from cumulative distances moved between VPS positions per day, was 265 m for all lionfish with a range of 93–807 m among individuals, capturing reef-associated movements as well as transiting behavior between reefs (Table 2). Maximum cumulative distance traveled per day ranged from 253 to 2513 m, and total cumulative distance tracked over the study ranged from 930 m over 4 days to 50 km over 61 days (Table 2).
VPS and COA position estimates, rate of movement, distance between consecutive movements, and height off bottom (V9P-only) were used to infer fate for fish with sporadic (days to weeks) or permanent VPS disappearances (Fig. 4). The timing of Hurricane Irma corresponded to temporary and permanent VPS losses of four fish, IDs 1096, 1860, 1866, and 1869, and the timing of Hurricane Nate corresponded to the loss of two fish, IDs 1869, and 1094 (Fig. 4). While patterns in movement and detection frequency appeared normal for some fish following sporadic VPS disappearances (e.g., 1862, 1863, 1866, 1094), we estimated some fish lost their tag or died (e.g., 1857, 1096) (Electronic Supplementary Fig. S5). One tag loss event was confirmed by divers on 3 Sept 2017 when a V9P transmitter (ID 1093) was retrieved from the seabed at reef C where a lionfish with a hole on its dorsal musculature was observed (Electronic Supplementary Fig. S7). Time-series position data indicate the known-shed tag was last located on 23 Aug 2017 (Fig. 4), but raw detections were recorded through 3 Sep 2017 (Figs. 4, 5).
Movement patterns of two tagged lionfish (ID 1860, 1871) for which tag detection ceased during the study indicate they emigrated from the study area (Electronic Supplementary Fig. S6). For fish that emigrated, ROM estimates <0.05 m s−1 on the last day of VPS detection were characteristic of normal lionfish swimming speeds (Electronic Supplementary Fig. S6). Fish 1860, tagged on low-density reef C, spent time at three other reefs >150 m away from its tagging reef. The final VPS detections of this fish (6 Sept 2017) were at a suspected reef nearly 200 m from reef C, and raw detection and COA data collected after VPS disappearance indicated detections on the westernmost array receivers before permanent disappearance of the tag from detection range (Fig. 4). Fish 1871 was tagged on reef H, a reef that had an intermediate lionfish density (~17 fish 100 m−2), and visited one suspected artificial reef more than 200 m away before traveling back to the tagging reef and emigrating from the array. The final VPS detections of this fish (12 Aug 2017) showed directional movement 115 m away from the tagging reef in a southeast direction, and raw detection and COA data collected after VPS disappearance indicated detections on easternmost receivers used for range testing (R1–R5) before permanent disappearance of the tag from detection (Electronic Supplementary Fig. S6).
Two lionfish (ID 1861, 1870) exhibited movement patterns characteristic of a tag that had been consumed by a fast-moving predator at the end of acoustic detection (Fig. 7). For fish that were depredated, ROM estimates during the last day of tag detection showed uncharacteristically high (>0.5 and up to 1.0 m s−1) swimming speeds before ultimately disappearing from detection (Electronic Supplementary Fig. S3). In addition to abnormally high ROM, consecutive VPS locations show atypically large (>800 m and up to 2 km) distances moved just prior to the end of the detection period for each fish (Fig. 7, Electronic Supplementary Fig. S4). Neither tag was detected again within the acoustic array following these events (Fig. 4).
Movement patterns inferred as predation events for two lionfish (ID 1861, 1870). Gray lines indicate tracks between successive lionfish detections, and black lines indicate tracks between successive predator detections. Insets are box plots of rate of movement (ROM) between tag detections pre- versus post-predation. Known reefs = triangles, suspected reefs = squares, and acoustic receivers = gray circles. Scale bars are indicated on each plot
No tagged lionfish were observed on any of the tagging reefs within the array during dives made on 11 Dec 2017. Although two tags remained functional within the array until the end of study (Fig. 4), 15 (75%) lionfish were ultimately assigned a fate of death or tag loss, and no fish remained alive, or actively tracked, at the end of the study period (Table 1).
Activity patterns and home range
Lionfish activity patterns were examined using distance from the nearest reef and height off bottom calculated with VPS-derived geoposition data. The final GLMM model predicting factors affecting lionfish distance from reef was Distance ~ Density + Diel period + Temperature + Wave height + (1|Tag) (Table 3, Electronic Supplementary Table S3), but it only explained a small fraction of the deviance in the data (pseudo R2 = 0.35). Bottom temperature and wave height had additive effects on lionfish distance from reef, while lionfish density had a negative effect (Table 3). Relative to dawn, lionfish were farther from reefs during the day and at dusk, but closer to reefs at night (Table 3). The final GLMM model predicting factors affecting lionfish height off bottom for fish tagged with V9P tags was Height off bottom ~ Density + Diel period + Temperature + Wave height + Wind speed + (1|Tag) (Table 3, Electronic Supplementary Table S3), which explained a large amount of the deviance in the data (pseudo R2 = 0.77). Lionfish density, bottom temperature, and wave height had additive effects on lionfish depth, while wind speed had a negative effect. Lionfish were higher in the water column during all diel periods of day, relative to dawn (Table 3).
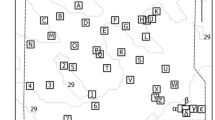
Lionfish home range estimated with VPS-derived geoposition data were highly overlapped among individuals tagged at the same reef and did not exhibit a significant relationship with number of days tracked (Fig. 8, Electronic Supplementary Fig. S8). Home range was sometimes confined to small areas surrounding tagging reefs (95% KUD <500 m2), but this varied considerably among individuals (Table 2). A maximum of six reefs were within the 95% KUD home range of a single lionfish, ID 1096 (Fig. 8f). Mean (± SE) MCP across all fish was 42,241.1 (± 33,181.8) m2, and ranged from 408.4 (ID 1858) to 670,995.4 m2 (ID 1096). Mean (± SE) 50% KUD of core area home range among all fish was 53.0 (± 10.0) m2, and ranged from 16.9 (ID 1092) to 225.6 m2 (ID 1096). Mean (± SE) 95% KUD of home range among all fish was 640.1 (± 187.2) m2, and ranged from 158.2(ID 1092) to 4050.7 m2 (ID 1096).
Estimated lionfish home ranges computed as 95% kernel utilization distribution (KUD). Panels A–E organized by tagging reef which is indicated in each plot: a Reef B, b Reef C, c Reef D, d Reef E, e Reef H. and f Reef H. Known reefs = triangles, suspected reefs = squares, and acoustic receivers = gray circles, Scale bars and tag IDs are indicated on each plot. One KUD contour in Panel F that was not visible is indicated by an asterisk (*)
Fish tagged on high lionfish density reefs had larger home ranges on average for 50% KUD and 95% KUD metrics (Table 2). However, GLMM analyses of KUD home range data indicated these differences were not statistically significant (50% KUD, p = 0.438; 95% KUD, p = 0.128). By contrast, lionfish density had a negative effect on MCP-estimated home range (p = 0.007) (Table 2). Similarly, while larger lionfish tended to have larger home ranges, lionfish TL was not statistically significant for either 50% KUD (p = 0.153) or 95% KUD (p = 0.224), but had a positive effect on lionfish MCP home range (p = 0.003) (Table 2).
Discussion
Lionfish movement dynamics
Home range and activity patterns
Fine-scale geoposition estimates provided evidence of diel patterns in foraging and sheltering behaviors displayed by nGOM lionfish. Compared to other time periods, lionfish exhibited greater horizontal space use during the day, where they were farther from reefs and lower in the water column. Patterns in lionfish activity reported here are consistent with those estimated from hard bottom reefs of comparable depth off North Carolina (Bacheler et al. 2015), but differ from shallower reefs in the Florida Keys where activity peaked during crepuscular hours (McCallister et al. 2018). Lionfish are generalist predators that consume a wide variety of prey from reef, sand, and pelagic habitats in the invaded range (Morris and Akins 2009; Dahl and Patterson 2014; Dahl et al. 2017), thus diel activity patterns may correspond to lionfish foraging on different prey sources during different time periods throughout the day. Lionfish on nGOM artificial reefs exploit a wide range of prey resources, regularly feeding on non-reef associated prey fish (e.g., lizardfishes, flounders, searobins) that inhabit open sand habitat, but also on transient pelagic prey items, such as schooling planktivorous fishes (e.g., scads, Decapterus sp., Trachurus sp.) and squids (Loligo spp.) that remain higher in the water column (Dahl and Patterson 2014; Dahl et al. 2017). Diel patterns in movement may also be driven by predator avoidance, where high reef association at night and directed movements between reefs (i.e., little time spent over open habitat) reduce vulnerability to predators (Black et al. 2014).
Home ranges produced from fine-scale geoposition data provide the most accurate representation of area use for invasive lionfish to date and show high variability in space use among individuals. For some individuals, home range was confined to the artificial reefs where tagging occurred, while others had home ranges that encompassed several artificial reefs within the array. Additional reefs were identified by aggregation and high site fidelity (i.e., homing behavior) of multiple tagged lionfish to areas without a known reef. Home range size often increases with body size in fishes (Kramer and Chapman 1999); however, only MCP home ranges were positively influenced by TL. Larger lionfish explored more area, but no relationship was found between fish length and lionfish activity patterns or KUD home ranges. The narrow range (~100 mm) of TL encountered in this study may have precluded the ability to discern variation in home range or activity patterns by fish length (Özgül et al. 2019). Furthermore, Tamburello and Côté (2015) found intermediate sized lionfish (200–280 mm TL) exhibited the greatest range of movement among tagged fish, thus potential density- and size-dependent effects on movement might be better examined in future studies by tagging a broader size range of lionfish.
High lionfish density (>15 fish per 100 m2) in nGOM artificial reefs was anticipated to influence their movement via increased competition (Tamburello and Côté 2015). While lionfish exhibited greater vertical space use at high densities, they were closer to reef habitats horizontally and density did not appear to influence the size of KUD home ranges. The relative large size of fish (288–395 mm TL) tagged in this study and size-dependent trade-offs in movement (Bowler and Benton 2005) could have prevented detection of density-dependent movement. For example, competitive interactions where larger fish outcompete smaller fish for similar prey items would result in density-dependent emigration of smaller, not larger fish, from high density reefs (Abesamis and Russ 2005). Furthermore, while interspecific aggression was not directly observed during pre-tagging surveys, some degree of territoriality may have contributed to reduced home ranges at higher lionfish densities (Fishelson 1975). Finally, the physical costs of moving may be higher than expected given a negative relationship has been documented between body condition and density (Dahl et al. 2019), which would explain the larger MCP home ranges observed at low densities (Bowler and Benton 2005).
Long-distance movements
Intraspecific competition on densely populated nGOM artificial reefs may promote lionfish movement on greater scales than has been reported elsewhere in the invaded range. Lionfish exhibited frequent movements away from reef structure, swam freely between paired reef modules spaced up to 25 m apart, and 40% of lionfish traveled to distant (>100 m and up to 2 km) reefs from their initial tagging location, demonstrating the capability and willingness of lionfish in the study area to swim over open sandy substrate that may normally be a barrier to fish movement (Turgeon et al. 2010). While conventionally tagged lionfish have been observed moving to adjacent coral reef patches in the Bahamas, such long-range (>100 m) movements were infrequent (~10 or fewer) compared to this study despite twice as many fish being tracked (Akins et al. 2014; Tamburello and Côté 2015). Furthermore, acoustically tagged lionfish on natural hard bottom reefs in North Carolina (Bacheler et al. 2015) and patch reefs in the Florida Keys (McCallister et al. 2018) rarely moved >100 m per day.
Movements to the edges of the acoustic receiver array by two lionfish swimming at typical (<0.05 m s−1) speeds before ultimately disappearing beyond the edge of the array signified emigration from the study array and provided additional evidence of long-distance movements. Neither body size nor density of lionfish at the tagging reef explained which individuals were more likely to move long distances or emigrate. However, individuals that ultimately emigrated moved more often and farther than lionfish that displayed high site fidelity to tagging reefs. They also exhibited regular transiting movements to reefs distant from their tagging reef prior to leaving the array. In the Bahamas, lionfish movements were further between patch reefs compared to movements along continuous reef habitat (Tamburello and Côté 2015). The network of nGOM artificial reefs within our acoustic array and the surrounding Okaloosa-C large area artificial reef system may explain the high scale of movement reported herein and prompt movement of lionfish in a stepping-stone pattern (Luiz et al. 2012; Dahl et al. 2019).
Comparison to previous studies
Lionfish movement dynamics on nGOM artificial reefs may differ from those reported in prior studies due to regional differences in habitat, prey availability, and competition (both inter- and intraspecific) (Kramer and Chapman 1999; Abesamis and Russ 2005), as well as methodological approach. Conventional tagging and mark–recapture limitations, including the need to recapture (i.e., resight) fish, temporal and spatial sampling biases, low rate of recapture, and a lack of inference on movement during time at liberty between recaptures, may result in underestimated or mischaracterized movement (Freiwald 2012). Indeed, mean lionfish home range (552 m2) reported using conventional tagging and minimum convex polygons during daytime hours (Tamburello and Côté 2015) were two orders of magnitude smaller than that estimated in this study (42,241 m2).
Acoustic tagging and passive telemetry provide copious amounts of information without the need to recapture tagged fish, but suffer from other limitations at low resolution (Heupel et al. 2006). For example, position estimates calculated as centers of activity (COAs) are sensitive to the time interval over which detections are averaged, and may fluctuate widely due to variable detection probabilities among acoustic receivers (Farmer et al. 2013). KUDs calculated by McCallister et al. (2018) using COA (3 locations day−1) likely resulted in over-smoothed (i.e., overestimated) lionfish home range estimates (Kie 2013), as opposed to KUDs calculated using up to 168 VPS locations day−1 (this study). Furthermore, common acoustic detections at the edges of an array consisting of overlapping receiver ranges can generate overestimated home ranges and include areas that fish did not use (Farmer et al. 2013; Skerritt et al. 2015), and the largest home ranges reported by McCallister et al. (2018) were estimated at sites with the most receivers that covered the largest area. Thus, the mean home range estimates for lionfish derived using passive telemetry and COAs (McCallister et al. 2018), which were larger (95% KUD: 3979 m2) than those estimated in this study (95% KUD: 729 m2), are likely overestimates, especially given the higher frequency and magnitude of long-range lionfish movements we observed using VPS. Finally, the search radius (i.e., bandwidth) and cell size parameters used in KUD estimation that differ between studies leads to less comparable home ranges. Whereas parameters were reported and standardized across all fish in this study, McCallister et al. (2018) used a least-squares cross-validation method that produced different bandwidth values to estimate each individual’s home range.
Fates of tagged lionfish
Depredation
Rapid swim speeds away from reef structure and abrupt changes in tag movement or depth before the disappearance of two tagged lionfish (345- and 365-mm TL) from our array provide strong evidence of lionfish being eaten by fast-moving predators that subsequently exited the array. One possibility is that lionfish were consumed by sharks, multiple species of which were observed visually in our nGOM array, or were detected on our receivers in the case of acoustically tagged individuals. For example, a bull shark (Carcharhinus leucas) tagged by researchers in the Bahamas (Maurits van Zinnicq Bergmann and Tristan Guttridge, Bimini Shark Lab, pers comm) was detected within the array on August 31, 2017 and traveled at a mean swim speed of 0.63 m s−1 (range 0.14–1.27 m s−1) before exiting several hours later. Bull sharks were also observed in nearby nGOM arrays exhibiting similar movement dynamics (Bohaboy et al. 2019), but a number of other coastal sharks are also common in the nGOM, including blacktip (C. limbatus), sandbar (C. plumbeus), tiger (Galeocerdo cuvier), and nurse sharks (Ginglymostoma cirratum) (Hannan et al. 2012; Bethea et al. 2014). Other possible predators of adult lionfish include large groupers, of which several species reside in the nGOM, including goliath grouper (Epinephelus itajara), red grouper (E. morio), and gag grouper (Mycteroperca microlepis). Two adult goliath groupers were observed in our array during pre-tagging surveys on June 29 and July 11, 2017, just prior to a suspected predation event on a tagged lionfish. Collins (2014) estimated ROM for goliath grouper moving between artificial reefs in connection with spawning migrations to be 0.42 m s−1 on average and as high as 0.8 m s−1; however, outside of spawning migrations, these fish exhibit high site fidelity and little movement (Koenig et al. 2011).
Depredation events have previously been inferred for other species via telemetry data and changes in movement patterns, depth, and acceleration data (Heupel and Simpfendorfer 2002; Gibson et al. 2015). While depredation of adult lionfish was unexpected given a lack of information that piscivores in their invaded region consume lionfish, and the physical and chemical defenses imparted by relatively large, venomous spines of adult lionfish (Morris and Whitfield 2009; Albins and Hixon 2013), anecdotal evidence does exist that large (>10 kg) piscivores, such as sharks and groupers, will consume adult lionfish in their invaded range (Maljković et al. 2008; Mumby et al. 2011; Diller et al. 2014). Furthermore, divers in some areas of the invaded range have conditioned sharks and groupers to opportunistically consume injured or dead lionfish in conjunction with organized removal (i.e., culling) efforts (Handwerk 2011; Albins and Hixon 2013; Diller et al. 2014). Depredation events detected via telemetry verify that no divers provoked opportunistic predation and represent one of few examples of detected natural depredation of adult invasive lionfish in the Atlantic Ocean. Still, it is unknown whether predators commonly consume adult lionfish in this system, or whether the electronic tags themselves attracted predators, such as sharks.
Tag loss and death
Apart from lionfish that were depredated, emigrated, or were confirmed to have shed their acoustic tag, the remaining fish (75%) were ultimately assigned a fate of tag loss or death within 3 months of tagging. In situ external tagging was used to avoid the negative effects of barotrauma, including mortality, when ascending from depths >30 m, to minimize handling stress experienced by fish, and to minimize flight responses from tagging (Starr et al. 2000; Lindholm et al. 2005; Akins et al. 2014). Furthermore, external tagging can result in higher detection probabilities compared to internally implanted tags (Dance et al. 2016). Unfortunately, external tag retention is highly variable and dependent on the method used to secure the tag (Jepsen et al. 2015). External acoustic tag attachment proved successful over a short period of time in this study; however, further research is required to determine its long-term efficacy. While data on internally attached tags were not collected in this study, it is assumed that external tags provided a higher likelihood of tag transmissions being heard by three or more receivers (Dance et al. 2016), but signal disappearances occurred intermittently and long range movements of lionfish between tagging reefs were not always detected in real time (i.e., tags were located via VPS at different reefs, but often not during transits between them). Additionally, VPS-calculated positions were not always possible due to lower than expected detection probabilities of tagged lionfish, revealed by acoustic tags still heard inside the study array in times of VPS disappearance. This was likely an artifact of lionfish behavior, where high reef association likely blocked the line of sight between acoustic tags and receivers, and detection probabilities of stationary reference tags were lowest near the seafloor where lionfish often reside. High tag retention estimates have been reported using the same methods as used here for lionfish to quickly attach acoustic and satellite tags to sharks (Rogers et al. 2013), mantas (Dewar et al. 2008), tunas (Domeier et al. 2005), and groupers (Angela Collins, pers comm), but the degree to which tag loss contributed to the reduced temporal scale of data collected in this study is unknown. Nevertheless, earlier acoustic studies with internally tagged lionfish reported similar durations of tag detections (Bacheler et al. 2015; McCallister et al. 2018).
Tropical cyclone activity in the nGOM triggered notable declines in reference tag and animal tag detections during the months of September and October 2017, corresponding with hurricanes Irma and Nate, respectively. The passage of Hurricane Irma resulted in a cold-water upwelling event which dropped bottom temperature drastically (>10 °C), nearly reaching the mean chronic lethal minimum temperature reported for lionfish (Kimball et al. 2004). While Kimball et al. (2004) concluded lionfish are robust to rapid changes in temperature, the rate of change experienced in the current study far exceeded the range they observed (− 1 to 3 °C day−1). Four lionfish were lost from acoustic detection following Irma, with geoposition data indicating two died (or lost tags), one emigrated, and one returned to detection, moving normally after 5 days. Lionfish that remained within the array showed behavioral changes in movement and were less active and closer to reef structure, both horizontally and vertically, following Irma. However, low detectability of reference tags during storm events (i.e., poor acoustic conditions) may have resulted in a failure to detect increased lionfish movement, including emigration, as an evacuation response to temperatures approaching a critically low threshold. Behavioral movement shifts in response to hurricane-caused changes in bottom temperature and environmental conditions, including evacuation and emigration, have been recently reported in other telemetered fishes (Secor et al. 2019; Bacheler et al. 2019b), and support the inference that hurricanes impacted lionfish movement, or caused mortality. Only two lionfish remained actively tracked in early October and were permanently lost to acoustic detection following Hurricane Nate.
The recent emergence of an ulcerative skin disease in the nGOM represents an additional stressor that may have impacted lionfish under study and caused premature mortality of some individuals (Harris et al. 2018). The first diseased lionfish were documented within the same region as our acoustic array in August 2017, and estimated disease prevalence was as high as 40% during summer and early fall 2017 (Harris et al. 2020). While the etiology and mortality effects of the disease remain unknown, a contemporaneous decline in regional lionfish populations was observed on both natural and artificial reefs in 2018, and the greatest magnitude of declines (>75%), was observed on high-density artificial reefs (Harris et al. 2020). Ultimately, the combination of strong tropical cyclones, thermal stress, and disease occurrence likely increased the likelihood of mortality for tagged lionfish, but no definitive conclusions could be made to differentiate whether disappearances were due to tag loss or death.
Conclusions
Acoustic telemetry using a VPS was highly effective at tracking fine-scale geoposition of lionfish in time and space, but the premature loss of tags due to hurricanes, predation, emigration, or tag loss reduced the temporal scale (<3 months) of data collected. Geoposition data revealed lionfish had relatively small home ranges around nGOM artificial reefs. However, lionfish also spent considerable time foraging tens of meters away from reefs and traveled between distant reefs at a higher frequency and scale than was previously reported. To examine the extent of lionfish movement and population connectivity in the nGOM, as well as provide estimates of lionfish home range nearer the true size, future acoustic studies should be designed to increase tag retention (e.g., via surgical implantation of tags), the areal extent of acoustic arrays, and the length of study. VPS data collection may be improved by more conservative receiver spacing resulting in a higher degree of receiver overlap; however there are trade-offs between spatial scale of coverage, detection frequency, and the high cost of additional acoustic equipment. Technological constraints of tag size and decibel output required that larger lionfish be tagged in the present study, but newly developed tags which produce higher output and pressure sensor data at smaller size (e.g., V7P, Vemco) will aid in future studies that aim to characterize a wider range of adult lionfish movement. Lastly, the use of novel acoustic transmitters designed specifically to identify predation events (e.g., V5D, Vemco) will benefit subsequent studies that aim to estimate the rate of depredation on lionfish by sharks or other large predators in this system (Halfyard et al. 2017).
References
Abesamis RA, Russ GR (2005) Density-dependent spillover from a marine reserve: long-term evidence. Ecol Appl 15:1798–1812. https://doi.org/10.1890/05-0174
Agafonkin V, Thieurmel B (2017) Suncalc: compute sun position, sunlight phases, moon position and lunar phase. https://cran.r-project.org/package=suncalc. Accessed 5 Dec 2018
Ahrenholz DW, Morris JA (2010) Larval duration of the lionfish, Pterois volitans along the Bahamian Archipelago. Environ Biol Fishes 88:305–309. https://doi.org/10.1007/s10641-010-9647-4
Akaike H (1974) A new look at the statistical model identification. Springer, New York
Akins JL, Morris JA, Green SJ (2014) In situ tagging technique for fishes provides insight into growth and movement of invasive lionfish. Ecol Evol 4:3768–3777. https://doi.org/10.1002/ece3.1171
Albins MA, Hixon MA (2013) Worst case scenario: Potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ Biol Fishes 96:1151–1157. https://doi.org/10.1007/s10641-011-9795-1
Albins MA, Lyons PJ (2012) Invasive red lionfish Pterois volitans blow directed jets of water at prey fish. Mar Ecol Prog Ser 448:1–5. https://doi.org/10.3354/meps09580
Anton A, Cure K, Layman C, Puntila R, Simpson M, Bruno J (2016) Prey naiveté to invasive lionfish Pterois volitans on Caribbean coral reefs. Mar Ecol Prog Ser 544:257–269. https://doi.org/10.3354/meps11553
Bacheler NM, Whitfield PE, Muñoz RC, Harrison BB, Harms CA, Buckel CA (2015) Movement of invasive adult lionfish Pterois volitans using telemetry: importance of controls to estimate and explain variable detection probabilities. Mar Ecol Prog Ser 527:205–220. https://doi.org/10.3354/meps11241
Bacheler NM, Michelot T, Cheshire RT, Shertzer KW (2019a) Fine-scale movement patterns and behavioral states of gray triggerfish Balistes capriscus determined from acoustic telemetry and hidden Markov models. Fish Res 215:76–89. https://doi.org/10.1016/j.fishres.2019.02.014
Bacheler NM, Shertzer KW, Cheshire RT, MacMahan JH (2019b) Tropical storms influence the movement behavior of a demersal oceanic fish species. Sci Rep 9:1481. https://doi.org/10.1038/s41598-018-37527-1
Ballew NG, Bacheler NM, Kellison GT, Schueller AM (2016) Invasive lionfish reduce native fish abundance on a regional scale. Sci Rep 6:32169. https://doi.org/10.1038/srep32169
Barbour AB, Allen MS, Frazer TK, Sherman KD (2011) Evaluating the potential efficacy of invasive lionfish (Pterois volitans) removals. PLoS ONE 6:e19666. https://doi.org/10.1371/journal.pone.0019666
Bethea DM, Ajemian MJ, Carlson JK, Hoffmayer ER, Imhoff JL, Grubbs RD, Peterson CT, Burgess GH (2014) Distribution and community structure of coastal sharks in the northeastern Gulf of Mexico. Environ Biol Fishes. https://doi.org/10.1007/s10641-014-0355-3
Beven II JL, Berg R (2018) National Hurricane Center tropical cylcone report: Hurricane Nate (AL162017). National Oceanic and Atmospheric Administration, 45 pp. www.nhc.noaa.gov/data/tcr/. Accessed 31 May 2019
Black AN, Weimann SR, Imhoff VE, Richter ML, Itzkowitz M (2014) A differential prey response to invasive lionfish, Pterois volitans: prey naiveté and risk-sensitive courtship. J Exp Mar Bio Ecol 460:1–7. https://doi.org/10.1016/j.jembe.2014.06.002
Bohaboy E, Guttridge T, Hammerschlag N, Van Zinnicq BM, Patterson WF (2019) Application of three-dimensional acoustic telemetry to assess the effects of rapid recompression on reef fish discard mortality. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsz202
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135. https://doi.org/10.1016/j.tree.2008.10.008
Bowler DE, Benton TG (2005) Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev 80:205–225. https://doi.org/10.1017/S1464793104006645
Burnham K, Anderson D (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Cangialosi JP, Latto AS, Berg R (2018) National Hurricane Center tropical cyclone report: Hurricane Irma (AL112017). National Oceanic and Atmospheric Administration, 111 pp. www.nhc.noaa.gov/data/tcr/. Accessed 31 May 2019
Chagaris D, Binion-Rock S, Bogdanoff A, Dahl K, Granneman J, Harris H, Mohan J, Rudd MB, Swenarton MK, Ahrens R, Patterson WF, Morris JA, Allen M (2017) An ecosystem-based approach to evaluating impacts and management of invasive lionfish. Fisheries 42:421–431. https://doi.org/10.1080/03632415.2017.1340273
Collins A (2014) An investigation into the habitat, behavior and opportunistic feeding strategies of the protected Goliath grouper (Epinephelus itajara). University of South Florida, Tampa
Crossin GT, Heupel MR, Holbrook CM, Hussey NE, Lowerre-Barbieri SK, Nguyen VM, Raby GD, Cooke SJ (2017) Acoustic telemetry and fisheries management. Ecol Appl. https://doi.org/10.1002/eap.1533
Dahl KA, Patterson WF (2014) Habitat-specific density and diet of rapidly expanding invasive red lionfish, Pterois volitans, populations in the northern Gulf of Mexico. PLoS ONE 9:e105852. https://doi.org/10.1371/journal.pone.0105852
Dahl KA, Patterson WF, Snyder RA (2016) Experimental assessment of lionfish removals to mitigate reef fish community shifts on northern Gulf of Mexico artificial reefs. Mar Ecol Prog Ser 558:207–221. https://doi.org/10.3354/meps11898
Dahl KA, Patterson WF, Robertson A, Ortmann AC (2017) DNA barcoding significantly improves resolution of invasive lionfish diet in the northern Gulf of Mexico. Biol Invasions 19:1917–1933. https://doi.org/10.1007/s10530-017-1407-3
Dahl KA, Portnoy DS, Hogan JD, Johnson JE, Gold JR, Patterson WF (2018) Genotyping confirms significant cannibalism in northern Gulf of Mexico invasive red lionfish, Pterois volitans. Biol Invasions 20:3513–3526. https://doi.org/10.1007/s10530-018-1791-3
Dahl K, Edwards M, Patterson W (2019) Density-dependent condition and growth of invasive lionfish in the northern Gulf of Mexico. Mar Ecol Prog Ser. https://doi.org/10.3354/meps13028
Dance MA, Moulton DL, Furey NB, Rooker JR (2016) Does transmitter placement or species affect detection efficiency of tagged animals in biotelemetry research? Fish Res 183:80–85. https://doi.org/10.1016/j.fishres.2016.05.009
Dewar H, Mous P, Domeier M, Muljadi A, Pet J, Whitty J (2008) Movements and site fidelity of the giant manta ray, Manta birostris, in the Komodo Marine Park, Indonesia. Mar Biol 155:121–133. https://doi.org/10.1007/s00227-008-0988-x
Diller JL, Frazer TK, Jacoby CA (2014) Coping with the lionfish invasion: evidence that naïve, native predators can learn to help. J Exp Mar Bio Ecol 455:45–49. https://doi.org/10.1016/j.jembe.2014.02.014
Domeier ML, Kiefer D, Nasby-Lucas N, Wagschal A, O’Brien F (2005) Tracking Thunnus thynnus orientalis with the aid of an automated algorithm. Fish Bull 103:292–306
Espinoza M, Farrugia TJ, Webber DM, Smith F, Lowe CG (2011) Testing a new acoustic telemetry technique to quantify long-term, fine-scale movements of aquatic animals. Fish Res 108:364–371. https://doi.org/10.1016/j.fishres.2011.01.011
Farmer NA, Ault JS, Smith SG, Franklin EC (2013) Methods for assessment of short-term coral reef fish movements within an acoustic array. Mov Ecol 1:7. https://doi.org/10.1186/2051-3933-1-7
Feeley MW, Morley D, Acosta A, Barbera P, Hunt J, Switzer T, Burton M (2018) Spawning migration movements of Mutton Snapper in Tortugas, Florida: spatial dynamics within a marine reserve network. Fish Res. https://doi.org/10.1016/j.fishres.2018.02.020
Fishelson L (1975) Ethology and reproduction of pteroid fishes found in the Gulf of Agaba (Red Sea), especially Dendrochirus brachypterus. Pubbl Staz Zool Napoli 39:635–656
Frazer TK, Jacoby CA, Edwards MA, Barry SC, Manfrino CM (2012) Coping with the lionfish invasion: can targeted removals yield beneficial effects? Rev Fish Sci 20:185–191. https://doi.org/10.1080/10641262.2012.700655
Freiwald J (2012) Movement of adult temperate reef fishes off the west coast of North America. Can J Fish Aquat Sci 69:1362–1374. https://doi.org/10.1139/f2012-068
Furey NB, Dance MA, Rooker JR (2013) Fine-scale movements and habitat use of juvenile southern flounder Paralichthys lethostigma in an estuarine seascape. J Fish Biol 82:1469–1483. https://doi.org/10.1111/jfb.12074
Gallien L, Münkemüller T, Albert CH, Boulangeat I, Thuiller W (2010) Predicting potential distributions of invasive species: where to go from here? Divers Distrib 16:331–342. https://doi.org/10.1111/j.1472-4642.2010.00652.x
Gibson AJF, Halfyard EA, Bradford RG, Stokesbury MJW, Redden AM (2015) Effects of predation on telemetry-based survival estimates: Insights from a study on endangered Atlantic salmon smolts. Can J Fish Aquat Sci. https://doi.org/10.1139/cjfas-2014-0245
Gittings SR, Fogg AQ, Frank S, Hart JV, Clark A, Clark B, Noakes SE, Fortner RL (2017) Going deep for lionfish: designs for two new traps for capturing lionfish in deep water. Silver Spring, MD
Green SJ, Akins JL, Maljković A, Côté IM (2012) Invasive lionfish drive Atlantic coral reef fish declines. PLoS ONE 7:e32596. https://doi.org/10.1371/journal.pone.0032596
Green SJ, Dulvy NK, Brooks AML, Akins JL, Cooper AB, Miller S, Côté IM (2014) Linking removal targets to the ecological effects of invaders: A predictive model and field test. Ecol Appl 24:1311–1322. https://doi.org/10.1890/13-0979.1
Hackerott S, Valdivia A, Green SJ, Côté IM, Cox CE, Akins L, Layman CA, Precht WF, Bruno JF (2013) Native predators do not influence invasion success of Pacific lionfish on Caribbean reefs. PLoS ONE 8:e68259. https://doi.org/10.1371/journal.pone.0068259
Halfyard EA, Webber D, Del Papa J, Leadley T, Kessel ST, Colborne SF, Fisk AT (2017) Evaluation of an acoustic telemetry transmitter designed to identify predation events. Methods Ecol Evol 8:1063–1071. https://doi.org/10.1111/2041-210X.12726
Handwerk B (2011) Shark’s lionfish lunch. In: Natl. Geogr. Mag. https://news.nationalgeographic.com/news/2011/03/pictures/110404-sharks-lionfish-alien-fish-invasive-species-science/. Accessed 5 Jul 2019
Hannan KM, Driggers WB, Hanisko DS, Jones LM, Canning AB (2012) Distribution of the nurse shark, Ginglymostoma cirratum, in the northern Gulf of Mexico. Bull Mar Sci. https://doi.org/10.5343/bms.2011.1033
Harris HE, Fogg AQ, Yanong RPE, Frasca S, Cody T, Waltzek TB, Patterson III WF (2018) First report of an emerging ulcerative skin disease in invasive lionfish (FA209). In: Gainesv. Univ. Florida Inst. Food Agric. Sci. https://edis.ifas.ufl.edu/fa209. Accessed 9 Oct 2018
Harris HE, Fogg AQ, Allen MS, Ahrens RNM, Patterson WF (2020) Precipitous declines in northern Gulf of Mexico invasive lionfish populations following the emergence of an ulcerative skin disease. Sci Rep 10:1934. https://doi.org/10.1038/s41598-020-58886-8
Herbig JL, Szedlmayer ST (2016) Movement patterns of gray triggerfish, Balistes capriscus, around artificial reefs in the northern Gulf of Mexico. Fish Manag Ecol 23:418–427. https://doi.org/10.1111/fme.12190
Heupel M, Simpfendorfer C, Lowe C (2005) Passive acoustic telemetry technology: current applications and future directions. Mote Tech Rep No 1066:98
Heupel MR, Simpfendorfer CA (2002) Estimation of mortality of juvenile blacktip sharks, Carcharhinus limbatus, within a nursery area using telemetry data. Can J Fish Aquat Sci 59:624–632. https://doi.org/10.1139/f02-036
Heupel MR, Semmens JM, Hobday AJ (2006) Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Mar Freshw Res 57:1–13. https://doi.org/10.1071/MF05091
Hooge P, Eichenlaub W, Solomon E (2001) Using GIS to analyze animal movements in the marine environment. Spat Process Manag Mar Popul. https://doi.org/10.3354/meps10225
Hussey NE, Kessel ST, Aarestrup K, Cooke SJ, Cowley PD, Fisk AT, Harcourt RG, Holland KN, Iverson SJ, Kocik JF, Mills Flemming JE, Whoriskey FG (2015) Aquatic animal telemetry: a panoramic window into the underwater world. Science (80-) 348:1255642–1255642. https://doi.org/10.1126/science.1255642
Jepsen N, Thorstad EB, Havn T, Lucas MC (2015) The use of external electronic tags on fish: an evaluation of tag retention and tagging effects. Anim Biotelem 3:49. https://doi.org/10.1186/s40317-015-0086-z
Jud ZR, Layman CA (2012) Site fidelity and movement patterns of invasive lionfish, Pterois spp., in a Florida estuary. J Exp Mar Bio Ecol 414:69–74. https://doi.org/10.1016/j.jembe.2012.01.015
Kessel ST, Cooke SJ, Heupel MR, Hussey NE, Simpfendorfer CA, Vagle S, Fisk AT (2014) A review of detection range testing in aquatic passive acoustic telemetry studies. Rev Fish Biol Fish 24:199–218. https://doi.org/10.1007/s11160-013-9328-4
Kie JG (2013) A rule-based ad hoc method for selecting a bandwidth in kernel home-range analyses. Anim Biotelem 1:13. https://doi.org/10.1186/2050-3385-1-13
Kie JG, Matthiopoulos J, Fieberg J, Powell RA, Cagnacci F, Mitchell MS, Gaillard J-M, Moorcroft PR (2010) The home-range concept: are traditional estimators still relevant with modern telemetry technology? Philos Trans R Soc B Biol Sci 365:2221–2231. https://doi.org/10.1098/rstb.2010.0093
Kimball ME, Miller JM, Whitfield PE, Hare JA (2004) Thermal tolerance and potential distribution of invasive lionfish (Pterois volitans/miles complex) on the east coast of the United States. Mar Ecol Prog Ser 283:269–278. https://doi.org/10.3354/meps283269
Kitchens LL, Paris CB, Vaz AC, Ditty JG, Cornic M, Cowan JH, Rooker JR (2017) Occurrence of invasive lionfish (Pterois volitans) larvae in the northern Gulf of Mexico: characterization of dispersal pathways and spawning areas. Biol Invasions. https://doi.org/10.1007/s10530-017-1417-1
Koenig CC, Coleman FC, Kingon K (2011) Pattern of recovery of the goliath grouper Epinephelus itajara population in the southeastern US. Bull Mar Sci. https://doi.org/10.5343/bms.2010.1056
Kramer DL, Chapman MR (1999) Implications of fish home range size and relocation for marine reserve function. Environ Biol Fishes 55:65–79
Lesser MP, Slattery M (2011) Phase shift to algal dominated communities at mesophotic depths associated with lionfish (Pterois volitans) invasion on a Bahamian coral reef. Biol Invasions 13:1855–1868. https://doi.org/10.1007/s10530-011-0005-z
Lindholm J, Fangman S, Kaufman L, Miller S (2005) In situ tagging and tracking of coral reef fishes from the Aquarius undersea laboratory. Mar Technol Soc J 39:68–73. https://doi.org/10.4031/002533205787521721
Luiz OJ, Madin JS, Ross Robertson D, Rocha LA, Wirtz P, Floeter SR (2012) Ecological traits influencing range expansion across large oceanic dispersal barriers: insights from tropical Atlantic reef fishes. Proc R Soc B Biol Sci. https://doi.org/10.1098/rspb.2011.1525
Maljković A, Van Leeuwen TE, Cove SN (2008) Predation on the invasive red lionfish, Pterois volitans (Pisces: Scorpaenidae), by native groupers in the Bahamas. Coral Reefs 27:501–501. https://doi.org/10.1007/s00338-008-0372-9
McCallister M, Renchen J, Binder B, Acosta A (2018) Diel activity patterns and movement of invasive lionfish (Pterois volitans/P. miles) in the Florida Keys identified using acoustic telemetry. Gulf Caribb Res 29:27–40. https://doi.org/10.18785/gcr.2901.13
Morris JA, Akins JL (2009) Feeding ecology of invasive lionfish (Pterois volitans) in the Bahamian archipelago. Environ Biol Fishes 86:389–398. https://doi.org/10.1007/s10641-009-9538-8
Morris JA, Whitfield P (2009) Biology, ecology, control and management of the invasive Indo-Pacific lionfish: an updated integrated assessment. NOAA Tech Memo NOS NCCOS 99:57
Mumby PJ, Harborne AR, Brumbaugh DR (2011) Grouper as a natural biocontrol of invasive lionfish. PLoS ONE 6:e21510. https://doi.org/10.1371/journal.pone.0021510
Muñoz RC, Currin CA, Whitfield PE (2011) Diet of invasive lionfish on hard bottom reefs of the Southeast USA: insights from stomach contents and stable isotopes. Mar Ecol Prog Ser 432:181–193. https://doi.org/10.3354/meps09154
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R 2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
National Oceanic and Atmospheric Administration, National Weather Service (2017) National Data Buoy Center: Station 42012-Orange Beach. https://www.ndbc.noaa.gov/station_page.php?station=42012. Accessed 15 June 2018
Özgül A, Lök A, Ulaş A, Düzbastilar FO, Tanrikul TT, Pelister C (2015) Preliminary study on the use of the Vemco Positioning System to determine fish movements in artificial reef areas: a case study on Sciaena umbra Linnaeus, 1758. J Appl Ichthyol. https://doi.org/10.1111/jai.12922
Özgül A, Lök A, Tansel Tanrıkul T, Alós J (2019) Home range and residency of Scorpaena porcus and Scorpaena scrofa in artificial reefs revealed by fine-scale acoustic tracking. Fish Res 210:22–30. https://doi.org/10.1016/j.fishres.2018.10.008
Patterson WF (2013) Acoustic tracking of reef fishes associated with EE-LAARS Refugia Reefs: Project Final Report Grant No. FWC-10340
Patterson WF, Dance MA, Addis DT (2009) Development of a remotely operated vehicle based methodology to estimate fish community structure at artificial reef sites in the northern Gulf of Mexico. In: Proc 61st Gulf Caibbean Fish Inst., vol 61, pp 263–270
Rogers PJ, Huveneers C, Goldsworthy SD, Mitchell JG, Seuront L (2013) Broad-scale movements and pelagic habitat of the dusky shark Carcharhinus obscurus off Southern Australia determined using pop-up satellite archival tags. Fish Oceanogr. https://doi.org/10.1111/fog.12009
Schofield P (2010) Update on geographic spread of invasive lionfishes (Pterois volitans [Linnaeus, 1758] and P. miles [Bennett, 1828]) in the Western North Atlantic Ocean, Caribbean Sea and Gulf of Mexico. Aquat Invasions 5:S117–S122. https://doi.org/10.3391/ai.2010.5.S1.024
Schofield PJ, Langston JN, Morris Jr. JA, Fuller P (2014) Pterois volitans/miles fact sheet. In: USGS Nonindigenous Aquat. Species Database. https://dx.doi.org/10.3133/fs20143032. Accessed 10 May 2018
Secor DH, Zhang F, O’Brien MHP, Li M (2019) Ocean destratification and fish evacuation caused by a Mid-Atlantic tropical storm. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsx241
Simpfendorfer CA, Heupel MR, Hueter RE (2002) Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Can J Fish Aquat Sci. https://doi.org/10.1139/f01-191
Skerritt DJ, Robertson PA, Mill AC, Polunin NVC, Fitzsimmons C (2015) Fine-scale movement, activity patterns and home-ranges of European lobster Homarus gammarus. Mar Ecol Prog Ser. https://doi.org/10.3354/meps11374
Smedbol S, Smith F, Webber D, Vallee R, King T (2014) Using underwater coded acoustic telemetry for fine scale positioning of aquatic animals. In: 20th Symposium of the International Society on Biotelemetry Proceedings, p 3
Smith F (2013) Understanding HPE in the VEMCO positioning system (VPS), Vemco. https://vemco.com/wp-content/uploads/2013/09/understanding-hpe-vps.pdf. Accessed 11 Apr 2019
Starr RM, Heine JN, Johnson KA (2000) Techniques for tagging and tracking deepwater rockfishes. North Am J Fish Manag 20:597–609. https://doi.org/10.1577/1548-8675(2000)020<0597:TFTATD>2.3.CO;2
Tamburello N, Côté IM (2015) Movement ecology of Indo-Pacific lionfish on Caribbean coral reefs and its implications for invasion dynamics. Biol Invasions 17:1639–1653. https://doi.org/10.1007/s10530-014-0822-y
Teets DA (2003) Predicting sunrise and sunset times. Coll Math J. https://doi.org/10.2307/3595771
Thompson MJ, Schroeder WW, Phillips NW, Graham BD (1999) Ecology of live bottom habitats of the northeastern Gulf of Mexico : a community profile. US Dept. of the Interior, US Geological Survey, Biological Resources Division, USGS/BRD/CR—l999–000l and Minerals Management Service, Gulf of Mexico OCS Region, New Orleans
Turgeon K, Robillard A, Grégoire J, Duclos V, Kramer DL (2010) Functional connectivity from a reef fish perspective: behavioral tactics for moving in a fragmented landscape. Ecology. https://doi.org/10.1890/09-2015.1
Walter WD, Fischer JW, Baruch-Mordo S, Vercauteren KC (2009) What is the proper method to delineate home range of an animal using today’s advanced GPS telemetry systems: the initial step. In: Modern Telemetry
Winemiller KO, Jepsen DB (1998) Effects of seasonality and fish movement on tropical river food webs. J Fish Biol. https://doi.org/10.1111/j.1095-8649.1998.tb01032.x
Yergey ME, Grothues TM, Able KW, Crawford C, DeCristofer K (2012) Evaluating discard mortality of summer flounder (Paralichthys dentatus) in the commercial trawl fishery: developing acoustic telemetry techniques. Fish Res. https://doi.org/10.1016/j.fishres.2011.11.009
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Statistics for biology and health, mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
This research was funded by Florida Fish and Wildlife Conservation Commission through the Division of Marine Fisheries Management and the Artificial Reef Program (FWC-14027). We thank Josh Livingston, Joe Livingston, and Erin Bohaboy for assistance with all major field operations and technical diving operations. We thank Steve Garner, Miaya Glabach, and Jordan Bajema for field assistance in ROV surveys and array deployment and retrieval. We thank Thomas TinHan and Steve Garner for assistance with R analyses and code.
Funding
This study was funded by Florida Fish and Wildlife Conservation Commission through the Division of Marine Fisheries Management and the Artificial Reef Program (FWC-14027).
Author information
Authors and Affiliations
Contributions
KAD and WFP conceived and designed the study; KAD and WFP developed the methodology; KAD conducted the fieldwork; KAD analyzed data and performed statistical analyses; KAD wrote the manuscript and WFP provided review and editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
All applicable international, national, and/or institutional guidelines for sampling, care, and experimental use of organisms for the study have been followed.
Consent for publication
All authors are in agreement to submit the manuscript for publication as written.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The R code generated during the current study is available from the corresponding author on reasonable request.
Additional information
Responsible Editor: E. Hunter.
Reviewed by D. Moulton and an undisclosed expert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dahl, K.A., Patterson, W.F. Movement, home range, and depredation of invasive lionfish revealed by fine-scale acoustic telemetry in the northern Gulf of Mexico. Mar Biol 167, 111 (2020). https://doi.org/10.1007/s00227-020-03728-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03728-4