Abstract
Echinoderms are widely used to investigate the relationship between egg size, energy content and larval developmental strategies in marine invertebrates; although there have been few studies on ophiuroids and holothuroids. In this paper, we provide the first detailed biochemical information on egg composition and utilization in the planktotrophic holothuroid, Australostichopus mollis. The egg ultrastructure, protein content (85.1 ng egg−1) and lipid:protein ratio of 0.42 were consistent with those of other planktotrophic echinoderms of similar egg size. However, the lipid content (35.6 ng egg−1) was outside the 95% prediction band for the relationship between egg size and lipid content for echinoderms. Triacylglycerol (TAG) was the main energetic lipid present in the egg, with ca 50% of the TAG being utilized to construct the feeding auricularia; the remaining TAG was estimated to be consumed over 114.8 h (4.8 days) of development. Feeding a microalgal diet during early larval development did not affect the rate of TAG utilization, but increased protein content in the 90-h auricularia. Biochemical information from A. mollis eggs/larvae suggests that TAG might be the ancestral maternally derived energetic lipid in the Echinodermata, but also that there may be different patterns of lipid utilization between different classes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The relationship between egg size, energy content and development in marine invertebrates has commonly been investigated in the echinoderms, particularly in the asteroids and echinoids (Jaeckle 1995; Podolsky and Strathmann 1996; Levitan 2000; McEdward and Miner 2006). Echinoderms are an ideal phylum for comparative analyses as they are abundant, found in all habitats, show all modes of development (planktotrophy, lecithotrophy, brooding), and in many taxa show rapid changes in development mode within short evolutionary times, allowing comparison of closely related species with different life-histories (Byrne et al. 1999; Byrne and Cerra 2000; Prowse et al. 2008). In addition, egg size in echinoderms is generally considered to be a reasonable predictor of maternal investment and developmental mode (Jaeckle 1995; George et al. 1997; Byrne et al. 2003). The smaller eggs of planktotrophic species usually contain more protein than lipid, greater amounts of presynthesized structural lipids for the formation of feeding larval structures (Jaeckle 1995; Sewell 2005; Falkner et al. 2006, 2015; Byrne et al. 2008a; Prowse et al. 2008), and triacylglycerol (TAG) as the primary energetic lipid to fuel larval development (Podolsky et al. 1994; Villinski et al. 2002; Sewell 2005; Falkner et al. 2006, 2015; Byrne et al. 2008a, b; Whitehill and Moran 2012). In contrast, the larger eggs of lecithotrophic species usually contain more lipid than protein and more energetic lipids to fuel development during the juvenile phase without feeding (Jaeckle 1995; Byrne et al. 2003, 2008a; Falkner et al. 2006, 2015; Prowse et al. 2008). These energetic lipids are present either as an hypertrophic accumulation of TAG or a switch to long-term energy-storage lipids such as wax ester (WE), methyl ester (ME) or diacylglycerol ether (DAGE) (Falkner et al. 2006, 2015; Lee et al. 2006; Prowse et al. 2008, 2009).
Within the echinoderms, differences in egg size, biochemical composition and utilization are best understood in the asteroids and echinoids, which show distinctly bimodal egg size distributions between planktotrophy and lecithotrophy (Emlet et al. 1987; Sewell and Young 1997). In contrast, in both the ophiuroids and holothuroids, there is considerable overlap in the egg size for species with planktotrophic and lecithotrophic development (Sewell and Young 1997; Falkner et al. 2006, 2015). Sewell and Young (1997) hypothesized that the eggs of holothuroids and ophiuroids might not be “equal” to the eggs of the other echinoderm classes in characteristics such as morphology, energetic content or biochemical composition. Previous studies have provided information on ophiuroid egg morphology (Tyler and Gage 1979; Grange et al. 2004; Byrne 1989, 1991; Moloney and Byrne 1994) and, more recently, on lipid class composition (Falkner et al. 2006, 2015) and utilization during larval development (Falkner 2007; Whitehill and Moran 2012). However, in holothuroids, there is only a single study of egg morphology in a lecithotrophic species (Kessel 1966), and no studies on egg lipid class composition and utilization in the holothuroids, in part due to the difficulty in spawning individuals in the laboratory (Sewell and Manahan 2001; Kato et al. 2009; Leonet et al. 2009). Further information on holothuroid egg characteristics would provide a more complete picture of egg evolution, larval nutritional strategy in the echinoderms (Falkner et al. 2015) and, as holothuroids most closely represent the ancestral type of larval development in the Deuterostomia, provide valuable insights into the evolution of egg size, larval development and developmental mode within this clade (Lacalli 2003; Nakano et al. 2003).
Here we examine in detail the egg morphology, biochemical composition and utilization of lipids during early larval development of the aspidochirote sea cucumber Australostichopus mollis, a commercial species found throughout New Zealand and in south-eastern Australia (Pawson 1970). A. mollis is a planktotrophic species with a relatively large egg size (~160 µm), providing an ideal species for comparison with lecithotrophic species of holothurians (egg size 150–950 µm) (Sewell and Young 1997) and other planktotrophic echinoderms. We describe (1) lipid and protein composition of A. mollis eggs in the context of the echinoderms, (2) microscopic distribution of lipid and protein in the egg, (3) lipid and protein utilization during early embryo/larval development, and (4) the effects of food availability on biochemical composition of early stage larvae.
Materials and methods
Collection and larval cultures
Adult sea cucumbers were collected within their reproductive season (Sewell 1992) from the rocky subtidal region at Ti Point (36º19′20.39″S; 174º47′28.7″E), on the northeast coast of New Zealand. Individuals were transported to the University of Auckland and left to acclimatize for a few days without food in aerated static tanks (H 30 cm, L 80 cm, W 55 cm) containing 1-µm filtered seawater (FSW). Around the main moon phases, 16 sea cucumbers of undetermined sex were placed into two separate smaller aerated tanks (H 20 cm, L 40 cm, W 20 cm), and spawning was induced gregariously using a combination of temperature shock and addition of microalgae (Agudo 1996; Archer 1996; Morgan 2009). The creamy coloured fertilized eggs, in suspension due to tank aeration, were carefully siphoned from the water column, concentrated, and then gently washed with 1-µm FSW. Half of the fertilized eggs from each tank were processed and stored separately at −80 °C for protein, lipid and microscopic analysis, while the other half was retained for embryo/larval cultures. The number of sea cucumbers that were parents of the fertilized eggs in each spawning tank is unknown; but will range from a minimum of 2 parents (1 male, 1 female) to a maximum of the 8 sea cucumbers in the tank (sex ratio of A. mollis close to 1:1, Sewell 1990). Thus, egg samples collected from a spawning tank are the pooled offspring of several parents.
Eggs for protein and lipid analysis were placed in a 50-ml Falcon tube on ice, and the concentration determined in a known volume (n = 6). Replicates of ~200 eggs per spawning tank were placed in 1.5 ml Eppendorf tubes (n = 16–20, half for protein and half for lipid analysis), centrifuged briefly (3000 RPM for 10 s), the excess seawater removed and samples stored at −80 °C until analysis. Mean egg diameter (d) (n = 60) for each spawning tank was estimated from digital images obtained under a Leica DMR upright microscope attached to a Nikon 500 digital sight cooled colour camera and images analysed with AnalySIS® 5 (Life Science). Egg volume was estimated as \(\left( {V = \frac{4}{3}\pi r^{3} } \right),\) where r = egg radius derived from d/2.
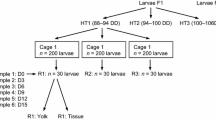
The remaining embryos from each spawning tank were cultured in 4-L cylindrical plastic culture vessels containing 1-µm FSW (n = 4 per spawning tank). In this experiment, we had two spawning tanks (pooled offspring from multiple parents) with the embryos from each spawning tank divided between the four culture vessels (for details of the sampling design, see Supplementary Figure S1). Culture vessels were stocked at an initial density of 5–10 eggs/embryos ml−1 and gradually decreased to 0.5 larvae ml−1 by the time of first feeding (2 days post-fertilization). Tanks were aerated using plastic tubing fitted with an air flow regulator and glass Pasteur pipette and were held under constant light (12:12 h light:dark cycle) and temperature conditions (19 ± 0.5 °C). Culture vessels were cleaned every 2 days by siphoning the larvae into a new 4-L vessel, avoiding debris accumulated at the bottom. Once collected in a clean vessel, 70% of the water containing the larvae was removed through reverse filtration (75-µm filter) and was replaced with fresh 1-µm FSW.
In initial experiments, Australostichopus mollis embryo/larval cultures were found to be highly susceptible to culture crashes. To ensure that we obtained egg-to-larval stages from each spawning event, we alternated sample collection between culture vessels at each sampling point (see Supplementary Figure S1). Samples of ~200 embryos (n = 16–20) were collected at 16–32 cells (4 h), blastula (14 h), early gastrula (19 h) and late gastrula (38 h); exact sample sizes used for biochemical analysis at each time point are given in Supplementary Table S1. Due to high levels of sampling prior to 48 h when the larvae showed a fully developed gut, larvae from both spawning tanks were combined and redistributed into four culture tanks; two tanks were assigned to fed and starved diet treatments, respectively (Supplementary Figure S1). Fed treatments received a 1:1:1 ratio of Isochrysis galbana, Dunaliella tertiolecta and Chaetoceros muelleri (CSIRO Australia) twice daily at a concentration of 3000 cells ml−1. Samples of ~200 early auricularia (65 and 90 h of development) from fed and starved treatments (n = 16–20) were collected as described for earlier stages (sample sizes used in biochemical analyses are provided in Supplementary Table S1).
Lipid analysis
Lipids from frozen egg, embryo and larval samples were extracted following Sewell (2005), with the minor difference being that we used methanol and chloroform from the LiChrosolv® Hypergrade for LC–MS range (Merck Millipore) and a lower concentration of ketone standard to estimate lipid recovery (10 µl of 500 µg ml−1). The isolated chloroform phase containing lipids was kept at −20 °C for a maximum period of 2 days until the lipid class identification and quantification procedure using the Iatroscan Mark V new Thin Layer Chromatography/Flame Ionization Detection system (TLC/FID) and silica gel S-III Chromarods. Stored samples were dried up under instrument-grade N2 gas and redissolved in 10 µl chloroform. To retain lipids in a narrow band at the origin, we placed a precleaned rack of 10 Chromarods (as in Sewell 2005) on top of a 175 × 125 cm heat plate (Chiltern Scientific) and directly spotted the full 10 µl of sample onto one Chromarod using a fixed-volume Drummond microdispenser and glass bores (Parrish 1987). The V-vial was then rinsed with another 10 µl of chloroform and this volume spotted on to the same Chromarod to ensure transfer of the entire lipid extract. Spotting the entire lipid extract, with vial washing, is a procedural improvement over the protocol described in Sewell (2005) where 1 µl samples of lipid extracts were spotted onto separate Chromarods as technical replicates. When the volume of the lipid extract was small (typically 10-µl), significant evaporation of the chloroform occurred in the unspotted lipid extract while the first Chromarod was being spotted (i.e. lipid concentrations increased in a 1-µl sample over time). In echinoderm tissues, a sample of ca 200 eggs/larvae per sample, and spotting of the entire sample onto the single Chromarod allows clear, consistent peak quantification; we collect a larger number of biological samples (egg/larvae) at each time point to compensate for any errors during the extraction process and to facilitate identification of unknown peaks by ‘spiking’ with known lipid standards if required.
On the rack of ten Chromarods, eight Chromarods were used to spot biological samples, one was used as a blank to monitor possible contamination of developing solvents, and the last Chromarod was spotted with a dilution of a composite of highly purified lipid standards (99%) to detect possible peak position shifts due to external conditions. Lipids within samples spotted on the Chromarods were chromatographically separated in a two-stage development process based on Parrish (1987): Development 1 = hexane-diethyl ether-formic acid (98.95:1:0.05, by vol.) for 24 min; constant humidity chamber containing a saturated solution of CaCl2 (Delmas et al. 1984; Parrish 1987) for 5 min; hexane development for a further 19 min, rack dried in the Iatroscan (5 min) and partially scanned (PPS27) until after the ketone peak. Development 2 = Constant humidity chamber (5 min); hexane-diethyl ether-formic acid (79:20:1, by vol) for 35 min; dried inside the Iatroscan (5 min); full scan to the origin. For both scans, the Iatroscan had flow rates of 2000 ml min−1 for air and 160 ml min−1 for hydrogen. The two-stage development produced two chromatograms per sample (partial scan, full scan) which were collected on a SESChromstar PC-board and quantified using SES-Chromstar version 4.10 (SES Analysesysteme). Lipid class quantification was based on quadratic regressions from multilevel lipid calibration curves using lipid standards as in Sewell (2005). Standards represented the neutral energetic lipids: aliphatic hydrocarbon (AH), wax ester (WE), methyl ester (ME), triacylglycerol (TAG), free fatty acids (FFA) and diacylglycerol (DAG); as well as the structural neutral lipid cholesterol (ST) and the structural polar lipids acetone mobile polar lipid (AMPL) and phospholipids (PL). Total lipid content as well as total energetic lipid and total structural lipid content were obtained by summing the total amounts of respective lipid classes contained in the samples.
Protein analysis
Total protein content of eggs, embryos and larvae was determined using the micro-Bradford method of Jaeckle and Manahan (1989) and a standard curve based on bovine serum albumin. To each frozen pellet, we added 200 µl Milli-Q water, sonicated the sample with four 2 s @ 60 Hz bursts on ice (Soniprep 150, MSE®), and precipitated the protein with two TCA steps: (1) 100 µl 15% TCA and centrifugation (20,000 RPM for 20 min at 4 °C), (2) 200 µl 5% TCA and centrifugation as above. The protein-containing pellet was redissolved in 500 µl 1 N NaOH, vortexed and heated at 56 °C for 30 min and neutralized with 300 µl 1.67 M HCl. 200 µl of a 1:4 filtered dilution of Bio-Rad Protein Assay dye reagent in Milli-Q water was added as per the manufacturer’s instructions. Total soluble protein was determined in two microplate wells per sample by measuring sample absorbance at 595 nm using a Synergy HT multidetection microplate reader (Bio-TEK®) and KC4™ Microplate data analysis software (Bio-TEK®).
Egg morphology
Histochemistry
Egg samples were processed for paraffin histology by fixing in Bouin’s fluid for one day, transferred through a graded ethanol series (70, 95, 100%, p-xylene), followed by Histosec®(Merck) embedding, 3 changes of Histosec®, sectioning (6 µm thick) and staining with periodic-acid Shiff’s (PAS) which stains echinoderm yolk (Byrne et al. 1999, 2003).
Microscopy and ultrastructure
Egg samples were fixed at 4 °C for 2 h in 3% glutaraldehyde in 0.2 M cacodylate buffer containing 30 mg ml−1 NaCl followed by serial rinses with the buffer containing reduced amounts of NaCl (30, 15, 5 mg ml−1, no NaCl, respectively), 2 h post-fixation in 1% osmium tetroxide in 0.2 M cacodylate buffer at 4 °C and a final wash in 0.2 M cacodylate buffer. Samples were then dehydrated in a series of graded ethanols and embedded in epoxy resin (Byrne et al. 1999; Byrne and Cerra 2000; Byrne et al. 2008a). Semi-thin sections (2 µm) of eggs were cut using a Leica EM UC6 ultra-microtome, dried onto factory coated glass slides (Superfrost® Plus, Menzel-Gläser) and stained using a polychromatic staining method consisting of methylene blue-azure II and basic fuchsine which stains cytoplasm blue, nuclei a darker blue, and fat or intracellular lipid droplets grey to green (D’Amico 2005). Semi-thin sections were observed on a Leica DMR upright microscope, photographed using a Nikon 500 digital sight cooled colour camera and images analysed with AnalySIS® 5 (Life Science).
Ultrathin sections (80 nm) were also prepared on a Leica EM UC6 ultra-microtome, placed on a copper grid and stained with 2% uranyl acetate for 20 min and 2% lead citrate for 4 min. Sections were observed on a Tecnai 12 (FEI) transmission electron microscope (TEM), photographed with an Ultrascan® 1000 (Gatan) camera and images processed with ImageJ 1.46r.
Data analyses
Egg biochemical composition was compared between Spawning Tanks 1 and 2 using a t test (FFA, CHOL, PL, Energetic lipids) or Mann–Whitney Rank test if assumptions of normality or equal variances failed (AH, TAG, DAG, AMPL, Total, Structural Lipids, Protein) using the Systat module within Sigmaplot 13.0.
Embryo and larval lipid and protein content were analysed in two parts, corresponding to the prefeeding period (4–38 h), and when the feeding treatments commenced (65, 90 h). In the prefeeding period, data were analysed with a two-way analysis of variance (factors: time, spawning tank) using the general linear models (GLM) procedure within SAS 9.4 (Cary, North Carolina). All analyses with significant effects of time also showed significant interaction effects, preventing interpretation of the main effects (Quinn and Keough 2002). For these biochemical constituents, one-way analysis of variance was performed for each spawning tank separately using the GLM procedure in SAS 9.4. Significant effects of time were examined using a Tukey’s Studentized Range (HSD) Test with α = 0.05. For the feeding treatments (65, 90 h), the PROC MIXED procedure within SAS 9.4 was used to test for differences in lipid and protein contents blocked by culture, with four cultures (1 = fed 65, 90 h; 2 = fed 65, 90 h; 3 = unfed 65, 90 h; 4 = unfed 65, 90 h; see Supplementary Figure S1) using the default options (Covariance structure = variance components; Estimation method = REML; Residual variance method = profile; Fixed effects SE method = model-based; Degrees of freedom method = containment). Assumptions of homogeneity of variance and normality for all analyses were checked using residual analysis; if required, appropriate transformations were performed and are listed in the respective results tables.
Results
Egg protein and lipid composition
Eggs of Australostichopus mollis were neutrally to negatively buoyant and measured 160.22 µm (±0.8 SE, n = 60) in diameter. There was no significant difference in egg size between spawning tanks (t (118) = 0.524, P = 0.601). Eggs contained a total of 35.6 ng lipid (±0.8 SD, n = 2 spawning tanks; Table 1) and 85.1 ng of protein (±19.4 SD, n = 2 spawning tanks), with a lipid:protein ratio of 0.42. When standardized to egg volume (2.15 nl), this corresponded to 16.6 ng nl−1 lipid and 39.6 ng nl−1 protein. When the biochemical composition of A. mollis eggs is compared with data available from other echinoderms, the lipid content is slightly below the 95% prediction band (Fig. 1a), while the protein content is within the 95% prediction band for this egg size (Fig. 1b).
Lipid and protein content of echinoderm eggs related to egg volume in µl a Lipid: regression y = 1.164x + 5.361, r 2 = 0.970, b Protein: regression y = 1.009x + 4.798, r 2 = 0.965. Data from Sewell and Manahan (2001) expanded with data from Falkner et al. (2006), Prowse et al. (2008), McAlister and Moran (2012), Moran et al. (2013) and this study. Shape indicates echinoderm class: triangle Echinoidea, star Asteroidea; circle Holothuroidea; cross Ophiuroidea. Open symbols planktotrophic development, grey-filled symbols lecithotrophic development and black-filled symbols brooding lecithotrophic development. Regression slope (black) is shown with 95% confidence interval (grey line) and 95% prediction band (dotted line). Insets in Panel a and b show relationships when lipid and protein contents of the 16–32 cell stage of A. mollis are used
A. mollis eggs contained seven lipid classes; four neutral lipids with energy-storage functions (AH, TAG, FFA, DAG), the neutral lipid with a structural function (ST), and the polar and structural AMPL and PL (Table 1). Structural lipids dominated the overall composition of the egg (71.50%, Table 1), with PL being the most abundant lipid class (88.6% of structural lipids). Energetic lipids represented 28.50% of the lipids present in the egg, with TAG being the most abundant (64.3% of energetic lipids) (Table 1). There were no significant differences between eggs from spawning tanks 1 and 2 in protein, total lipid, structural lipid, and energetic lipids or any of the lipid classes (PL, AMPL, ST, TG, FFA and DAG and AH; full details in Supplementary Table S1).
Egg morphology
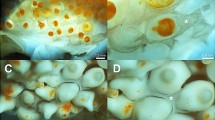
Paraffin sections of fertilized A. mollis eggs showed abundant deep-red PAS+ protein granules evenly distributed throughout the cytoplasm (Fig. 2a). The deep blue dividing nucleus positioned towards the centre of the egg was a clear indicator of successful fertilization (Fig. 2a). Plastic sections revealed evenly distributed large and abundant yolk granules (blue) and few small lipid droplets (green), along with scattered optically empty vesicles located mostly towards the periphery of the egg (Fig. 2b). TEM showed abundant and evenly distributed yolk granules which were dark grey to grey in colour and had a well-defined delimiting membrane (Fig. 2c, d) and very few lipid droplets, generally observed as optically empty vesicles (especially when larger in size) or as granules less electron-dense than protein granules that lacked a well-defined delimited membrane (Fig. 2c, d). Optically empty vesicles were observed towards the periphery of the egg, likely corresponding to empty cortical granules (Fig. 2c).
Fertilized egg from A. mollis. a Paraffin section stained with periodic acid–Schiff. PAS+ yolk granules (Y) occur throughout the cytoplasm. Dividing nucleolus (N) is also visible. Scale bar 25 µm. b Plastic section stained with methylene blue-azure II and basic fuchsine. Cytoplasmic material stains blue, and lipid droplets stain grey to green. Scale bar 15 µm. c TEM of periphery of the egg. Scale bar 5 µm. d TEM of interior of the egg. Scale bar 3 µm. Y yolk granule, V empty vesicles, L lipid droplet, C cortical granule, F fertilization membrane
Lipid and protein utilization
The lipid and protein content of A. mollis embryos from both spawning tanks showed an unexpected increase between the fertilized egg and the 16–32 cell stage (4 h, Fig. 3), particularly in spawning tank 2. Because the 16–32 cell stage represents the maximum amount of energetic lipid and protein in the embryo, we have used this value when examining changes in lipid and protein content during the prefeeding period. Note too that use of the 16–32 cell values for lipid and protein in the examination of the relationship between egg size and biochemical content (Fig. 1) does not change the findings reported with fertilized eggs; lipid content is still outside the 95% prediction band (Fig. 1 insets). The lipid class DAG was detected in only some embryo samples at low levels (71/92 with 0 ng individ−1), so is not considered an important lipid class during early development in A. mollis.
Lipid and protein composition (mean ± SE) during early development of embryos and larvae of A. mollis. Squares indicate prefeeding development. Dashed vertical line indicates commencement of fed/unfed treatment (48 h). Feeding treatments: fed filled, unfed open symbol. Sample sizes for each developmental stage are provided in Supplementary Table S1. Data points at 65 and 90 h are offset for clarity
During embryonic and unfed larval development, there was a significant interaction between spawning tank and time in levels of total, structural and energetic lipids, TAG, PL and protein; all other lipid classes showed no significant differences in the two-way analysis of variance (Table 2). One-way analysis of variance was then conducted separately for spawning tanks 1 and 2, whose embryos were derived from different sets of biological parents. Significant differences with time in total, structural and energetic lipids were driven by significant differences in their major component lipid classes, TAG (included in total, energetic) and PL (included in total, structural, Table 3), but with lipid utilization patterns differing between spawning tanks. Spawning tank 1 showed no difference in PL content between the 16–32 cell embryo (4 h) and the early gastrula (19 h); significant Tukey’s HSD difference between means were seen from the early to late gastrula (38 h), and between the 4 and 38 h stage (Table 3). TAG remained non-significantly different over time until the early gastrula (19 h), before declining significantly to the late gastrula stage (Tables 2, 3; Fig. 4). Spawning tank 2, however, showed maximal PL and TAG at the 16–32 cell embryo before showing a significant decline to a stable level between 14 and 38 h (Tables 2, 3; Fig. 4).
Lipid class composition (mean ± SE) during development of embryos and larvae of A. mollis. Squares indicate prefeeding development. Dashed vertical line indicates commencement of fed/unfed treatment (48 h). Feeding treatments: fed filled, unfed open symbol. Sample sizes for each developmental stage are provided in Supplementary Table S1. Data points at 65 and 90 h are offset for clarity
The protein content of A. mollis prefeeding embryos showed no significant difference with time in spawning tank 1, but there was a significant difference in spawning tank 2 (Table 3). Here, protein content was significantly lower between the 16–32 cell embryo (4 h) and the early gastrula stage (19 h), but by the late gastrula (38 h) had returned to a level more similar to that of the blastula stage at 14 h (Table 3; Fig. 3).
Development of the auricularia larvae in A. mollis was fuelled by the main maternally derived energetic lipid TAG, which significantly decreased with time in both embryonic (Table 3) and unfed larval development (Table 4; Fig. 4a). From maximum maternally derived TAG values at the 16–32 cell stage, unfed A. mollis larvae utiized TAG at a rate of 0.0824 ng h−1 (Based on grand mean of spawning tanks 1, 2; and unfed cultures 1, 2: y = −0.0824x + 9.4587; r 2 = 0.9454; F 1,5 = 87.51 P < 0.001; see Supplementary Figure S2). If the rate of TAG utilization remains constant, TAG reserves would be exhausted at approximately 114.8 h (4.8 day; Supplementary Figure S2). FFA and AH were minor contributors to total energetic lipid, and their contents did not significantly change with time during embryonic (Table 2) and unfed larval development (Table 4; Figs. 4b, c).
Structural lipids and their main component lipid class, PL, showed a significant decrease during embryonic (Table 3) and unfed larval development (Table 4; Figs. 3, 4e). ST and AMPL were minor components of structural lipid and their content did not significantly change through development (Tables 2, 4), although levels of AMPL were particularly variable in the egg and 16–32 cell stage (Figs. 4d, f).
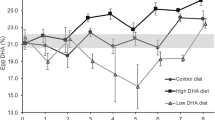
After the larvae were able to feed, both fed and unfed A. mollis larvae showed a marked decrease in energetic lipids and TAG between 65 and 90 h (Table 4; Figs. 3a, 4a). The percent decrease in TAG, based on the grand mean of the two cultures per feeding treatment, was 53.3% for fed and 62.2% for unfed larvae. Over this period there was also a significant decline in total, structural and PL in the unfed treatment only (Table 4; Figs. 3b, c, 4e). There were no significant differences in any lipid class, total, structural or energetic lipids between fed and unfed treatments (Table 4).
Protein content, in contrast, showed different patterns with both time and diet (Table 4). Unfed A. mollis auricularia showed a significant decrease in protein content in unfed larvae (based on grand means = 29.5%, Fig. 3d), while fed larvae showed a significant 40.6% increase in protein content (Table 4; Fig. 3d).
Discussion
Detailed comparison of the eggs and embryos/early larvae of the sea cucumber Australostichopus mollis with other planktotrophic echinoderms has revealed four major findings: (1) that A. mollis eggs showed typical echinoderm egg morphology; (2) that relative to egg size, A mollis eggs had a typical ratio of lipid:protein but relatively low lipid content; (3) that TAG was the main maternally derived energetic lipid, and the majority was utilized in the construction of the auricularia; and (4) that feeding of the auricularia did not affect the rate of utilization of TAG, but increased the protein content in the fed larvae.
The mean A. mollis egg diameter of 160 µm reported here is close to an average size for planktotrophic aspidochirotes (169 µm, Sewell and Young 1997), with an egg morphology typical of planktotrophic echinoderms containing conspicuous yolk protein droplets and scattered lipid droplets (e.g. Byrne et al. 1999; Byrne and Cerra 2000; Byrne et al. 2003; Falkner et al. 2013). Proteinaceous yolk granules depicted in TEM images by Kessel (1966) in the oocytes of the lecithotrophic sea cucumber Thyone briareus are similar to those described here in A. mollis (Fig. 2). In addition, the protein droplets are PAS+ as described in previous histological studies of mature oocytes within the holothurian ovary (e.g. Sewell and Chia 1994; Ramofafia et al. 2000). Thus, in terms of morphology, the eggs of A. mollis are considered “equal” to those from other echinoderm classes.
However, compared with echinoderms with similar egg size (Fig. 1), A. mollis had the lowest lipid content reported with 35.6 ng egg−1 of lipid (Sewell and Manahan 2001; Prowse et al. 2008; Falkner et al. 2006, 2015; McAlister and Moran 2012; Moran et al. 2013). Planktotrophic asteroid and echinoid species with similar egg size have considerably higher lipid content [Pentaceraster cumingi, 150.6 µm egg diameter, 57.4 ng lipid egg−1 (Moran et al. 2013); Strongylocentrotus droebachiensis, 155.2 µm egg diameter, 119 ng lipid egg−1 (Jaeckle 1995) and Patiriella regularis, 165 µm egg diameter, 121.5 ng lipid egg−1 (Prowse et al. 2008)].
Although, based on data from only a single planktotrophic holothurian, it is probably premature to suggest why sea cucumbers might have a lower maternal investment for a particular egg size, there are two, non-mutually exclusive, hypotheses that might prove useful to stimulate further research. First, as the adult diet can have marked effects on egg quality and biochemical composition (George 1990; Bertram and Strathmann 1998), the detritivorous diet of A. mollis may affect the biochemical content of the eggs. This hypothesis is based on comparison with the omnivorous P. regularis, with a similar egg size and phospholipid content, but higher amounts of total lipid and protein (Prowse et al. 2008). Alternatively, as hypothesised by Sewell and Young (1997) and supported by recent empirical data (Falkner et al. 2006, 2015; Whitehill and Moran 2012), the eggs of holothuroids and ophiuroids may have a different relationship between egg size and biochemical composition from that of other echinoderms, and/or egg size may be independently responding to other selective pressures, such as to increase fertilization success (Moran et al. 2013).
TAG and PL were the main energetic and structural lipids in the egg, as previously described in other planktotrophic echinoderms (Podolsky et al. 1994; Villinski et al. 2002; Sewell 2005; Byrne et al. 2008a, b; Prowse et al. 2008; Whitehill and Moran 2012). Because we spotted the entire lipid extract, we were also able to detect lipid classes, such as FFA and DAG, which were present at very low levels in A. mollis eggs (<1.6 ng/egg), but not consistently reported in other studies using the Iatroscan TLC/FID system (e.g. Sewell 2005; Falkner et al. 2006, 2015; Meyer et al. 2007; Byrne et al. 2008a, b; Prowse et al. 2008). DAG (<0.4% of egg lipids) is found at trace levels in tissues of living plants and animals as cellular messengers and key intermediaries in the biosynthesis of TAG from phospholipids (Harwood 2012; Christie 2014). FFA (<5% of egg lipids) are commonly present in living systems for energy production, during lipid biosynthesis and as cellular messengers, particularly when bound to protein carriers, and were detected at low levels in the eggs of six echinoid and five asteroid species through traditional TLC (Villinski et al. 2002). High levels of FFA may also indicate sample degradation (Christie 2014), but as egg samples were collected immediately after spawning, kept on ice during processing (max. 1 h) and immediately stored at −80 °C, this seems an unlikely explanation for the presence of FFA in A. mollis eggs.
The energetic lipid diacylglycerol ether (DAGE) has been reported in the eggs of three asteroids with planktotrophic development (Coscinasterias muricata, Patiriella regularis, and Meridiastra mortenseni), but not in four species of planktotrophic ophiuroids, Ophiactis resiliens, Ophionereis fasciata, Ophiocoma dentata and Ophiopteris antipodum (Prowse et al. 2009; Falkner et al. 2015). In contrast, DAGE is found abundantly in asteroids and ophiuroids with lecithotrophic development (Prowse et al. 2009; Falkner et al. 2015). Those authors have proposed that the evolution of non-feeding development in the Echinodermata has involved increased oogenic deposition of DAGE in asteroids and echinoids (Prowse et al. 2009; Falkner et al. 2015), whereas the ophiuroids provide lecithotrophic eggs with larger quantities of WE and DAGE (Falkner et al. 2015). Sea cucumbers, which are the sister group to the echinoids (Telford et al. 2014; Reich et al. 2015), might also be expected to have DAGE present in planktotrophic species, and an increased DAGE deposition in lecithotrophs. Preliminary analysis of A. mollis eggs with traditional TLC did not, however, detect DAGE (data not shown), but further research on the presence of DAGE in both planktotrophic and lecithotrophic holothurians is warranted.
The increased amounts of several lipid classes (TAG, DAG, ST, PL) and protein in the development stage from the fertilized egg to the 16–32 cell (4 h) parallel the patterns seen in initial lipid amounts during the early development in the echinoid E. chloroticus (Sewell 2005). As this pattern appeared only in certain lipid classes and protein, it is unlikely this is a result of a sampling or analysis error, and might instead be related to the stress used to induce spawning, resulting in the release of immature oocytes that have not yet completed vitellogenesis. For this reason, the 16–32 cell stage was considered as a more reliable indicator of egg TAG content than the actual egg samples, and therefore used in Fig. 1, as a starting point for estimates of TAG utilization and for calculation of the facultative feeding period (FFP) as discussed below.
As this was the first analysis of the biochemical composition of eggs from a planktotrophic holothurian, we were particularly interested in comparing the patterns of utilization of maternally derived TAG in early development with that of other echinoderms. Echinoids and ophiuroids have been shown to utilize large amounts of TAG during development of the pluteus larvae, with only trace amounts remaining in some species [Evechinus chloroticus: Sewell (2005); Strongylocentrotus purpuratus: Meyer et al. (2007); Ophiocoma alexandri, Arbacia puctulata: Whitehill and Moran (2012)], or with 25–50% of the TAG utilized in others [Ophiocoma dentata, Ophionereis fasciata, Ophiactis resiliens: Falkner (2007); Tripneustes gratilla: Byrne et al. (2008a, b)]. Asteroids utilize 53 and 34% of the egg TAG during development to the bipinnaria stage in Patiriella regularis and Meridiastra mortenseni (Prowse et al. 2008). The auricularia larvae of Australostichopus mollis studied here is similar to the asteroids, using about 50% of the TAG from the 16–32 cell embryo to a fully formed auricularia at 65 h.
Both fed and unfed larvae of A. mollis continue to utilize TAG reserves after the auricularia is fully formed. At 90 h of development, 81% of the TAG reserves in the 16–32 cell stage had been utilized, and these reserves were estimated to be exhausted ca 115 h (4.8 days) after fertilization. Following Miner et al. (2005) in defining the FFP from the point of fertilization, and with time to metamorphosis ca 22 days (unpub. data), A. mollis larvae would spend 0.22 (4.8/22) of their larval life facultatively feeding. Support for this relatively long FFP in A. mollis comes from experimental work by Morgan (2008), where there was a divergence in larval size [proposed as an indicator of FFP by Miner et al. (2005) and Byrne et al. (2008b)] between high and low phytoplankton diets in the period between 3 and 7 days. The FFP in planktotrophic echinoids is generally shorter, from 48 to 72 h, but longer FFP are reported in Tripneustes gratilla (Byrne et al. 2008b), in the enhanced planktotroph Encope michelini (Eckert 1995) and the facultative planktotroph Clypeaster rosaceus (Emlet 1986; see also Miner et al. 2005, their Table 3).
McEdward (1997) hypothesised that the length of FFP was positively correlated with egg size, and this was supported in the study of 6 tropical sand-dollar species by Miner et al. (2005). Recently, however, Byrne et al. (2008a) have shown that the echinoid Tripneustes gratilla has a small egg (85.2 µm), but a long FFP (>8 days), perhaps related to the extremely high TAG content of the egg (54% of total lipid). The suggestion that it is “egg quality” rather than egg size per se that is important to FFP (Byrne et al. 2008a) is further supported by Whitehill and Moran (2012) who examined the relationship between egg size and FFP in an ophiuroid Ophiocoma alexandri and an echinoid Arbacia punctulata with similar egg sizes (71.0 and 73.8 µm, respectively). The ophiuroid utilized its TAG reserves at a slower rate (0.05 ng h−1) than the echinoid (0.10 ng h−1), with a corresponding difference in FFP: 137 h (5.7 days) vs 55 h (2.3 days). The latter study highlights the importance of rate of utilization of the energetic reserves in setting FFP: A. mollis females may invest a relatively low amount of energetic lipids in the eggs, but the rate of utilization of TAG (0.08 ng h−1) allows this maternal investment to fuel larval growth and development for a considerably longer period of time (nearly 5 days).
Phospholipids and proteins, precursors of larval structures (Jaeckle 1995), decreased slightly or remained relatively constant before feeding started, though the patterns differed slightly with the source of the biological material (spawning tank 1 and 2). Further sea cucumber spawnings will be required to clarify if the pattern of PL decrease seen in Spawning Tanks 1 or 2 is the more “typical” pattern for this species. For protein, there was no significant change with time in Spawning Tank 1, as generally observed in echinoderm larvae (George et al. 1997; Sewell 2005; Meyer et al. 2007; Pace and Manahan 2007; Prowse et al. 2008), or an increase in protein in the late gastrula stage (Spawning Tank 2).
Once feeding commenced, proteins were the first biochemical component to increase, almost doubling 2 days after first feeding. A similar phenomenon has been observed in S. purpuratus, where protein content almost tripled in fed larvae 2 days after first feeding (Meyer et al. 2007). In Asterina miniata, protein content in fed and unfed larvae differed around 10 days after first feeding, with an associated increase in total lipid content and larval length in the fed treatment (Pace and Manahan 2007). Unfed T. gratilla larvae have shown a 30% protein increase fuelled by a lipid decrease 4 days after being able to feed (Byrne et al. 2008a), suggesting that the fast protein increase seen in A. mollis and S. purpuratus only 2 days after first feeding might be related to an early response to food availability. This early response might not be directly related to larval growth, given that we observed no significant increase in structural lipids or the components of this (PL, AMPL, ST) in the fed treatment, although PL and ST showed a slight decrease in the unfed treatment.
Similar observations have been made in T. gratilla, where neither phospholipid content nor size differed between fed and starved larval treatments up to 8 days of development (Byrne et al. 2008b). Morgan (2008) did not find significant morphological differences in A. mollis auricularia larvae under low- and high-food regimes until 7 days after first feeding, while George (1999) reported size differences between fed and starved asteroid larvae 10–14 days post-fertilization. In the planktotrophic sea cucumber Apostichopus japonicus, a species that reaches metamorphic competency 6 days before A. mollis [16 days post-fertilization (Ito and Kitamura 1997; Matsuura et al. 2009)], fed/starved larvae showed significant size differences 5 days after first feeding, where stomach size proved to be the best indicator of larval condition (Sun and Li 2012, 2014). Further, Byrne et al. (2008b) noted that nutrient reserves acquired by T. gratilla larvae during the FFP were not utilized for growth, but instead were allocated for storage. Further research would be required to determine possible drivers of protein increase during early larval development in A. mollis, which could be related to an increase in the secretion of digestive enzymes, or the synthesis of high-energy protein storage products such as lipoproteins (Lee 1991; Lee et al. 2006).
The present investigation provides the first biochemical data on eggs of a planktotrophic holothurian, with previous values available only in a brooding species (Jaeckle 1995). Our results support the hypothesis that TAG is the ancestral energy-storage lipid fuelling the formation of planktotrophic larvae in all echinoderm classes (Podolsky et al. 1994; Villinski et al. 2002; Sewell 2005; Falkner et al. 2006, 2015; Meyer et al. 2007; Byrne et al. 2008a, b; Prowse et al. 2008, 2009; Whitehill and Moran 2012), and provide preliminary evidence that ophiuroids and holothuroids might have different energetic adaptations for early development compared with the asteroids and echinoids. Further studies in more ophiuroid and holothuroid species will be fundamental to demonstrate differing early development energetic adaptations in the Echinodermata.
References
Agudo N (1996) Sandfish hatchery techniques. Secretariat of the Pacific Community, Noumea
Archer JE (1996) Aspects of the reproductive and larval biology and ecology of the temperate holothurian Stichopus mollis (Hutton). MSc dissertation, University of Auckland, New Zealand
Bertram DF, Strathmann RR (1998) Effects of maternal and larval nutrition on growth and form of planktotrophic larvae. Ecology 79:315–327
Byrne M (1989) Ultrastructure of the ovary and oogenesis in the ovoviviparous brittlestar Ophiolepis paucispina. (Echinodermata: Ophiuroidea). Biol Bull 176:79–95
Byrne M (1991) Reproduction, development and population biology of the Caribbean ophiuroid Ophionereis olivacea, a protandric hermaphrodite that broods its young. Mar Biol 111:387–399
Byrne M, Cerra A (2000) Lipid dynamics in the embryos of Patiriella species (Asteroidea) with divergent modes of development. Dev Growth Differ 42:79–86
Byrne M, Cerra A, Villinski JT (1999) Oogenic strategies in the evolution of development in Patiriella (Echinodermata: Asteroidea). Invert Reprod Develop 36:195–202
Byrne M, Selvakumaraswamy P, Villinski JT, Raff RA (2003) Evolution of maternal provisioning in ophiuroids, asteroids and echinoids. In: David B (ed) Feral JP. Echinoderm Research AA Balkema, Lisse, pp 171–175
Byrne M, Prowse TAA, Sewell MA, Dworjanyn S, Williamson JE, Vaïtilingon D (2008a) Maternal provisioning for larvae and larval provisioning for juveniles in the toxopneustid sea urchin Tripneustes gratilla. Mar Biol 155:473–482
Byrne M, Sewell MA, Prowse TAA (2008b) Nutritional ecology of sea urchin larvae: influence of endogenous and exogenous nutrition on echinopluteal growth and phenotypic plasticity in Tripneustes gratilla. Funct Ecol 22:643–648
Christie WW (2014) AOCS lipid library http://lipidlibrary.aocs.org/index.html
D’Amico F (2005) A polychromatic staining method for epoxy embedded tissue: a new combination of methylene blue and basic fuchsine for light microscopy. Biotech Histochem 80:207–210
Delmas RP, Parrish CC, Ackman RG (1984) Determination of lipid class concentrations in sea water by Thin-Layer Chromatography with Flame Ionization Detection. Anal Chem 56:1272–1277
Eckert GL (1995) A novel larval feeding strategy of the tropical sand dollar, Encope michelini (Agassiz): adaptation to food limitation and an evolutionary link between planktotrophy and lecithotrophy. J Exp Mar Biol Ecol 187:103–128
Emlet R (1986) Facultative planktotrophy in the tropical echinoid Clypeaster rosaceus (Linnaeus) and a comparison with obligate planktotrophy in Clypeaster subdepressus (Gray) (Clypeasteroida: Echinoidea). J Exp Mar Biol Ecol 95:183–202
Emlet R, McEdward L, Strathmann R (1987) Echinoderm larval ecology viewed from the egg. In: Jangoux M, Lawrence J (eds) Echinoderm studies 2. Balkema, Rotterdam, pp 55–136
Falkner I (2007) Evolution of maternal provisioning in ophiuroids: characterisation of egg nutrients and their roles in development. PhD dissertation, University of Sydney, Australia
Falkner I, Byrne M, Sewell MA (2006) Maternal provisioning in Ophionereis fasciata and O. schayeri: brittle stars with contrasting modes of development. Biol Bull 211:204–207
Falkner I, Barbosa S, Byrne M (2013) Reproductive biology of four ophiocomid ophiuroids in tropical and temperate Australia—reproductive cycle and oogenic strategies in species with different modes of development. Invert Reprod Develop 57:189–199
Falkner I, Sewell MA, Byrne M (2015) Evolution of maternal provisioning in ophiuroid echinoderms: characterisation of egg composition in planktotrophic and lecithotrophic developers. Mar Ecol Prog Ser 525:1–13
George SB (1990) Population and seasonal differences in egg quality of Arbacia lixula (Echinodermata: Echinoidea). Invert Reprod Dev 17:111–121. doi:10.1080/07924259.1990.9672098
George SB (1999) Egg quality, larval growth and phenotypic plasticity in a forcipulate seastar. J Exp Mar Biol Ecol 237:203–224
George SB, Young CM, Fenaux L (1997) Proximate composition of eggs and larvae of the sand dollar Encope michelini (Agassiz): the advantage of higher investment in plankotrophic eggs. Invert Reprod Dev 32:11–19
Grange LT, Tyler PA, Peck LS, Cornelius N (2004) Longterm interannual cycles of the gametogenic ecology of the Antarctic brittle-star Ophionoctus victoriae. Mar Ecol Prog Ser 278:141–155
Harwood JL (2012) Lipid metabolism. In: Gunstone FD, Harwood JL, Dijkstra AJ (eds) The lipid handbook with CD room. CRC Press, Boca Raton, pp 637–702
Ito S, Kitamura H (1997) Induction of larval metamorphosis in the sea cucumber Stichopus japonicus by periphitic diatoms. Hydrobiologia 358:281–284
Jaeckle WB (1995) Variation in size, energy content, and biochemical composition of invertebrate eggs: correlates to the mode of larval development. In: McEdward LR (ed) Marine invertebrate larvae. CRC, Boca Raton, pp 49–77
Jaeckle WB, Manahan DT (1989) Growth and energy imbalance during the development of a lecithotrophic molluscan larva (Haliotis rufescens). Biol Bull 177:237–246
Kato S, Tsurumaru S, Taga M, Yamane T, Shibata Y, Ohno K, Fujiwara A, Yamano K, Yoshikuni M (2009) Neuronal peptides induce oocyte maturation and gamete spawning of sea cucumber, Apostichopus japonicus. Dev Biol 326:169–176
Kessel RG (1966) Some observations on the ultrastructure of the oocyte of Thyone briareus with special reference to the relationship of the Golgi complex and endoplasmic reticulum in the formation of yolk. J Ultra R 16:305–319
Lacalli T (2003) Developmental biology: a larval revelation. Nature 421:120–121
Lee RF (1991) Lipoproteins from the hemolymph and ovaries of marine invertebrates. In: Gilles R (ed) Advances in comparative and environmental physiology, vol 7. Springer, Berlin, pp 187–207
Lee RF, Hagen W, Kattner G (2006) Lipid storage in marine zooplankton. Mar Ecol Prog Ser 307:273–306
Leonet A, Rasolofonirina R, Wattiez R, Jangoux M, Eeckhaut I (2009) A new method to induce oocyte maturation in holothuroids (Echinodermata). Invert Reprod Dev 53:13–21
Levitan DR (2000) Optimal egg size in marine invertebrates: theory and phylogenetic analysis of the critical relationship between egg size and development time in echinoids. Am Nat 156:175–192
Matsuura H, Yazaki I, Okino T (2009) Induction of larval metamorphosis in the sea cucumber Apostichopus japonicus by neurotransmitters. Fish Sci 75:777–783
McAlister JS, Moran AL (2012) Relationships among egg size, composition, and energy: a comparative study of geminate sea urchins. PLoS One 7:e41599
McEdward LR (1997) Reproductive strategies of marine benthic invertebrates revisited: facultative feeding by planktotrophic larvae. Am Nat 150:48–72. doi:10.1086/286056
McEdward LR, Miner BG (2006) Estimation and interpretation of egg provisioning in marine invertebrates. Integr Comp Biol 46:224–232
Meyer E, Green A, Moore M, Manahan D (2007) Food availability and physiological state of sea urchin larvae Strongylocentrotus purpuratus. Mar Biol 152:179–191
Miner BG, McEdward LA, McEdward LR (2005) The relationship between egg size and the duration of the facultative feeding period in marine invertebrate larvae. J Exp Mar Biol Ecol 321:135–144
Moloney P, Byrne M (1994) Histology and ultrastructure of the ovaries and oogenesis in the ophiuroid Ophionereis schayeri. In: David B, Guille A, Feral J-P, Roux M (eds) Echinoderms through time. AA Balkema, Rotterdam, pp 463–469
Moran AL, McAlister JS, Whitehill EAG (2013) Eggs as energy: revisiting the scaling of egg size and energetic content among echinoderms. Biol Bull 224:184–191
Morgan AD (2008) The effect of food availability on phenotypic plasticity in larvae of the temperate sea cucumber Australostichopus mollis. J Exp Mar Biol Ecol 363:89–95
Morgan AD (2009) Spawning of the temperate sea cucumber, Australostichopus mollis (Levin). J World Aquacult Soc 40:363–373
Nakano H, Hibino T, Oji T, Hara Y, Amemiya S (2003) Larval stages of a living sea lily (stalked crinoid echinoderm). Nature 421:158–160
Pace DA, Manahan DT (2007) Efficiencies and costs of larval growth in different food environments (Asteroidea: Asterina miniata). J Exp Mar Biol Ecol 353:89–106
Parrish CC (1987) Separation of aquatic lipid classes by chromarod thin-layer chromatography with measurement by Iatroscan flame ionization detection. Can J Fish Aquat Sci 44:722–731
Pawson DL (1970) The marine fauna of New Zealand: sea cucumbers (Echinodermata: Holothuroidea). New Zealand Oceanographic Institute Memoir 52
Podolsky RD, Strathmann RR (1996) Evolution of egg size in free-spawners: consequences of the fertilization-fecundity trade-off. Am Nat 148:160–173. doi:10.2307/2463076
Podolsky RD, Virtue P, Hamilton T, Vavra J, Manahan DT (1994) Energy metabolism during development of the antarctic sea urchin Sterechinus neumayeri. Antarct J US 29:157
Prowse TAA, Sewell MA, Byrne M (2008) Fuels for development: evolution of maternal provisioning in asterinid sea stars. Mar Biol 153:337–349
Prowse TAA, Falkner I, Sewell MA, Byrne M (2009) Long-term storage lipids and developmental evolution in echinoderms. Evol Ecol Res 11:1069–1083
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Ramofafia C, Battaglene SC, Bell JD, Byrne M (2000) Reproductive biology of the commercial sea cucumber Holothuria fuscogilva in the Solomon Islands. Mar Biol 136:1045–1056
Reich A, Dunn C, Akasaka K, Wessel G (2015) Phylogenomic analyses of Echinodermata support the sister groups of Asterozoa and Echinozoa. PLoS One 10(3):e0119627
Sewell MA (1990) Aspects of the ecology of Stichopus mollis (Echinodermata: Holothuroidea) in north-eastern New Zealand. N Z J Mar Fresh 24:87–93
Sewell MA (1992) Reproduction of the temperate Aspidochirote Stichopus mollis (Echinodermata: Holothuroidea) in New Zealand. Ophelia 25:103–121
Sewell MA (2005) Utilization of lipids during early development of the sea urchin Evechinus chloroticus. Mar Ecol Prog Ser 304:133–142
Sewell MA, Chia FS (1994) Reproduction of the intraovarian brooding apodid Leptosynapta clarki (Echinodermata: Holothuroidea) in British Columbia. Mar Biol 121:285–300
Sewell MA, Manahan DT (2001) Echinoderm eggs: biochemistry and larval biology. In: Barker M (ed) Echinoderms 2000: proceedings of the 10th international conference, Dunedin, New Zealand. Swets and Zeitlinger, Lisse, pp 55–58
Sewell MA, Young CM (1997) Are echinoderm egg size distributions bimodal? Biol Bull 193:297–305
Sun X, Li Q (2012) Effects of temporary starvation on larval growth, survival and development of the sea cucumber Apostichopus japonicus. Mar Biol Res 8:771–777
Sun X, Li Q (2014) Effects of delayed first feeding on larval growth, survival and development of the sea cucumber Apostichopus japonicus (Holothuroidea). Aquacult Res 45:278–288
Telford MJ, Lowe CJ, Cameron CB, Ortega-Martinez O, Aronowicz J, Oliveri P, Copley RR (2014) Phylogenomic analysis of echinoderm class relationships supports Asterozoa. Proc R Soc B 281:20140479
Tyler PA, Gage JD (1979) Reproductive ecology of deep-sea ophiuroids from the Rockall Trough. In: Naylor E, Hartnoll RG (eds) Cyclic phenomena in marine plants and animals: proceedings of the 13th European marine biology symposium. Pergamon Press, Oxford, pp 215–222
Villinski JT, Villinski JC, Byrne M, Raff RA (2002) Convergent maternal provisioning and life-history evolution in echinoderms. Evolution 56:1764–1775
Whitehill EAG, Moran AL (2012) Comparative larval energetics of an ophiuroid and an echinoid echinoderm. Invertebr Biol 131:345–354
Acknowledgements
We greatly appreciate the help with sea cucumber collections, lipid/protein analysis and microscopy from Adrian Turner, Erica Zarate, Angela Little and Emily Douglas. We gratefully acknowledge the comments of the three anonymous reviewers, which have greatly improved the manuscript, and the statistical advice of Dr. Katya Ruggiero during the manuscript revision. This research was also supported by a Chilean Government scholarship (Becas-Chile, National Commission for Scientific and Technological Research, CONICYT) awarded to JPD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
All applicable guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: M. Byrne.
Reviewed by M. Gibbs, P. Virtue and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peters-Didier, J., Sewell, M.A. Maternal investment and nutrient utilization during early larval development of the sea cucumber Australostichopus mollis . Mar Biol 164, 178 (2017). https://doi.org/10.1007/s00227-017-3209-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3209-7