Abstract
The physical and chemical environment around corals, as well as their physiology, can be affected by interactions with neighboring corals. This study employed small colonies (4 cm diameter) of Pocillopora verrucosa and Acropora hyacinthus configured in spatial arrays at 7 cm s−1 flow speed to test the hypothesis that ocean acidification (OA) alters interactions among them. Interaction effects were quantified for P. verrucosa using three measures of growth: calcification (i.e., weight), horizontal growth, and vertical growth. The study was carried out in May–June 2014 using corals from 10 m depth on the outer reef of Moorea, French Polynesia. Colonies of P. verrucosa were placed next to conspecifics or heterospecifics (A. hyacinthus) in arrangements of two or four colonies (pairs and aggregates) that were incubated at ambient and high pCO2 (~1000 µatm) for 28 days. There was an effect of pCO2, and arrangement type on multivariate growth (utilizing the three measures of growth), but no interaction between the main effects. Conversely, arrangement and pCO2 had an interactive effect on calcification, with an overall 23 % depression at high pCO2 versus ambient pCO2 (i.e., pooled among arrangements). Within arrangements, there was a 34–45 % decrease in calcification for solitary and paired conspecifics, but no effect in conspecific aggregates, heterospecific pairs, or heterospecific aggregates. Horizontal growth was negatively affected by pCO2 and arrangement type, while vertical growth was positively affected by arrangement type. Together, our results show that conspecific aggregations can mitigate the negative effects of OA on calcification of colonies within an aggregation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spatial arrangement of sessile organisms can influence the outcomes of species interactions (Tilman 1994) and therefore plays a role in shaping community structure. The influence of sessile neighboring organisms on the growth, fecundity, and survival of a given individual constitutes ‘neighborhood effects’ (Mack and Harper 1977) that have been comprehensively studied in plant communities (Bonan 1988; Stoll and Weiner 2000), as well as benthic marine communities such as coral reefs (Cornell and Karlson 2000). On coral reefs, space is a limiting resource on benthic surfaces (Jackson and Buss 1975), and sessile organisms compete for this resource through an array of mechanisms including overgrowth, digestive aggression, allelopathy, and agonistic behaviors (reviewed in Chadwick and Morrow 2011). For scleractinians, the spatial arrangement of colonies can influence the outcome of competition for space, as shown for Porites lobata (a weak competitor) competing against Porites rus (a strong competitor) in Moorea (Idjadi and Karlson 2007). In this interaction, P. lobata benefited from conspecific aggregations when competing against P. rus (Idjadi and Karlson 2007).
The implications for community dynamics of the distribution of corals on reef surfaces, and the extent to which corals aggregate with con- and heterospecifics, have been explored for historic and present-day coral reefs (Lang 1973; Rinkevich and Loya 1985; Karlson et al. 2007; Elahi 2008), but little is known of how these effects are modulated by impending environmental changes. One physical factor of great interest for contemporary and future reefs is seawater pH, which is declining as a result of ocean acidification [OA (Hoegh-Guldberg et al. 2007)], and potentially could change how corals interact (Evensen et al. 2015). As OA differentially affects coral growth depending on abiotic (e.g., flow speeds; Comeau et al. 2014c) and biotic conditions (e.g., presence of nearby competitors; Evensen et al. 2015), it is reasonable to hypothesize that con- and heterospecific interactions among corals will mediate the impacts of OA on coral communities (Hurd 2015).
Negative effects of OA on the calcification of scleractinian corals initially were reported around the start of the current millennium (Langdon et al. 2000; 2003), but recent studies have identified more varied calcification responses (Chan and Connolly 2013), and a greater number of physiological processes that are affected by high pCO2 and depressed pH (Hofmann et al. 2010; Comeau et al. 2014a). While calcification of some corals is strongly depressed (44–80 %) by OA (i.e., 781–789 µatm pCO2; Langdon and Atkinson 2005), others are more modestly affected (e.g., Comeau et al. 2013), and a few appear resistant (e.g., Comeau et al. 2014b), at least at ≤1000 µatm pCO2. The diversity of calcification responses shown by reef corals to declining pH indicates that more resistant species can be found on at least some reefs, which raises the possibility that OA-resistant species would have a selective advantage over OA-susceptible species at high pCO2 (Gaylord et al. 2015). In this case, space occupation on the reef will be dictated in part, by OA tolerance, in addition to ecological processes that mediate present-day coral community structure. If these effects favor a gradual replacement of corals that are susceptible to low pH with corals that are resistant to low pH, then evolutionary changes might allow coral communities to endure longer in the high pCO2 seas of the future than has been proposed (Fabricius et al. 2011). Likewise, differential susceptibility among corals to bleaching has also supported predictions of changes in coral community structure as tropical seawater continues warms (Marshall and Baird 2000), and in a few places this appears already to have occurred (Marshall and Baird 2000; Loya et al. 2001; Pratchett et al. 2013).
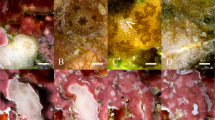
The present study evaluated the effects of high pCO2 on interactions among colonies of Pocillopora verrucosa (sensu Veron 2000) and Acropora hyacinthus in Moorea, French Polynesia. P. verrucosa was selected for this experiment as it is common in present-day, shallow (10–12 m depth) reefs of Moorea (Edmunds et al. 2016, N. Evensen pers. obs.), and has been common in this location for at least 30 years (Chevalier and Kühlmann 1983). A. hyacinthus was selected as a heterospecific competitor, as interactions among adjacent colonies of Pocillopora and Acropora were common on the outer reefs of Moorea when this study was conducted in 2014 (Fig. 1). We quantified the response of P. verrucosa to coral–coral interactions through con- and heterospecific encounters (with A. hyacinthus) created by arranging pairs or aggregates of two or four colonies in aquaria, and incubating them at ambient and high pCO2 for 28 days. We tested the hypotheses that: (1) OA alters the outcome of coral–coral interactions as measured by changes in colony growth (calcification, and both horizontal and vertical linear extension) and (2) coral–coral interactions modulate the extent to which OA affects the growth of P. verrucosa.
Examples of Pocillopora verrucosa and Acropora hyacinthus interacting on the outer reef of Moorea. a A. hyacinthus (left) indirectly interacting (overtopping) with multiple P. verrucosa colonies, b P. verrucosa (top) and A. hyacinthus directly interacting (tissue in contact), c A. hyacinthus (center) directly interacting with two P. verrucosa colonies. Photographs taken in May 2014 at ~10 m depth
Materials and Methods
Field observations
This study was conducted in May and June 2014 in Moorea. Pocillopora verrucosa was used to create spatial arrangements in which small colonies (~4 cm diameter) were placed adjacent to colonies of conspecifics or Acropora hyacinthus (the heterospecific treatment). The ecological relevance of these interactions was determined by assaying colonies of P. verrucosa and A. hyacinthus on the outer reef of Moorea for interactions with other corals. These analyses were conducted using 0.25 m2 photoquadrats randomly placed along a 50-m transect running parallel to the reef crest at 10 m depth. Sampling occurred in 2014 and was conducted at ‘LTER1 and LTER2’ (~1.5 km apart; n = 40 site−1) on the north shore of Moorea (Edmunds 2015). Corals were identified to genus, as species-level identification of members of this genus is equivocal in images (e.g., Edmunds et al. 2016). Photoquadrats were used to quantify the frequency of interactions between adjacent Pocillopora colonies, and between adjacent colonies of Pocillopora and Acropora. Adjacent colonies of each genus were inferred to be interacting and competing for space when they were <5 mm apart, which included cases where they were touching. It was not possible to evaluate the veracity of inferred competition based on proximity of colonies in photoquadrats. However, inspection of adjacent colonies (also <5 mm apart) at the same sites while scuba diving in 2014 revealed areas of dead tissue at the zone of interaction which suggests competition was underway. The number of colonies inferred to be engaged in spatial competition based on analyses of the photoquadrats was expressed as a percentage of the total number of Pocillopora and Acropora colonies in each photoquadrat, and the results were averaged among photoquadrats.
Sample collection and preparation
Spatial arrangements were created using colonies of P. verrucosa and A. hyacinthus collected from the outer reef, with the arrangements designed to mimic encounters among Pocillopora colonies, and between Pocillopora and Acropora colonies found at 10 m depth in 2014 (Fig. 1). Coral–coral configurations were prepared to create five arrangements: (1) controls consisting of a single colony of P. verrucosa; (2) conspecific pairings, in which a colony of P. verrucosa was adjacent to a conspecific of equal size; (3) conspecific aggregations, in which a colony of P. verrucosa was surrounded by three conspecifics of equal size; (4) heterospecific pairings, in which a colony of P. verrucosa was adjacent to a fragment of A. hyacinthus; and (5) heterospecific aggregations, in which a colony of P. verrucosa was surrounded by three fragments of A. hyacinthus (Fig. S1). In all cases, the effect of the arrangements following incubations was measured on one colony of P. verrucosa, hereafter referred to the as the ‘primary colony.’ In all types of arrangements, adjacent colonies were positioned 3–5 mm apart to provide a gap large enough to allow linear skeletal growth, but small enough to be bridged by agonistic structures (i.e., mesenterial filaments, sweeper polyps, and sweeper tentacles), which are employed in competitive encounters among corals (Chadwick and Morrow 2011).
To prepare the arrangements, colonies of P. verrucosa (n = 120, ~4 cm in diameter) and A. hyacinthus (n = 20, ~10 cm in diameter) were collected from 10 m depth on the outer reef in May 2014. Colonies were removed from the reef using a hammer and chisel, and returned submerged in seawater to the Richard B. Gump South Pacific Research Station. Initially we had planned to create arrangements using colonies of similar size, since colony size affects competitive outcomes (Zilberberg and Edmunds 2001). However, when the study was initiated, the population size structure of A. hyacinthus at 10 m depth was negatively skewed, and small colonies (≤4 cm diameter), suitable for pairing with 4-cm-diameter P. verrucosa (which were abundant), were rare. Therefore, small colonies of A. hyacinthus were created by fragmenting colonies that were ~10 cm diameter. Each colony of A. hyacinthus was fragmented into 5–6 pieces consisting of 7–8 branch tips, and each piece was secured individually in its natural growth orientation to 4 × 4 cm plastic bases using epoxy (Z Spar A788). In this procedure, multiple fragments from each colony shared a common genotype, but the potential effects of this were minimized by randomly selecting fragments for use in preparing coral–coral arrangements. Colonies of P. verrucosa were attached upright to the same type of plastic bases as described for A. hyacinthus.
Following attachment to their plastic bases, fragments of A. hyacinthus and colonies of P. verrucosa were left to recover for 7 days in a shallow tank supplied with running seawater pumped from Cook’s Bay. Following recovery, most fragments (~80 %) of A. hyacinthus showed signs of recovery from the preparation process as evaluated by cessation of stress-related mucus secretion, and the growth of new tissue over fractured surfaces. The corals attached to their bases were used to prepare the aforementioned coral–coral arrangement by securing colonies of P. verrucosa and A. hyacinthus in different configurations to 15 × 15 × 1 cm terracotta tiles using non-toxic silicone aquarium sealant (Aqueon, USA). Three replicates of each arrangement were randomly allocated to one of four outdoor flumes (after Comeau et al. 2014c, described below) that were maintained at either ambient pCO2 or high pCO2 (targeted at 1000 µatm) for 28 days. The objective of this experimental design was to assess the effect of pCO2, and spatial arrangement on the growth of P. verrucosa. pCO2 was a fixed, between-plot effect; flume was a random factor nested in each pCO2 treatment, and arrangement was a fixed, split-plot effect in each flume.
The flumes consisted of a working section (5.0 × 0.3 × 0.3 m) in which water was re-circulated using pumps (W. Lim Wave II) to obtain a unidirectional flow of 7 cm s−1. Water in the flume was ~25 cm deep, with flow measured 4 cm off the bottom of the flume to estimate the flow experienced by the coral colonies in the flume. This flow speed is similar to the mean flow speed of 6.89 ± 0.01 cm s−1 (±SE, n = 76) recorded at 10 m depth on the fore reef of the north shore of Moorea in 2006–2014 using a bottom-mounted, Acoustic Doppler Current Profiler (Sentinel ADCP; Teledyne RD Instruments, Poway, California, USA; Washburn 2015). In the flumes, flow speed was measured across the working section using an Acoustic Doppler Velocimeter (ADV; Nortek AS, Vectrino, Norway). The flumes were filled with filtered seawater pumped from Cook’s Bay at 12 m depth, and supplied at 5 L min−1. Seawater passed through an 88-cm transition section at the upstream end of the flume, which housed 20-cm-long flow straighteners made of stacked, 3-cm-diameter PVC pipes.
The flumes were exposed to sunlight attenuated using neutral density shading to 374 ± 9 µmol photons m−2 s−1 (mean ± SE, n = 5), as measured at 12:00 h at the start of the incubation and every 7 days thereafter (using a 4π quantum sensor LI-193 and a LiCor LI-1400 m).
Carbonate chemistry and physical parameters
Two CO2 treatments were created in pairs of flumes maintained at ambient pCO2 (~400 μatm) and high pCO2 (~1000 μatm) to match the atmospheric pCO2 expected by the end of the current century in a pessimistic scenario characterized by representative concentration pathway (RCP) 8.5 (Moss et al. 2010). pCO2 was controlled using a pH-controlled system (Apex Aquacontroller, Neptune Systems, USA) that employed a solenoid to regulate the bubbling of either pure CO2 or CO2-free air. CO2-free air was obtained by scrubbing CO2 from ambient air using a column packed with soda lime. Using chillers (DA-500B Arctica, JBJ, USA), temperature in the flumes was regulated at 27.01 ± 0.04 °C (mean ± SE, n = 96) which is similar to the mean seawater temperature on the fore reef of Moorea at ~10 m depth during the experiment (27.3 °C; Washburn 2015). pH was measured daily using a portable meter (Orion 3-stars) fitted with a DG 115-SC probe (Mettler-Toledo, Switzerland) calibrated every 2 days using 2-amino-2-hydroxymethyl-1,3-propanediol (TRIS) buffers at a salinity of 35.0. pH was also measured weekly with a spectrophotometric procedure using m-cresol dye (SOP 6b, Dickson et al. 2007), with results obtained with the portable pH meter falling within ± 0.01 units of those obtained using the more accurate m-cresol procedure. Total alkalinity (A T) of the seawater in the flumes was measured every 2 days by open-cell potentiometric titrations using an automated titrator (model T50, Mettler-Toledo) with 50 mL samples of seawater. Titrations of certified reference materials (CRM) provided by A.G. Dickson yielded A T values within 5 μmol kg−1 (0.2 %) of the certified value. Parameters of the carbonate system were calculated from salinity, seawater temperature, A T and pHT using the R package Seacarb (Lavigne and Gattuso 2013).
Dependent variables
Growth of the primary colony in each treatment was measured using three dependent variables: calcification as mass of CaCO3, horizontal growth (change in diameter), and vertical growth (change in height). Horizontal growth was used as a proxy for the ability to compete for space with an adjacent coral, based on the rationale that corals expand toward neighbors during competitive encounters to occupy additional space (Fine and Loya 2003). Vertical growth was measured to test for a trade-off with horizontal growth and to assess whether growth was redirected vertically in the presence of neighboring colonies.
Calcification was measured by buoyant weighing (±1 mg) corals before and after incubation (Spencer-Davies 1989), with the difference between initial and final buoyant weight converted to dry weight using the density of aragonite (2.93 g cm−3). Calcification was normalized to tissue area (mg cm−2 days−1), which was estimated using wax dipping (Stimson and Kinzie 1991). To measure horizontal growth, corals were photographed in planar view under natural lighting in seawater before and after incubation. Images were divided into four quadrants centered on the primary P. verrucosa colony in order to codify the measurement process, with the quadrants created by the diagonals of the PVC tile on which the corals were epoxied (Fig. S2). Horizontal growth was obtained by measuring the growth of the tip of the longest branch in each quadrant. The growth of the branch tips in each of the four quadrants (i.e., the growth in four directions) cumulatively provided a measure of horizontal growth for the colony (μm days−1). Finally, vertical growth was obtained from lateral images of the corals, in which the distance from the base to the apex of the colony was measured before and after incubation. Vertical growth was expressed as change in height over time (μm days−1). While the growth measurements were reported as daily growth, these were measured once after 28 days in the treatments, and standardized to daily growth to facilitate comparisons across studies.
Photographs were used to measure linear growth, and were taken using a Nikon D70 camera (6.1-megapixel resolution) fitted with a Nikon 60-mm f/2.8D AF Micro-Nikkor lens. Each image contained a scale bar to standardize measurements that were obtained using ImageJ version 1.46 software (Rasband 1997). With this procedure, the precision of measurements was ±50 µm as determined by repeatedly (4 times) photographing a single coral on one day.
Statistical analysis
Physical conditions in the flumes were analyzed with a two-way ANOVA, with pCO2 as a fixed effect and flume a random factor nested in each treatment. Calcification, horizontal growth, and vertical growth collectively were analyzed in multivariate framework using mixed-effects PERMANOVA, with pCO2 as a fixed, between-plot effect, flume as a random factor nested in each treatment, and arrangement as a fixed, split-plot effect in each flume. As growth variables were measured using different metrics, data were standardized as z-scores (Quinn and Keough 2002). PERMANOVA was performed on a similarity matrix of Euclidean distances among replicates, with tests based on 9999 permutations of the residuals under the reduced model (Anderson et al. 2008). If the PERMANOVA was significant, the three response variables were separately analyzed using univariate split-plot ANOVAs with the same fixed and random factors described for the PERMANOVA. Univariate ANOVAs were used to test for the effects of pCO2 and arrangement on the dependent variables individually. In all cases, flume was dropped from the statistical analyses when not significant at P ≥ 0.250 (Quinn and Keough 2002). Tukey’s honestly significant difference (HSD) post hoc tests were conducted when significant differences (P ≤ 0.05) were detected by ANOVAs.
PERMANOVA was conducted using PRIMER-E version 6 software (Clarke and Gorley 2006) with the PERMANOVA+ extension (Anderson et al. 2008). ANOVAs and post hoc analyses were performed using R statistical software, with assumptions of normality and equality of variance evaluated through graphical analyses of residuals.
Results
Field observations
In April 2014, mean coral cover at 10 m depth on the outer reef was 26 ± 1 % (±SE, n = 77), and of this cover, 67 % was Pocillopora spp. and 3 % Acropora spp. Colonies of Pocillopora spp. occurred at a mean density of 13 ± 0.5 colonies m−2 (±SE, n = 77) and frequently engaged in coral–coral interactions (Fig. 1). Forty-two percent (n = 419 colonies) of Pocillopora spp. colonies were interacting with congenerics (i.e., colonies were ≤0.5 cm apart), of which 28 % was interacting with two congeneric colonies, and 5 % was interacting with three or more congenerics. Acropora spp. occurred at a mean density of 0.5 ± 0.1 colonies m−2 (±SE, n = 77), and Acropora–Pocillopora interactions affected 3 % of Pocillopora spp. colonies (n = 1001), and 74 % of Acropora spp. colonies (n = 39).
Manipulative experiment
Treatments were controlled precisely during the incubations, with analysis of seawater chemistry (Table 1) revealing that pCO2, pH, and Ωarag differed between treatments (F 1,4 ≥ 818.7, P ≤ 0.001), but not flumes (F 4,92 ≤ 0.409, P ≥ 0.524). A T and seawater temperature did not vary among flumes (F 4,92 ≤ 0.017, P ≥ 0.896) or between treatments (F 1,4 ≤ 0.552, P ≥ 0.459). Overall, the treatments contrasted 411 ± 6 μatm pCO2 with 1033 ± 21 μatm pCO2 (±SE, n = 48).
PERMANOVA showed an effect of pCO2 (pseudo-F 1,4 = 9.64, P perm = 0.002), and arrangement type (pseudo-F 4,50 = 9.91, P perm = 0.001) on multivariate growth, but there was no interaction between the main effects (pseudo-F 4,50 = 0.727, P perm = 0.596). Flume effects were not significant (P perm > 0.25) and were therefore dropped from the statistical model. Arrangement type explained the greatest proportion of the variance in the statistical model, with 37 % of the total variance attributable to this source, and 15 % to pCO2.
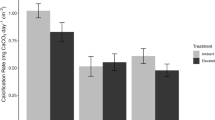
Univariate analysis of growth showed that the response of primary colonies to the treatments depended on the response variable (Table 2). For calcification, there was an interactive effect of pCO2 and arrangement type (Fig. 2a) (Table 2). Overall (i.e., pooled among arrangements), high pCO2 depressed mean calcification of primary colonies of P. verrucosa 23 %, from 0.60 ± 0.04 mg cm−2 days−1 in ambient pCO2, to 0.46 ± 0.03 mg cm−2 days−1 in high pCO2 (±SE, n = 30). Within arrangements, however, high pCO2 depressed calcification 34 % for control corals (i.e., single colonies), and 45 % for corals in conspecific pairings (Tukey’s HSD, P ≤ 0.03). There was no effect of pCO2 for in the other three arrangements (Fig. 2a), although there was a trend (P = 0.66) for corals in conspecific aggregations to calcify faster at high pCO2 compared to ambient pCO2. Subsequently, calcification rates for corals in conspecific aggregations under high pCO2 were not significantly different from the calcification rates of control corals under ambient pCO2 or high pCO2 (Tukey’s HSD, P ≥ 0.12).
Growth of Pocillopora verrucosa placed in arrangements simulating interactions found naturally on the outer reef of Moorea, and incubated for 28 days in ambient or high pCO2. Corals were positioned in the following arrangements: (1) ‘controls’, a single P. verrucosa colony; (2) conspecific pairings, P. verrucosa adjacent to conspecific of equal size; (3) conspecific aggregations, P. verrucosa surrounded by three conspecifics of equal size; (4) heterospecific pairings, P. verrucosa adjacent to A. hyacinthus fragment; (5) heterospecific aggregations, P. verrucosa surrounded by three A. hyacinthus fragments. A diagram of each arrangement is located above the top panel. Growth is expressed on three scales: (a) calcification, (b) horizontal linear growth, and (c) vertical linear growth. Differing letters denote significant differences among arrangement types, and asterisks denote significant differences between pCO2 treatments within an arrangement type (P < 0.05). Values displayed are mean ± SE (n = 6)
Horizontal growth of primary colonies was affected by pCO2, and arrangement type, but there was no interaction between the two (Table 2). Mean horizontal growth decreased 53 % from 65.99 ± 6.13 μm days−1 in ambient pCO2 to 31.27 ± 4.23 μm days−1 in high pCO2 (±SE, n = 30; Fig. 2b). Horizontal growth decreased across all spatial arrangements compared to the control treatment (Tukey’s HSD, all P ≤ 0.01), except for the conspecific pairings (Tukey’s HSD, P = 0.32).
Vertical growth of primary colonies was affected by arrangement, but not pCO2 or the interaction between the two (Table 2). Vertical growth increased 73 % in the conspecific aggregation compared to the control treatment (Tukey’s HSD, P = 0.03), while none of the other arrangements differed from the control (Tukey’s HSD, P ≥ 0.72) (Fig. 2c). Further, vertical growth for corals in conspecific aggregations was also significantly higher (79 %) than for corals in heterospecific pairings (Tukey’s HSD, P = 0.02).
Discussion
This study tested the hypotheses that ocean acidification alters interactions among corals arranged in conspecific and heterospecific pairings and aggregations, and that adjacent colonies affect the ways in which OA modulates the growth of primary colonies. Our results support both hypotheses, with the effect of OA on the calcification of P. verrucosa modulated by neighboring colonies (‘arrangement’), and overall colony growth (and thus the ability of colonies to compete for space) affected by OA. For P. verrucosa exposed to high pCO2, calcification was depressed to a lesser extent for colonies in conspecific aggregations relative to colonies placed in other arrangements, and indeed, was similar to that of single colonies. To our knowledge, our study is the first to demonstrate that conspecific aggregations of corals have the potential to alleviate the negative effects of OA on coral calcification.
On coral reefs and in terrestrial plant communities, conspecific aggregations can stimulate growth of organisms on the edge of an aggregation when faced with stronger competitors (Stoll and Prati 2001; Idjadi and Karlson 2007). Moreover conspecific aggregations can improve environmental conditions for downstream conspecifics in marine systems, such as occurs when upstream branches of the coral Madracis mirabilis facilitate particle capture for downstream branches through reduced flow speeds (Sebens et al. 1997). In the context of OA, photosynthesizing organisms (e.g., macroalgae in temperate and tropical coastal communities) can improve conditions for downstream calcifiers (i.e., through increases in pH and Ωarag) in habitats with largely unidirectional flow (Anthony et al. 2013; Hurd 2015). Together with the present findings, these observations suggest that it might be valuable to further investigate the potential for biologically mediated processes attributed to neighboring organisms to affect seawater chemistry. One advantage of such investigations could be a better understanding of the means by which the dynamic physical and chemical conditions routinely experienced by corals in their natural environment could affect their susceptibility to OA (Mumby and van Woesik 2014; Hurd 2015).
In the present study, calcification rates were greatest for solitary colonies at ambient pCO2, with calcification lower under all conditions involving high pCO2 and neighbors. The rapid calcification of solitary colonies probably reflects freedom from competition with neighboring colonies for light or other resources (Chadwick and Morrow 2011), and in the case of solitary colonies under ambient pCO2, exposure to an aragonite saturation state favoring calcification (Hoegh-Guldberg et al. 2007). Although OA depressed the calcification rate of solitary corals, the depressed rate still was greater than the OA-depressed calcification rates of colonies of P. verrucosa in all aggregates tested, except for corals placed in conspecific aggregations.
While the present study cannot reveal the mechanism(s) driving the higher calcification rates for colonies in conspecific aggregations relative to other competitive arrangements, a leading hypothesis for these effects involves the role of closely spaced coral branches in modifying the flow of seawater among colonies in conspecific aggregations. Though the flow of seawater among coral branches within the aggregations was not measured, the biological consequences of the unidirectional flow speed experienced by P. verrucosa in the flumes probably were different for aggregates versus solitary colonies or pairings. These differences would be created in aggregates from upstream colonies, which would have accentuated turbulent flow around downstream colonies (Reidenbach et al. 2006), including the primary P. verrucosa colony on which growth was measured. Such turbulence would favor increased retention times of seawater around the primary colony through eddies forming in the wake of upstream coral branches (Sebens et al. 1997; Reidenbach et al. 2006). Increased retention of seawater within the aggregations could lead to the retention and increased concentration (and thus perhaps improved uptake; Crossland and Barnes 1983) of dissolved and particulate organic carbon (DOC and POC) within conspecific aggregations, which are sources of carbon routinely released in large quantities by corals in shallow water (Naumann et al. 2010; Wild et al. 2010). Likewise, it is also possible that water retention among coral branches would have favored the capture by expanded polyps of small particulates (<100 μm) (Sebens et al. 1997) in the seawater. Further, Levas et al. (2015) showed that the coral Turbinaria reniformis reduced its DOC losses under high pCO2 (741 μatm), which suggests that corals modify their organic carbon budget when exposed to OA. Thus, particle capture and uptake of DOC might have contributed to a modified carbon budget, and subsequently higher rates of calcification, for corals placed in conspecific aggregations under high pCO2, as both food particles and DOC can function as important nutritional inputs to corals (Houlbrèque and Ferrier-Pagès 2009; Naumann et al. 2010; Wild et al. 2010).
An additional flow-related consequence of placing corals in aggregates is that such colonies probably experienced reduced flow speeds relative to solitary or paired corals (Sebens et al. 1997). While increased seawater flow can benefit coral calcification under ambient and high pCO2 in certain circumstances (Jokiel 1978; Comeau et al. 2014c), it has recently been suggested that reduced flow speeds could benefit coral calcification under high pCO2. Chan et al. (2016) demonstrated that pH adjacent to coral tissues can be elevated relative to ambient seawater through enhanced diffusive boundary layer at low flow speeds, thereby potentially reducing the negative consequences of OA (i.e., reduced seawater pH) on calcification, as appears to be the case in the present study. While the present results also indicate a mitigating effect of neighboring corals on calcification of colonies in heterospecific aggregations under high pCO2, likely also due to modified seawater flow as discussed above, the effects are not identical to those observed in conspecific aggregates (Fig. 2a). In the case of heterospecific aggregations, calcification rates under high pCO2 were lower than those of the control and conspecific aggregation treatments under high pCO2, and potentially this accentuated effect might reflect the negative consequences of competition between the closely spaced colonies of different species (Chadwick and Morrow 2011).
While conspecific aggregations had a positive effect on coral calcification under high pCO2, at least relative to coral pairings and heterospecific aggregates, coral aggregations have also been shown to accentuate the negative effects of high pCO2 on calcification in the dark (Anthony et al. 2013). For example, when colonies of Acropora aspera were placed in aggregates mimicking those found on the shallow reefs of Heron Island, Australia, and exposed in the dark to 560–700 µatm pCO2 at 8 cm s−1, their metabolic activity (i.e., respiration and calcification) accentuated the depression of Ωarag caused by high ambient seawater pCO2 (Anthony et al. 2013). This effect is likely to be detrimental to coral calcification at night when there is no photosynthesis to counteract the effects of respiration in depressing Ωarag through the production of CO2 (Shamberger et al. 2014). However, during the day, when calcification in symbiotic scleractinian corals is typically 3–4 times higher than at night (Chalker and Taylor 1975; Moya et al. 2006), the aggregates of A. aspera created by Anthony et al. (2013) caused Ωarag to increase during the day under high pCO2, thereby limiting the depressive effect of OA on coral calcification. Thus, the potential benefits of coral aggregations that we describe during the day, when physical conditions remain favorable for calcification, may offset the unfavorable conditions created by coral aggregations at night.
Despite conspecific aggregations mitigating the effect of OA on calcification in the present study, there was a clear overall decrease in horizontal growth as a result of high pCO2, suggesting that the ability of P. verrucosa to compete with other taxa through overgrowth may be impaired (Box and Mumby 2007) at high pCO2. Conversely, vertical growth was unaffected by high pCO2, but was affected by the number of neighboring colonies, increasing 73 % in conspecific aggregations versus control colonies. While corals in the present study were placed far enough apart at the beginning of the experiment to allow space for growth, it is possible that colonies in conspecific aggregations redirected growth away from the neighboring colonies (sensu Romano 1990), favoring vertical growth over horizontal growth. Notably, for colonies in heterospecific aggregations, the strong reduction in horizontal growth rates is likely due to A. hyacinthus employing mesenterial filaments to attack the tissue of the central P. verrucosa colonies during the experiment (N. Evensen pers. obs.), thus further preventing colony growth.
Nonetheless, rapid linear growth is a common mechanism used to overgrow or overtop neighboring corals for branching corals (Fine and Loya 2003; Connell et al. 2004), such as P. verrucosa. Thus, the negative effects of OA on horizontal growth may alter the ability of P. verrucosa to compete for space under high pCO2. Furthermore, scleractinians involved in competitive encounters can often employ rapid linear growth in concert with agonistic structures such as sweeper polyps, sweeper tentacles, and mesenterial filaments (Chadwick and Morrow 2011). Little is known about the effects of OA on cnidarian tissue (Renegar et al. 2008), though experimental studies have demonstrated no effect of OA on tissue biomass in several corals (Edmunds 2011; Schoepf et al. 2013). The effects of OA on the agonistic structures employed by reef corals remain unknown, however, and the extent to which OA influences the formation and deployment of these structures may affect the competitive ability of corals under high pCO2. It might be productive, therefore, to explicitly address the effect of OA on the production and use of agonistic structures in reef corals in order to better understand the ecological importance of coral–coral competition under high pCO2.
Our findings complement recent studies on the effects of OA on ecological interactions among benthic taxa on coral reefs (Connell et al. 2013) by considering the effects of OA on coral–coral interactions. To date, studies of the effects of OA on interactions among reef taxa have focused on coral–macroalgal interactions (Diaz-Pulido et al. 2011). Such interactions are of clear importance given the abundance of macroalgae on many reefs (Done 1992; Bruno et al. 2009), but it is also relevant to ask how coral–coral interactions will be affected by OA on reefs where coral cover remains relatively high (e.g., 26 % in Moorea at the time of the study). The mitigating effects of conspecific aggregations on the growth of corals under OA may prove important on reefs like the ones in Moorea, where, at the time of study (2014), 14 % of Pocillopora colonies on the fore reef were interacting with two or more congenerics. The percentage of Pocillopora interacting with other corals now is likely to be higher given the high rates of coral recruitment on this reef and its ongoing rapid rate of recovery of coral cover and community structure (Bramanti and Edmunds 2016). Finally, as the hypothesized mechanisms driving the effects described herein are likely to apply to a variety of taxa, the notion of neighboring organisms modulating the negative effects of OA on calcifying organisms may have general application to other marine benthic communities (Andersson et al. 2014; Hurd 2015).
References
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: Guide to software and statistical methods. PRIMER-E, Plymouth
Andersson AJ, Yeakel KL, Bates NR, de Putron SJ (2014) Partial offsets in ocean acidification from changing coral reef biogeochemistry. Nat Clim Change 4:56–61
Anthony KRN, Diaz-Pulido G, Verlinden N, Tilbrook B, Andersson AJ (2013) Benthic buffers and boosters of ocean acidification on coral reefs. Biogeosciences 10:4897–4909
Bonan GB (1988) The size structure of theoretical plant populations: spatial patterns and neighborhood effects. Ecology 69:1721–1730
Box SJ, Mumby PJ (2007) Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar Ecol Prog Ser 342:139–149
Bramanti L, Edmunds PJ (2016) Density-associated recruitment mediates coral population dynamics on a coral reef. Coral Reefs. doi:10.1007/s00338-016-1413-4
Bruno JF, Sweatman H, Precht WF, Selig ER, Schutte VG (2009) Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology 90:1478–1484
Chadwick NE, Morrow KM (2011) Competition among sessile organisms on coral reefs. In: Dubinsky Z, Stambler N (eds) Coral reef: an ecosystem in transition. Springer, Dordrecht, pp 347–371
Chalker BE, Taylor DL (1975) Light-enhanced calcification, and the role of oxidative phosphorylation in calcification of the coral Acropora cervicornis. Proc R Soc B 190:323–331
Chan NCS, Connolly SR (2013) Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob Change Biol 19:282–290
Chan NCS, Wangpraseurt D, Kühl M, Connolly SR (2016) Flow and coral morphology control coral surface pH: implications for the effects of ocean acidification. Front Mar Sci 3:10
Chevalier JP, Kühlmann DHH (1983) Les scléractiniaires de Moorea. Île de la Société (Polynésie Française). J de la Société des océanistes 77:55–75
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Comeau S, Edmunds PJ, Spindel NB, Carpenter RC (2013) The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol Oceanogr 160:1127–1134
Comeau S, Edmunds PJ, Spindel NB, Carpenter RC (2014a) Fast coral reef calcifiers are more sensitive to ocean acidification in short-term laboratory incubations. Limnol Oceanogr 59:1081–1091
Comeau S, Carpenter RC, Nojiri Y, Putnam HM, Sakai K, Edmunds PJ (2014b) Pacific-wide contrast highlights resistance of reef calcifiers to ocean acidification. Proc R Soc B 281:20141339
Comeau S, Edmunds PJ, Lantz CA, Carpenter RC (2014c) Water flow modulates the response of coral reef communities to ocean acidification. Sci Rep 4:6681
Connell JH, Hughes TP, Wallace CC, Tanner JE, Harms KE, Kerr AM (2004) A long-term study of competition and diversity of corals. Ecol Monogr 74:179–210
Connell SD, Kroeker KJ, Fabricius KE, Kline DI, Russell BD (2013) The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Philos Trans R Soc B Biol Sci 368:20120442. doi:10.1098/rstb.2012.0442
Cornell HV, Karlson RH (2000) Coral species richness: ecological versus biogeographical influences. Coral Reefs 19:37–49
Crossland CJ, Barnes DJ (1983) Dissolved nutrients and organic particulates in water flowing over coral reefs at Lizard Island. Aust J Mar Freshw Res 34:835–844
Diaz-Pulido G, Gouezo M, Tilbrook B, Dove S, Anthony KRN (2011) High CO2 enhances the competitive strength of seaweeds over corals. Ecol Lett 14:156–162
Dickson A, Sabine C, Christian J (2007) Guide to best practices for ocean CO2 measurement. In: Dickson A, Sabine C, Christian J (eds) PICES Special Publication 3
Done TJ (1992) Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247:121–132
Edmunds PJ (2011) Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol Oceanogr 56:2402–2410
Edmunds PJ (2015) MCR LTER: coral reef: long-term population and community dynamics: corals, ongoing since 2005. knb-lter-mcr.4.33. doi:10.6073/pasta/1f05f1f52a2759dc096da9c24e88b1e8
Edmunds PJ, Leichter JJ, Johnston EC, Tong EJ, Toonen RJ (2016) Ecological and genetic variation in reef-building corals on four Society Islands. Limnol Oceanogr 61:543–557
Elahi R (2008) Effects of aggregation and species identity on the growth and behavior of mushroom corals. Coral Reefs 27:881–885
Evensen NR, Edmunds PJ, Sakai K (2015) Effects of pCO2 on spatial competition between the corals Montipora aequituberculata and massive Porites spp. Mar Ecol Prog Ser 541:123–134
Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De’ath G, Okazaki R, Muehllehner N, Glas MS, Lough JM (2011) Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat Clim Change 1:165–169
Fine M, Loya Y (2003) Alternate coral–bryozoan competitive superiority during coral bleaching. Mar Biol 142:989–996
Gaylord B, Kroeker KJ, Sunday JM, Anderson KM, Barry JP, Brown NE, Connell SD, Dupont S, Fabricius KE, Hall-Spencer JM, Klinger T, Milazzo M, Munday PI, Russell BD, Sanford E, Schreiber SJ, Thiyagarajan V, Vaughan MLH, Widdicombe S, Harley CDG (2015) Ocean acidification through the lens of ecological theory. Ecology 96:3–15
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hofmann GE, Barry JP, Edmunds PJ, Gates RD, Hutchins DA, Klinger T, Sewell MA (2010) The effect of ocean acidification on calcifying organisms in marine ecosystems: an organism-to-ecosystem perspective. Annu Rev Ecol Evol Syst 41:127–147
Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev 84:1–17
Hurd CL (2015) Slow-flow habitats as refugia for coastal calcifiers from ocean acidification. J Phycol 51:599–605
Idjadi JA, Karlson RH (2007) Spatial arrangement of competitors influences coexistence of reef-building corals. Ecology 88:2449–2454
Jackson JBC, Buss L (1975) Allelopathy and spatial competition among coral reef invertebrates. Proc Natl Acad Sci USA 72:5160–5163
Jokiel P (1978) Effects of water motion on reef corals. J Exp Mar Biol Ecol 35:87–97
Karlson RH, Cornell HV, Hughes TP (2007) Aggregation influences coral species richness at multiple spatial scales. Ecology 88:170–177
Lang JC (1973) Interspecific aggression by scleractinian corals. 2. Why the race is not only to the swift. Bull Mar Sci 23:260–279
Langdon C, Atkinson MJ (2005) Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J Geophys Res 110:1–16
Langdon C, Takahashi T, Sweeney C, Chipman D, Goddard J, Marubini F, Aceves H, Barnett H, Atkinson MJ (2000) Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Glob Biogeochem Cycles 14:639–654
Langdon C, Broecker WS, Hammond DE, Glenn E, Fitzsimmons K, Nelson SG, Peng TH, Hajdas I, Bonani G (2003) Effect of elevated CO2 on the community metabolism of an experimental coral reef. Glob Biogeochem Cycle 17:1011–1025
Lavigne H, Gattuso JP (2013) Seacarb: seawater carbonate chemistry with R, R package version 2.4.8. http://cran.r-project.org/web/packages/seacarb/index.html
Levas S, Grottoli AG, Warner ME, Cai WJ, Bauer J, Schoepf V, Baumann JH, Matsui Y, Gearing C, Melman TF, Hoadley KD, Pettay DT, Hu X, Li Q, Xu H, Wang Y (2015) Organic carbon fluxes mediated by corals at elevated pCO2 and temperature. Mar Ecol Prog Ser 519:153–164
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and losers. Ecol Lett 4:122–131
Mack RN, Harper JL (1977) Interference in dune annuals: spatial pattern and neighborhood effects. J Ecol 65:345–363
Marshall PA, Baird AH (2000) Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19:155–163
Moss RH, Edmonds JA, Hibbard KA, Manning MR, Rose SK, van Vuuren DP, Carter TR, Emori S, Kainuma M, Kram T, Meehl GA, Mitchell JFB, Nakicenovic N, Riahi K, Smith SJ, Stouffer RJ, Thomson AM, Weyant JP, Wilbanks TJ (2010) The next generation of scenarios for climate change research and assessment. Nature 463:747–756
Moya A, Tambutté S, Tambutté E, Zoccola D, Caminiti N, Allemand D (2006) Study of calcification during a daily cycle of the coral Stylophora pistillata: implications for “light enhanced calcification”. J Exp Biol 209:3413–3419
Mumby PJ, van Woesik R (2014) Consequences of ecological, evolutionary and biogeochemical uncertainty for coral reef responses to climatic stress. Curr Biol 24:413–423
Naumann MS, Haas A, Struck U, Mayr C, El-Zibdah M, Wild C (2010) Organic matter release by dominant hermatypic corals of the Northern Red Sea. Coral Reefs 29:649–659
Pratchett MS, McCowan DM, Maynard JA, Heron SF (2013) Changes in bleaching susceptibility among corals subject to ocean warming and recurrent bleaching in Moorea French Polynesia. PLoS One 8:e70443
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Rasband WS (1997) ImageJ. National Institutes of Health, Bethesda
Reidenbach MA, Koseff JR, Monismith SG, Steinbuck JV, Genin A (2006) The effects of waves and morphology on mass transfer within branched reef corals. Limnol Oceanogr 51:1134–1141
Renegar DA, Blackwelder PL, Moulding AL (2008) Coral ultrastructural response to elevated pCO2 and nutrients during tissue repair and regeneration. In: Proceedings of the 11th international coral reef symposium, Ft. Lauderdale, Florida, vol 2, pp 1320–1324
Rinkevich B, Loya Y (1985) Intraspecific competition in a reef coral: effects on growth and reproduction. Oecologia 66:100–105
Romano SL (1990) Long-term effects of interspecific aggression on growth of the reef building corals Cyphastrea ocellina (Dana) and Pocillopora damicornis (Linnaeus). J Exp Mar Biol Ecol 140:135–146
Schoepf V, Grottoli AG, Warner ME, Cai WJ, Melman TF, Hoadley KD, Pettay DT, Hu X, Li Q, Xu H, Wang Y, Matsui Y, Baumann JH (2013) Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS One 8:e750469
Sebens KP, Witting J, Helmuth B (1997) The effects of water flow and branch spacing on particle capture by the reef coral Madracis mirabilis (Duchaissaing and Michelotti). J Exp Mar Biol Ecol 211:1–28
Shamberger KEF, Cohen AL, Golbuu Y, McCorkle DC, Lentz SJ, Barkley HC (2014) Diverse coral communities in naturally acidified waters of a Western Pacific reef. Geophys Res Lett 41:499–504
Spencer-Davies P (1989) Short-term growth measurements of coral growth using an accurate buoyant weighing technique. Mar Biol 101:389–395
Stimson J, Kinzie RA III (1991) The temporal pattern and rate of release of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus) under nitrogen-enrichment and control conditions. J Exp Mar Biol Ecol 153:63–74
Stoll P, Prati D (2001) Intraspecific aggregation alters competitive interactions in experimental plant communities. Ecology 82:319–327
Stoll P, Weiner J (2000) A neighborhood view of interactions among plants. In: Dieckmann U, Law R, Metz JAJ (eds) The geometry of ecological interactions: simplifying spatial complexity. Cambridge University Press, Cambridge, pp 11–27
Tilman D (1994) Competition and biodiversity in spatially structured habitats. Ecology 75:2–16
Veron J (2000) Corals of the world. Australian Institute of Marine Science, Townsville
Washburn L (2015) MCR LTER: Coral Reef: Ocean Currents and Biogeochemistry: salinity, temperature and current at CTD and ADCP mooring FOR01 from 2004 ongoing. knb-lter-mcr.30.30. doi:10.6073/pasta/da2321da9139f3cba86e883c8f7a36a3
Wild C, Niggl W, Naumann MS, Haas AF (2010) Organic matter release by Red Sea coral reef organisms—potential effects on microbial activity and in situ O2 availability. Mar Ecol Prog Ser 411:61–71
Zilberberg C, Edmunds PJ (2001) Competition among small colonies of Agaricia: the importance of size asymmetry in determining competitive outcome. Mar Ecol Prog Ser 221:125–133
Acknowledgments
This research was supported by funding from the National Science Foundation (NSF) to the Moorea Coral Reef, Long-Term Ecological Research site (OCE 12-36905), NSF funding for OA research (OCE 10-41270 and OCE 14-15268), and gifts from the Gordon and Betty Moore Foundation. We are grateful to the staff at the Richard B. Gump South Pacific Research Station for hosting our visit to Moorea, to C. Lantz, V. Moriarty, M. Ho for field assistance in Moorea, and S. Comeau and C. Doropoulos for discussions that improved the quality of this work. We are also grateful to the reviewers for their help to improve the quality of the manuscript. This is contribution number 242 of the California State University, Northridge, Marine Biology Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: J. Grassle.
Reviewed by Undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Evensen, N.R., Edmunds, P.J. Interactive effects of ocean acidification and neighboring corals on the growth of Pocillopora verrucosa . Mar Biol 163, 148 (2016). https://doi.org/10.1007/s00227-016-2921-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2921-z