Abstract
Partial migration is considered ubiquitous among vertebrates, but little is known about the movements of oceanodromous apex predators such as sharks, particularly at their range extents. PAT-Mk10 and SPOT5 electronic tags were used to investigate tiger shark (Galeocerdo cuvier) spatial dynamics, site fidelity and habitat use off eastern Australia between April 2007 and May 2013. Of the 18 tags deployed, 15 recorded information on depth and/or temperature, and horizontal movements. Tracking times ranged between four and 408 days, with two recovered pop-up archival tags allowing 63 days of high-resolution archived data to be analysed. Overall mean proportions of time-at-depth revealed that G. cuvier spent the majority of time-at-depths of <20 m, but undertook dives as deep as 920 m. Tagged sharks occupied ambient water temperatures from 29.5 °C at the surface to 5.9 °C at depth. Deep dives (>500 m) occurred mostly around dawn and dusk, but no definitive daily dive patterns were observed. Horizontal movements were characterised by combinations of resident and transient behaviour that coincided with seasonal changes in water temperature. While the majority of movement activity was focused around continental slope waters, large-scale migration was evident with one individual moving from offshore Sydney, Australia, to New Caledonia (c. 1,800 km) in 48 days. Periods of tiger shark residency outside of Australia’s fisheries management zones highlight the potential vulnerability of the species to unregulated fisheries and the importance of cross-jurisdictional arrangements for species’ management and conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An understanding of spatio-temporal movements of animals is of central importance when assessing the dynamics and interactions within and between populations (Skov et al. 2010). Migration is a specific type of movement that may be persistent or seasonal, often featuring highly directional, long-distance travel (Dingle and Drake 2007; Papastamatiou et al. 2013). Acting at multiple scales, animal migration may influence population structure, govern ecosystem dynamics and influence evolutionary processes and patterns of local and global biodiversity (Nathan et al. 2008). However, in most animal populations, only a proportion of the population migrates—where individuals display either a resident or migrant behaviour—a process known as partial migration (Skov et al. 2010; Broderson et al. 2011; Chapman et al. 2011). In addition to the well-studied variation among species and between populations within a species, often there is within-population migratory behavioural variation that is seldom considered (Chapman et al. 2011). In a growing number of examples, individuals within populations show differences in migratory behaviour, with some individuals migrating between habitats while others remain resident in a single habitat (Dingle 1996).
With recent advances in tagging technology, our capacity to follow the individual movements of animals has led to the growing observation of partial migration and the factors driving it, particularly in aquatic species (Skov et al. 2010; Papastamatiou et al. 2013). These factors include environmental conditions, resource partitioning, ontogenetic diet shift, body condition, reproductive state and predation vulnerability (Chapman et al. 2011; Papastamatiou et al. 2013). As a result, both the timing of migration and the resident/migratory fraction in partially migrating populations are likely to vary between years and between populations (Cagnacci et al. 2011; Mysterud et al. 2011; Broderson et al. 2011). Within aquatic communities, recent studies suggest that all groups of fishes demonstrate partial migration, including oceanodromous top predators such as sharks (Chapman et al. 2012; Papastamatiou et al. 2013). Indeed, the partial migration of predatory elasmobranchs, whose movement patterns may be shaped by the dynamics of the surrounding environment, will have consequences for ecological processes and may influence fisheries management and conservation strategies (Forchhammer et al. 2002; Papastamatiou et al. 2013).
The global decline in shark populations due to overfishing has been documented extensively (Baum et al. 2003; Myers et al. 2007; Lam and Sadovy de Mitcheson 2011; Dulvy et al. 2013). However, the scale of commercial, artisanal and illegal fishing practices differs greatly among oceanic regions. More recently, the implementation of marine protected areas (MPAs) has been successful in reducing shark population declines in some areas (Dulvy et al. 2006; Bond et al. 2012; da Silva et al. 2013), but these areas seldom encompass the full home range of larger shark species (Knip et al. 2012). The southwest Pacific Ocean, comprising the east coast of Australia, New Zealand and many South Pacific Islands, encompasses a complex of exclusive economic zones (EEZ) and territorial sea and archipelago waters interspersed with international high seas. The lack of fishing regulation in international waters, coupled with the vastly different management and monitoring regimes of neighbouring jurisdictions, such as areas in the southwest Pacific, has resulted in increased threats to migratory shark species (Dulvy et al. 2008).
The tiger shark, Galeocerdo cuvier (Péron and Lesueur 1822), is a cosmopolitan species that occurs throughout the tropical and warm-temperate coastal and epipelagic waters of the world (Last and Stevens 2009). Currently listed as ‘Near Threatened’ on the International Union for the Conservation of Nature’s (IUCN) Red List of Threatened Species (Simpfendorfer 2009), G. cuvier can grow to around 550 cm total length (TL) and is the largest species in the family Carcharhinidae (Meyer et al. 2014). As an apex predator, tiger sharks have the ability to exert top-down pressure on marine ecosystems (Heithaus et al. 2008), such that the timing and extent of their movements may affect both population and trophic dynamics across a range of habitats (Skov et al. 2010). Studies of tiger sharks at various locations around the world have reported long-distance movements across the open ocean (Holland et al. 1999; Heithaus et al. 2007; Hammerschlag et al. 2012; Werry et al. 2014) and have revealed that individuals return to specific areas on a regular basis (Lowe et al. 2006; Fitzpatrick et al. 2012). Such site fidelity by some shark species has been attributed to foraging (Meyer et al. 2009), mating (Pratt and Carrier 2001), parturition (Baker et al. 1995) and the use of natal nurseries (Knip et al. 2012). In addition, habitat use may be related to size, with smaller sharks occupying different habitats to larger sharks in order to avoid predation (Lowe et al. 1996). As tiger sharks mature, their movements presumably include elements of exploration that enable them to discover new foraging grounds over time (Meyer et al. 2009). Holland et al. (1999) concluded that individual tiger sharks in Hawaii routinely utilised certain long-distance ‘travel paths’. At high latitudes, seasonal migrations have also been identified (Heithaus 2001; Wirsing et al. 2006). The drivers for these migrations are thought to be changes in water temperature and prey abundance, although the degree to which each of these factors contribute to movement behaviours and habitat use is unknown (Heithaus et al. 2001; Heithaus and Dill 2002; Meyer et al. 2009). More recently, Papastamatiou et al. (2013) surmised that tiger sharks in Hawaii are discretionary partial migrators that use conditional strategies based on both fixed intrinsic states (i.e. age and sex) and flexible extrinsic states (i.e. prey abundance and water temperature) to determine habitat use. However, the study concluded that using horizontal movement data alone could not verify the factors that drive partial migration and that collection of other behavioural data were needed.
On the east coast of Australia, tiger sharks occur seasonally to Merimbula (36°54′S 149°55′E) in southern New South Wales (NSW) during late summer (Last and Stevens 2009). Water conditions during these warmer months are characterised by increased surface flow over the continental shelf by the southward-flowing East Australian Current (EAC), with deeper thermoclines (50–200 m) and stronger eddies into the Coral and Tasman seas (Ridgway and Godfrey 1997; Steinberg 2007). Tiger sharks in these waters are targeted by commercial and recreational fishers (Chan 2001; Williams 2002; Macbeth et al. 2009), as well as by foreign vessels fishing illegally (Field et al. 2009). Governmental ‘culling’ operations through managed shark control programmes have also been in place at selected coastal regions of Queensland (QLD) and NSW for over 50 years (Paterson 1990; Reid and Krough 1992). Despite the range of fisheries that interact with G. cuvier, a lack of species-specific reporting in most Australian commercial fisheries, coupled with the species’ broad geographic distribution, movement capabilities and solitary nature, has made it difficult to determine accurate catch rate and population estimates. While tiger shark populations in tropical north-eastern Australian waters appear stable (Simpfendorfer 1992; Holmes et al. 2012), catch rate declines have been identified in the southern subtropical QLD and warm-temperate regions of NSW (Park 2007; Reid et al. 2011; Holmes et al. 2012). The scale, duration and periodicity of movements of individual tiger sharks likely influence the inter-annual variability observed in local catch rates, particularly at higher-latitude locations (Holmes et al. 2012). Therefore, identifying the extent of resident versus migratory behaviour in these areas is imperative, particularly as catch rates are often used as a proxy for population abundance (Maunder and Punt 2004; Lynch et al. 2012; Tavares et al. 2012). Further, collection of biological and environmental data may identify the intrinsic (i.e. sex and age) and extrinsic (i.e. water temperature) factors needed to develop future population models, better interpret catch rate data and implement region-specific conservation initiatives in the future.
To better understand the movement and behavioural ecology of tiger sharks on the east coast of Australia, the objectives of this study were to: (1) assess tiger shark spatial dynamics, site fidelity and habitat use off eastern Australia, (2) determine whether horizontal and vertical habitat use patterns vary according to shark size and/or sex and (3) identify migratory paths and investigate tiger shark connectivity across the broader western Pacific Ocean.
Materials and methods
Study sites
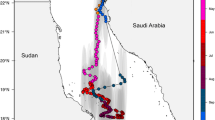
Tiger sharks were captured for tagging in both nearshore and offshore shelf waters at a number of locations throughout south-eastern Australia between April 2007 and April 2012. In QLD, tagging locations included the Sunshine Coast (25° 52′S 152°33′E), Bundaberg (24°30′S 153°15′E) and one location further north at Batt Reef (16°23′S 145°46′E) on the Great Barrier Reef. In NSW, tiger sharks were tagged at four locations on the Central Coast (33°17′S 151°11′E) and at two locations on the South Coast (34°35′S 150°52′E) (Fig. 1).
Specimen capture and electronic tagging
Sharks in nearshore waters were captured using single 18/0 J-hook drumlines baited with either mullet (Mugil cephalus) or unidentified shark flesh. Tiger sharks in offshore waters were attracted using chum (mixed fish mince) and captured on rod and line equipped with a single 10/0 tuna circle hook baited with an oily fish such as small mackerel tuna (Euthynnus affinis) or bonito (Sarda australis). Captured sharks were secured in a head-forward position next to the vessel and secured by a tail rope to ensure the animals remained close to the boat in preparation for tagging. An additional rope was also secured immediately posterior to the pectoral fins. A slow forward boat speed of 1 kn was maintained to ensure that water continued to pass over and the gills to oxygenate the blood. Total length (TL), fork length (FL), pre-caudal length (PCL), sex, tagging location, time and sea surface temperature (SST) were recorded for each shark.
Two types of electronic tags were used to track the movements and habitat utilisation of tiger sharks: the Wildlife Computers Mk10 Pop-up Archival Tag (PAT) and the Smart Position and Temperature Transmitting Tag (SPOT5). A 40-cm hand-held tagging pole was used to attach the PAT to the shark lateral to the base of the dorsal fin. Each PAT was tethered by two 6-cm strands of 135 lb nylon-coated stainless steel leader, crimped to a SPRO heavy swivel and attached to a 5-cm stainless steel dart head that was inserted into the dorsal musculature of the shark. Crimps were further covered using blue heat-shrink plastic tubing. SPOT5s were fitted to the upper portion of the first dorsal fin using nylon bolts passed through the fin and secured with stainless steel nuts. These tags were fitted so that the wet/dry sensor was out of the water when the upper dorsal fin broke the surface of the water.
Tag programming
All PATs were programmed to release after 180 days and then transmit archived data to the Argos system of polar orbiting satellites (www.argos-system.org). Time-at-temperature (°C) and time-at-depth (m) histograms were programmed in 14 user-defined bins. Temperature was measured in 2 °C increments from 6 to >30 °C (resolution = 0.05 °C; accuracy = ±0.1 °C), while depth was measured from 0 m to >1,000 m (resolution = 0.5 m; accuracy = ±1 m for 0–100 m range, ±1 % of reading for 100–1,000 m range). Each tag was programmed to record ambient light, temperature and depth at 10-s intervals. Although these tags could only deliver data aggregated over 4-hourly periods for the specified data bins via the Argos satellites, the entire high-resolution data record could be retrieved from recovered tags. A premature release mechanism was programmed to indicate a mortality event, whereby the tag would detach from the tether if the tag recorded a constant depth (±2 m) for a period greater than 96 h. For the SPOT5s, temperature (°C) was recorded in 12 bins in 2.5 °C increments from <5 to >32 °C. Tags were programmed to transmit location and temperature data to Argos satellites whenever the tag was exposed to the air when the shark was on the surface.
Data analyses
Igor Pro V6.22A and R V2.15.1 (R Core Team 2012) were used to plot vertical habitat use against temperature. Daily positions for PATs were estimated from raw ambient light data using the Wildlife Computers Global Position Estimator (WC-GPE) software (www.wildlifecomputers.com). Dawn/dusk light-level data were extracted using Wildlife Computers Argos Message Processor (WC-AMP), and daily latitudes and longitudes were estimated using WC-GPE. Daily records with poor dawn/dusk light-level curves were excluded from the analyses. Daily positions were estimated using an unscented Kalman filter (UKF), a state-space model applied to light-level measurements using R statistical software (Lam et al. 2008). Position estimates can be improved by matching sea surface temperature (SST) recorded by a tag when near the surface with remotely sensed SST data (Nielsen et al. 2006). Although this was undertaken for our tags, it proved unsuccessful either in improving position estimates or even reducing the variance around the light-only estimates. The reconstruction of movement tracks derived from the position estimates from light-only data was plotted using ArcGIS 10 (www.esri.com). Tracks were then regularized to one position per day to reduce variability associated with temporal frequency of positions (Aebischer et al. 1993), and kernel density was calculated to observe habitat use in ArcGIS [spatial analyst/kernel density]. Step lengths were calculated using geodetic distances calculated in R (Jaine 2013). Rate of movement (ROM) was calculated by dividing step length (in kilometres) between pairs of mean estimated positions by the time (in hours) between position fixes.
Tiger shark diel movements were examined using binned depth data collected by the PAT deployments. Data bins comprised 4 h of recorded information starting at 2000, 0000 hours (midnight), 0400, 0800, 1200 hours (midday) and 1600 hours. Some day/night overlap occurred between the approximate hours of 0400–0600 hours in the morning and 1600–1800 hours in the evening, depending on the time of year. In order to further adjust for potential errors associated with the overlap periods, day/night graphs of depth and temperature were plotted using Igor Pro software. A fast Fourier transform (FFT) analysis was conducted on the full archive data from recovered tags to examine diel periodicity in vertical movements within the water column.
For the SPOT5s, the position of the tag was determined during each transmission by the Argos satellite system. The accuracy of position estimates is reported in seven location classes (LC) of 3, 2, 1, 0, A, B and Z, with LC3 the most reliable (error <250 m), while LC2 = 250–500 m, LC1 = 500–1,500 m, LC0 to LCB = >1,500 m, LCZ = no position (CLS 2011). Position fixes were used from location data with a LC of 3–1 only. Movement and kernel density data were calculated using the same method applied to PATs. Individuals were separated into large (>2.5 m TL) and small (<2.5 m TL) sharks on map plots to establish horizontal movement patterns in relation to size. The magnitude and distribution of errors from Kalman filter location estimates have been assessed previously, with modelled PAT data providing comparable geolocation estimates to SPOT5 data (Holdsworth et al. 2009; Sippel et al. 2011). As such, kernel density is presented as a percentage of daily average positions for all sharks, with the 95 and 75 % contours highlighting core regions of occupancy.
Results
Tag deployments
A total of 18 tiger sharks were tagged with either PATs or SPOT5s off the coastal areas of QLD and NSW (Fig. 1; Table 1). Of these, two appeared lethargic at release (TS#16 and TS#18) and were subsequently presumed to have died as the deployed SPOT5s did not transmit. In addition, despite the release of TS#11 in apparently healthy condition, the deployed PAT failed to transmit. TS#9 appeared to have died after 5 days at liberty as the depth profile indicated a dive of over 1,760 m for longer than 96 h, prompting the premature release of the PAT. Of the 15 tagged sharks yielding data, geolocation maps were estimated for 13 sharks, with TS#5 and TS#6 having insufficient dawn/dusk information to allow the geolocation models to converge. Depth and temperature profiles were, however, obtained from all 15 sharks.
Days at liberty for PAT-tagged tiger sharks were between four and 58 days (mean = 20 days). Fin-mounted SPOT5s remained on fish for longer, transmitting for periods of between 103 and 408 days (mean = 190 days) (Table 1). The quality of the location classes was noticeably reduced after approximately 300 days at liberty, with several weeks lapsing between retrieval of usable LC3–1 quality data. As a result, the distance calculations for TS#15 (Table 1) were restricted to the first 300 days of deployment to reduce the potential error in overall estimates. Two of the PATs were recovered, providing 63 days of high-resolution archived data. Of the remaining nine PATs, 91 ± 4 % (mean and SD) of the transmitted data were successfully decoded. Mean daily movements ranged from 0.42 to 135.9 km day−1 (±40 km day−1), with an average speed of 2.6 km h−1 (±1.74 km h−1).
Season, size and sex
Fishing trips were conducted across all seasons, with most tiger shark catches occurring in NSW from spring to early autumn (11 of 13 captures). Three of five captures in QLD occurred during the winter months. Of the 18 sharks tagged, only three were males, of which only two yielded tracking data. The majority of tiger sharks tagged in this study were either juvenile or sub-adults, with possible exception of five individuals over 310 cm TL (Table 1). While the calcification state of the claspers was not noted, mating scars were not obvious on any of the females examined during tag deployment. Shark size did not influence distances travelled (χ 2 = 0.41, df = 1, p > 0.05) or inshore/offshore habitat preferences. TS#7, however, did show strong site fidelity to the waters near the Noosa region (26°23′S 153°9′E) throughout its 103-day tag deployment. As a result, TS#7 had the highest percentage (28 %) of time at cooler temperatures (18.1–20.6 °C) during winter, in contrast to other sharks that moved offshore into warmer shelf waters.
Vertical habitat use: depth and temperature
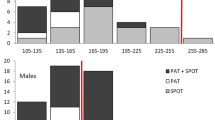
Tiger sharks spent considerable time in the epipelagic zone above the thermocline in depths of less than 100 m, but were recorded diving to depths of up to 920 m. Water temperature ranged from 29.5 °C at the surface and 5.9 °C at depth (Table 1). Overall, binned depth and temperature profile data indicated that tiger sharks spent the majority of their time at shallow depths and warm temperatures. Mean proportions of time-at-depth for PAT-tagged individuals (n = 9) revealed that 59 % of their time was spent at depths of <20 m and 87 % of their time in <50 m (Fig. 2a). The use of the upper water column was also reflected in the water temperature profiles, with over 50 % of the deployment time spent in the 24°–26 °C temperature bin (Fig. 2b). Conversely, SPOT5-tagged individuals (n = 4) spent approximately 60 % of the time in the slightly cooler 20.7–23.2 °C temperature bin (Fig. 2c).
Depth profiles obtained from four-hourly binned data did not indicate any clear diel patterns, though the vertical range was broader during the night (night: 322 ± 138 m; day: 220 ± 263 m; paired t test, t = −4.238, n = 128, p < 0.001) (Fig. 3 for (a) TS#1, (b) #2, (c) #13, (d) #14). Analysis of the full high-resolution archived data from TS#3 indicated that deep dive (>500 m) durations were often brief. For example, the deepest return dive from the surface to 872 m occurred at 0555 h on 21/12/07 and took just 37 min to complete (Fig. 4a). Patterns of oscillatory or yo–yo diving behaviour appeared evident at shallower depths (c. 150–200 m). These dives were characterised by longer durations at depth (≈15 min per dive) and interspersed with regular returns to the surface over many hours (Fig. 4c). Similar dive types were also evident from recovered archived data from the shorter deployment on TS#6 (Fig. 4b, d). The FFT analysis conducted on TS#3 archival data did not identify any diel pattern in diving behaviour.
Horizontal movements and kernel density
High-quality long-term horizontal movement tracks were obtained for two small and two large (±250 cm TL) SPOT5-tagged tiger sharks (TS#7, #10, #15, #17; Fig. 1). The smallest of these individuals (TS#7) remained within 80 km of its tagging location. Two sharks (TS#15 and TS#17) were tagged in temperate NSW waters in the austral autumn and travelled north to subtropical QLD waters during the winter months (June–August). The longest tag deployment (TS#15; 408 days) then returned to NSW waters in the austral spring (October) and continued to move down the east Australian coastline throughout summer to offshore from Eden (37°4′S, 149°54′E) near the NSW/Victoria state border. By April (austral autumn), TS#15 had returned to the offshore waters adjacent to Sydney where it had been tagged at the same time the year before. TS#10 stayed predominantly in QLD waters for the duration of the tag deployment and moved to warmer (>20 °C) offshore shelf waters in the winter months. When transiting the coast, TS#10, #15 and #17 all travelled along the shelf edge, making infrequent visits to nearshore waters. The average ROM for these four sharks over the duration of their tag deployments was 14.8 ± 13 km day−1 (TS#7), 46 ± 61 km day−1 (TS#10), 50 ± 346 km day−1 (TS#15) and 29 ± 60 km day−1 (TS#17), respectively.
Histograms of percentage time-at-depth (4-hourly binned data) for day (grey bars) and night (black bars) for duration of deployment for PAT-tagged a TS#1, b TS#2, c TS#13 and d TS#14. Associated depth-temperature graphs produced by Igor Pro show selected 11-day summary periods for each deployment. White bars indicate day time
The UKF state-space model provided corrected movement tracks for 11 PAT-tagged sharks (Fig. 1). The majority of sharks tagged off NSW maintained a localised (c. 400 km) range between Port Macquarie (31°25′S, 152°54′E) and Bega (36°40′S, 149°49′E). Three sharks (TS#8, TS#13 and TS#14) moved further south past the Victorian state border to offshore Tasman Sea waters. TS#2 undertook the greatest migration (c. 1,800 km straight line distance) from offshore Sydney to Nereus Reef, east of the Chesterfield Islands group in New Caledonian waters. Both PAT-tagged sharks in QLD (TS#9 and TS#12) moved immediately to deeper waters after tagging, with TS#9 travelling from Noosa (26°22′S, 153°9′E) to the QLD/NSW border in 9 days.
Kernel density analysis using the daily locations of each tiger shark showed that the main areas of activity (between 95 and 100 %) for the sharks tagged in this study were off Seal Rocks (32°27′S, 152°31′E) and Port Macquarie in NSW. Other areas of high use (between 50 and 94.9 %) were identified on the Sunshine Coast between Noosa and Double Island Point (25°56′S, 153°11′E) in QLD (Fig. 5). High-use areas in NSW were predominantly confined to continental slope waters, whereas activity in QLD waters was typically in offshore continental shelf waters.
Discussion
This study represents the first to observe migratory behaviours of tiger sharks near to the southern extent of their latitudinal range in the south-western Pacific Ocean. Although the sample size was small (n = 15) and restricted to predominantly female sub-adults, there were several broad similarities in behaviour among individuals including wide-ranging patterns of movement and visitation to the same locations. This is consistent with studies elsewhere that have shown tiger sharks to alternate between localised and extensive movements that may encompass a variety of habitats (Holland et al. 1999; Heithaus et al. 2007; Meyer et al. 2010; Hammerschlag et al. 2012). Further, horizontal movements were similarly characterised by transient behaviour, through directional swimming of up to several hundred kilometres (Holland et al. 1999; Jorgensen et al. 2010; Meyer et al. 2010; Papastamatiou et al. 2011), coupled with smaller scale (<25 km) resident behaviour, through area restricted swimming that repeatedly cover the same areas (Meyer et al. 2009; Jorgensen et al. 2010). Seasonal pole-ward movements into waters over 40°S were also identified, which is further south than previously reported for the species in this region. Daily step lengths recorded in this study provided estimates of movement speed for G. cuvier. Due to the potential statistical errors arising from the accuracy of PAT geolocation data, the geodetic distances calculated for PAT-tagged individuals are indicative only and may not be accurate. Nonetheless, the mean speed (km hour−1) and mean step lengths (km day−1) observed were similar to those reported in other tiger shark tagging studies elsewhere (Holland et al. 1999; Stevens et al. 2000; Kohler and Turner 2001).
In this study, movement paths on the Australian east coast were most often associated with the 200 m shelf-edge isobath or mid-continental shelf areas, with infrequent visits to nearshore waters. Bathymetric features such as the shelf-edge isobath or underwater seamounts may serve as navigational aids, particularly during broad-scale movements (Klimley 1993; Litvinov 2007). This was evident during the migration of TS#2, which after leaving the Australian EEZ travelled along the Lord Howe Rise, a deep-sea marginal plateau surmounted by small volcanic islands and seamounts that is influenced by eddies shed from the EAC (Harris et al. 2012). Ocean currents may also influence tiger shark movements (Hazin et al. 2013), and the seasonal fluctuations in the strength of the southward-flowing EAC likely contribute to the time spent in offshore waters in this region. Indeed, the unique oceanography parallel to the Australian coast between 32° and 39°S is known as ‘Eddy Avenue’, an area commonly containing large anticyclonic eddies causing sea surface temperature anomalies in the region. Smaller cyclonic eddies are also common and promote higher chlorophyll a levels (Everett et al. 2012). Warmer temperatures and higher levels of primary productivity may explain the use of these habitats by tiger sharks, particularly during the austral summer.
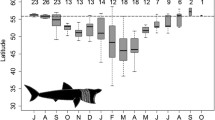
Latitudinal range extension during the summer months was realised through long-term (>100 day) deployments of SPOT5 tags. Retraction from temperate NSW waters into subtropical QLD waters occurred when water temperatures dropped below 19 °C (July–September) and when the southward-flowing EAC is at its weakest (Ridgway and Godfrey 1997). Interestingly, both sharks that were SPOT5-tagged in NSW undertook very similar travel paths north into QLD waters at the same time of year. These movements, coupled with reduced commercial and recreational catches of sharks in NSW in the winter months, indicate that perhaps year-round residency at latitudinal extremes (>30°S) for this species is rare. Indeed, targeting behaviour of shark game fishers in NSW shifts to short fin mako (Isurus oxyrinchus) in the colder months due to the scarcity of tiger sharks (Stevens 1984; Pepperell 2008). Further, due to historically low catches of ‘dangerous’ sharks in winter, including tiger sharks, since 1982 the NSW Shark Meshing Program has routinely removed shark nets from beaches in the May–August period each year to mitigate against whale entanglements (Green et al. 2009). Although latitudinally lower than NSW, Heithaus (2001) also reported a significant reduction in winter catch rates of tiger sharks when water temperatures dropped below 20 °C in Shark Bay, Western Australia (25°45′S, 113°44′E). Of the two long-term SPOT5s deployed in QLD, both individuals remained in the subtropics into late spring when other tagged sharks were observed returning to NSW. This residency behaviour is supported by year-round commercial fishing and QLD shark control captures of tiger shark in southern QLD (DEEDI, unpublished data; Holmes et al. 2012), indicating that individual decisions to move within warmer subtropical waters may not be influenced by extrinsic temperature factors alone. Indeed, Papastamatiou et al. (2013) found that variations in warmer water temperatures (23–26 °C), coupled with chlorophyll a concentrations, were probably proxies for marine productivity, thus influencing tiger shark utilisation of other areas.
Despite a seemingly clear correlation with water temperature, there are other extrinsic factors that may influence shark movements, such as prey availability. Such factors are harder to identify, although annual, seasonal movement of tiger sharks to particular foraging areas has been documented (Lowe et al. 1996; Fitzpatrick et al. 2012). Indeed, the stronger EAC currents flowing southward during the warmer months mark a seasonal biome shift in this region, which influences the distribution of pelagic fishes such as tunas, kingfish, mackerels and billfishes (Kailola et al. 1993; Gillanders et al. 2001; Lowry and Murphy 2003; Zischke et al. 2012), as well as spawning activity by deep-sea fishes on seamounts (Rowling et al. 2010). Offshore movements of tiger sharks in Hawaii have also been linked to patterns in oceanic productivity (Meyer et al. 2010). Seasonal habitat use is likely employed by tiger sharks as an important feeding strategy as it can facilitate the exploitation of different prey arenas, reduces competition among conspecifics and may afford them a level of surprise on unwary prey (Papastamatiou et al. 2006; Meyer et al. 2009).
Given the maturity state of the individuals in this study, it is unlikely that the use of the southern sites by these sharks was due either to mating or parturition. Consideration of other intrinsic states (i.e. age and sex) on the collective movement of tiger sharks was difficult due to the limited number of long-term tagged animals of both sexes. The strong site fidelity to inshore habitat exhibited by TS#7, a 180-cm TL male, was markedly different from other small female tiger sharks tagged offshore in this study (e.g. TS#8, TS#13) which exhibited much wider-ranging movements. Although the ability for juveniles to undertake wide-ranging movements has been documented elsewhere (Meyer et al. 2009; Papastamatiou et al. 2013), intraspecific differences in habitat use may also be a behavioural feature of tiger sharks (Vaudo et al. 2014). Meyer et al. (2010) surmised that different patterns of behaviour may result from unique, individual learning experiences, such as learning to exploit a particular prey patch. This might serve as a mechanism for intraspecific resource partitioning and may give rise to prey specialisation among individuals. Vertical habitat use of surface and deep waters (>500 m) was a ubiquitous trait exhibited by all sharks regardless of size. While tiger sharks in this region made occasional excursions below the thermocline (>100 m), the majority of time (72 %) was spent in the upper mixed layer between 0 and 30 m. Frequent use of the upper 5 m of the water column (29 %) was consistent with other tiger sharks from the north-west Atlantic (Vaudo et al. 2014), but was surprisingly in contrast to other G. cuvier tagged off of northern Australia and Hawaii, which spent the majority of the time-at-depths around 50–100 m. Minimum temperatures recorded during deep diving excursions of around 6–7 °C in this study were unexpected given the species’ tropical classification. In addition to their deep diving behaviour, occasional residence in cooler nearshore waters in winter and movements south into temperate waters indicate that tiger sharks are clearly capable of occupying cooler water masses for reasonable periods, but still spend the majority of their time at the highest water temperature ‘available’ during their migrations (Brill et al. 1993).
Analysis of the full archived dive data from TS#3 and TS#6 revealed complex vertical use of the water column. Brief deep dives to over 500 m were made throughout the tag deployments and typically occurred around dawn and dusk. These dives were often characterised by rapid, almost vertical descents to well below the mixed layer followed by more gradual ascents to the surface, which is consistent with the powered swimming performed by tiger sharks in Hawaii (Nakamura et al. 2011). By contrast, continual shallower dives to around the thermocline (150–200 m) over several hours were also observed, with periods of up to 15 min spent at depth before returning to the surface. Previous studies have collectively coined these vertical movements as oscillatory or ‘yo–yo’ dives and attributed them to a range of potential behaviours, including thermoregulation, swimming efficiency, foraging and navigation (Carey and Scharold 1990; Holland et al. 1999; Klimley et al. 2002; Heithaus et al. 2002; Weng et al. 2007; Nakamura et al. 2011; Vaudo et al. 2014). The distinctive differences between singular ‘deep’ diving and concurrent ‘oscillating’ dive behaviour identified in this study may be indicative of two discrete diving behaviours in tiger sharks. Holland et al. (1999) postulated that brief deep dives of tiger sharks in Hawaii served as a mechanism of orientation between shallow banks. Indeed, the most consistent deep diving behaviour observed in the present study was undertaken by migrating TS#2, which correlated directly with the time of its directional swimming along the Lord Howe Rise strongly suggesting that it may have been using the plateau topography as a navigational aid. Although data were binned for this animal, the recovered tags from TS#3 and TS#6 revealed that all dives below the thermocline were brief, with total excursions not exceeding ≈ 30 min. Deep orientation diving to the platform edge has also been identified in north-west Atlantic tiger sharks (Vaudo et al. 2014), indicating that this behaviour is probably ubiquitous across the species. Oscillating or ‘yo–yo’ dive behaviours were characterised by a sequence of shallower dives followed by regular returns to surface waters over several hours. Based on location data for TS#3, dives to 150–200 m were not associated with the continental shelf edge. More likely, these dives are undertaken to the edge of the deep thermocline created by the summer flow of the EAC along this coastline (Steinberg 2007). Other studies focusing on tiger shark dive behaviours using data loggers and high-rate data recording tags also found that depth distributions did not appear to be related to horizontal movements, thermoregulation, sex or size factors and that yo–yo diving might be an optimal search strategy to detect prey (Nakamura et al. 2011; Vaudo et al. 2014). As such, we suggest that the yo–yo diving observed here is also consistent with prey searching behaviour, whereby olfactory cues that disperse along the horizontal layers may be encountered with the highest probability (Klimley et al. 2002; Weng et al. 2007). Concomitantly, tiger sharks appear to rely on stealth as a foraging tactic and using the water column vertically in this way allows them to attack prey from below, reducing the number of escapes routes particularly for near surface-dwelling prey (Heithaus et al. 2002). A diet study by Chan (2001) revealed that the major taxonomic group found in the stomachs of tiger sharks in NSW was shearwaters (Puffinus spp.) (41.9 %), with a high proportion also consisting of cetaceans (19.4 %). Selection for these prey in temperate waters when other favoured warm-water species are not available (i.e. marine turtles, sea snakes; Simpfendorfer 1992) likely influences the diving and hunting strategies employed by tiger sharks in this region.
By deploying satellite tags on tiger sharks to identify patterns of habitat use and the environmental determinants of movement and migration, it is immediately apparent that they are tolerant of a range of environments while also able to actively respond to environmental dynamics and disperse into new habitats. This was evident from the residency behaviour interspersed with the highly directional transient movements observed in this study. Coupled with the vertical habitat use data, tiger sharks in this region clearly undertake migrations from subtropical QLD to exploit the seasonally warm and prey abundant waters of NSW. There was no evidence of year-round residency in these southern waters, perhaps indicating that contrary to the partial migration observed in subtropical waters, ‘complete’ migration may occur at the latitudinal extent for this species. While we acknowledge that the small sample size of this study may not have identified winter residents in NSW, we demonstrate that inter-annual variability in local abundances is considerable. Further, considering the inter-individual variation in both horizontal and vertical movements, it is likely that tiger sharks are incidental in their exposure to most fisheries and only as subset of the population will be vulnerable to local fisheries at a given time (Vaudo et al. 2014). As such, care should be taken when interpreting catch rate information as an indicator of population abundance of highly mobile marine animals, particularly at their latitudinal extent. With the identification of broad-scale migration occurring across the Coral Sea, local conservation initiatives alone may not be adequate in reducing the threats facing migratory sharks in this region. Our findings further emphasise the need to address marine conservation issues at both a local and an international scale.
References
Aebischer NJ, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74:1313–1325
Baker JD, Antonelis GA, Fowler CW, York AE (1995) Natal site fidelity in northern fur seals, Callorhinus ursinus. Anim Behav 50:237–247
Baum JK, Myers RA, Kehler DG, Worm B, Harley SJ, Doherty PA (2003) Collapse and conservation of shark populations in the northwest Atlantic. Science 299:389–392
Bond ME, Babcock EA, Pikitch EK, Abercrombie DL, Lamb NF, Chapman DD (2012) Reef sharks exhibit site fidelity and higher relative abundance in marine reserves on the Mesoamerican barrier reef. PLoS One 7(3). doi:10.1371/journal.pone.0032983
Brill RW, Holts DB, Chang RKC, Sullivan S, Dewar H, Carey FG (1993) Vertical and horizontal movements of striped marlin (Tetrapturus audax) near the Hawaiian Islands, determined by ultrasonic telemetry, with simultaneous measurement of oceanic currents. Mar Biol 117:567–574
Broderson J, Nicolle A, Nilsson PA, Skov C, Brönmark C, Hansson L (2011) Interplay between temperature, fish partial migration and trophic dynamics. Oikos 120:1838–1846. doi:10.111/j.1600-0706.2011.19433.x
Cagnacci F, Focardi S, Heurich M, Stache A, Hewison AJM, Morellet N, Kjellander P, Linnell JDC, Mysterud A, Neteler M, Delucchi L, Ossi F, Urbano F (2011) Partial migration in roe deer,: migratory and resident tactics are end points of a behavioural gradient determined by ecological factors. Oikos 120:1790–1802. doi:10.1111/j.1600-0706.2011.19441.x
Carey FG, Scharold JV (1990) Movements of blue shark (Prionace glauca) in depth and course. Mar Biol 106:329–342
Chan RWK (2001) Biological studies on sharks caught off the east coast of New South Wales. PhD thesis. University of New South Wales, Australia
Chapman BB, Brönmark C, Nilsson J, Hansson L (2011) The ecology and evolution of partial migration. Oikos 120:1764–1775. doi:10.1111/j.1600-0706.2011.20131.x
Chapman BB, Hulthén K, Broderson J, Nilsson PA, Skov C, Hansson LA, Brönmark C (2012) Partial migration in fishes: causes and consequences. Fish Biol 81:456–478. doi:10.1111/j.1095-8649.2012.03342.x
CLS (2011) Argos Users Manual. http://www.argos-system.org. Accessed 17 Aug 2011
da Silva C, Kerwath SE, Attwood CG, Thorstad EB, Cowley PD, Okland PD, Wilke CG, Naesje TF (2013) Quantifying the degree of protection afforded by no-take marine reserve on an exploited shark. Africa J Mar Sci 35:57–66. doi:10.2989/1814232X.2013.769911
Dingle H (1996) Migration: the biology of life on the move. Oxford University Press, New York
Dingle H, Drake VA (2007) What is Migration? BioOne 57(2):113–121
Dulvy NK, Jennings S, Rogers SI, Maxwell DL (2006) Threat and decline in fishes: an indicator of marine biodiversity. Can J Fish Aquat Sci 63:1267–1275
Dulvy NK, Baum JK, Clarke S, Compagno LJV, Cortés E, Domingo A, Fordham S, Fowler S, Francis MP, Gibson C, Martínez J, Musick JA, Soldo A, Stevens JD, Valenti S (2008) You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays. Aquat Conserv 18:459–482
Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM, Harrison LR, Carlson JK, Davidson LNK, Fordham SV, Francis MP, Pollock CM, Simpfendorfer CA, Burgess GH, Carpenter KE, Compagno LJV, Ebert DA, Gibson C, Heupel MR, Livingstone SR, Sanciangco JC, Stevens JD, Valenti S, White WT (2013) Extinction risk and conservation of the world’s sharks and rays. eLIFE 3. doi:10.7554/eLife.00590
Everett JD, Baird ME, Oke PR, Suthers IM (2012) An avenue of eddies: quantifying the biophysical properties of mesoscale eddies in the Tasman Sea. Geophys Res Lett 39:LI6608. doi:10.1029/2012GL053091
Field IC, Meekan MG, Buckworth RC, Bradshaw CJA (2009) Protein mining the world’s oceans: Australasia as an example of illegal expansion-and-displacement fishing. Fish Fish 10(3):323–328
Fitzpatrick R, Thums M, Bell I, Meekan MG, Stevens JD, Barnett A (2012) A comparison of the seasonal movements of tiger sharks and green turtles provides insight into their predator-prey relationship. PLoS One 7(12). doi:10.1371/journal.pone.0051927
Forchhammer MC, Post E, Stenseth NC (2002) North Atlantic Oscillation timing of long- and short-distance migration. J Anim Ecol 71:1002–1014
Gillanders BM, Ferrell DJ, Andrew NL (2001) Estimates of movements and life-history parameters of yellow-tail kingfish (Seriola lalandi): how useful are data from a cooperative tagging programme? Mar Fres Res 52:179–192
Green M, Ganassin C, Reid D (2009) Report into the NSW shark meshing (bather protection) program. New South Wales, Australia
Hammerschlag N, Gallagher AJ, Wester J, Luo J, Ault JS (2012) Don’t bite the hand that feeds: assessing ecological impacts of provisioning ecotourism on an apex marine predator. Funct Ecol 26:567–576
Harris PT, Nichol SL, Anderson TJ, Heap AD (2012) Habitats and Benthos of a Deep-Sea Marginal Plateau, Lord Howe Rise, Australia. In: Harris PT, Baker EK (eds) Seafloor geomorphology as benthic habitat. Elsevier, USA. ISBN: 978-0-12-385140-6
Hazin FHV, Afonso AS, De Castilho PC, Ferreira LC, Rocha BCLM (2013) Regional movements of the tiger shark, Galeocerdo cuvier, off northeastern Brazil: inferences regarding shark attack hazard. Ann Braz Acad Sci 85(3):1053–1062
Heithaus MR (2001) The biology of tiger sharks, Galeocerdo cuvier, in Shark Bay, Western Australia: sex ratio, size distribution, diet, and seasonal changes in catch rates. Environ Biol Fish 61(1):25–36
Heithaus MR, Dill LM (2002) Food availability and tiger shark predation risk influence bottlenose dolphin habitat use. Ecology 83(2):480–491
Heithaus MR, Marshall GJ, Buhleier BM, Dill LM (2001) Employing Crittercam to study habitat use and behavior of large sharks. Mar Ecol Prog Ser 209:307–310
Heithaus MR, Dill LM, Marshall GJ, Buhleier B (2002) Habitat use and foraging behavior of tiger sharks (Galeocerdo cuvier) in a seagrass ecosystem. Mar Biol 140(2):237–248
Heithaus MR, Wirsing AJ, Dill LM, Heithaus LI (2007) Long-term movements of tiger sharks satellite-tagged in Shark Bay, Western Australia. Mar Biol 151:1455–1461
Heithaus MR, Frid A, Wirsing AJ, Worm B (2008) Predicting ecological consequences of marine top predator declines. Trends Ecol Evol 23(4):202–210
Holdsworth JC, Sippel TJ, Block BA (2009) Near real time satellite tracking of striped marlin (Kajikia audax) movements in the Pacific Ocean. Mar Biol 156:505–514
Holland KN, Wetherbee BM, Lowe CG, Meyer CG (1999) Movements of tiger sharks (Galeocerdo cuvier) in coastal Hawaiian waters. Mar Biol 134(4):665–673
Holmes BJ, Sumpton WD, Mayer DG, Tibbetts IR, Neil DT, Bennett MB (2012) Declining trends in annual catch rates of the tiger shark (Galeocerdo cuvier) in Queensland, Australia. Fish Res 129–130:38–45
Jaine FRA (2013) The movement ecology of the reef manta ray Manta alfredi in eastern Australia. PhD Thesis, The University of Queensland, Australia
Jorgensen SJ, Reeb CA, Chapple TK, Anderson S, Perle C, Van Sommeran SR, Fritz-Cope C, Brown AC, Klimley AP, Block BA (2010) Philopatry and migration of Pacific white sharks. Proc R Soc B-Biol Sci 277:679–688. doi:10.1098/rspb.2009.1155
Kailola PJ, Williams MJ, Stewart PC, Reichelt RE, McNee A, Grieve C (1993) Australian fisheries resource. FRDC, Canberra
Klimley AP (1993) Highly directional swimming by scalloped hammerhead sharks, Sphyrna lewini, and subsurface irradiance, temperature, bathymetry, and geomagnetic field. Mar Biol 117:1–22
Klimley AP, Beavers SC, Curtis TH, Jorgensen SJ (2002) Movements and swimming behaviour of three species of sharks in La Jolla Canyon, California. Environ Biol Fish 63:117–135
Knip DM, Heupel MR, Simpfendorfer CA (2012) To roam or to home: site fidelity in a tropical coastal shark. Mar Biol 159:1647–1657
Kohler NE, Turner PA (2001) Shark tagging: a review of conventional methods and studies. Environ Biol Fish 60:191–223
Lam VYY, Sadovy de Mitcheson Y (2011) The sharks of South East Asia—unknown, unmonitored and unmanaged. Fish Fish 12:51–74. doi:10.1111/j.1467-2979.2010.00383.x
Lam CH, Nielsen A, Sibert JR (2008) Improving light and temperature based geolocation by unscented Kalman filtering. Fish Res 91:15–25
Last PR, Stevens JD (2009) Sharks and rays of Australia, 2nd edn. CSIRO Publishing, Australia
Litvinov F (2007) Fish visitors to seamounts: aggregations of large pelagic sharks above seamounts. Fish Aqua Res Ser 12:202–206
Lowe CG, Wetherbee BM, Crow GL, Tester AL (1996) Ontogenetic dietary shifts and feeding behavior of the tiger shark, Galeocerdo cuvier, in Hawaiian waters. Environ Biol Fish 47(2):203–211
Lowe CG, Wetherbee BM, Meyer CG (2006) Using acoustic telemetry monitoring techniques to quantify movement patterns and site fidelity of sharks and giant trevally around French Frigate Shoals and Midway Atoll. Atoll Res Bull 543:281–303
Lowry M, Murphy J (2003) Monitoring the recreational gamefish fishery off southeastern Australia. Mar Fresh Res 54:425–434
Lynch PD, Shertzer KW, Latour RJ (2012) Performance of methods used to estimate indices of abundance for highly migratory species. Fish Res 125–126:27–39. doi:10.1016/j.fishres.2012.02.005
Macbeth WG, Geraghty PT, Peddemors VM, Gray CA (2009) Observer-based study of targeted commercial fishing for large shark species in waters off northern New South Wales. Industry and Investment New South Wales, Cronulla
Maunder MN, Punt AE (2004) Standardizing catch and effort: a review of recent approaches. Fish Res 70:141–159. doi:10.1016/j.fishres.2004.08.002
Meyer CG, Clark TB, Papastamatiou YP, Whitney NM, Holland KN (2009) Long-term movement patterns of tiger sharks Galeocerdo cuvier in Hawaii. Mar Ecol Prog Ser 381:223–235
Meyer CG, Papastamatiou YP, Holland KN (2010) A multiple instrument approach to quantifying the movement patterns and habitat use of tiger (Galeocerdo cuvier) and Galapagos sharks (Carcharhinus galapagensis) at French Frigate Shoals, Hawaii. Mar Biol 157:1857–1868
Meyer CG, O’Malley JM, Papastamatiou YP, Dale JJ, Hutchinson MR, Anderson JM, Royer MA, Holland KN (2014) Growth and maximum size of tiger sharks (Galeocerdo cuvier) in Hawaii. PLoS One 9(1):e84799. doi:10.1371/journalpone.0084799
Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH (2007) Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315:1846–1850
Mysterud A, Loe LE, Zimmermann B, Bischof R, Veiberg V, Meisingset E (2011) Partial migration in expanding red deer populations in northern latitudes—a role for density dependence? Oikos 120:1817–1825. doi:10.1111/j.1600-0706.2010.19439.x
Nakamura I, Wantanabe YY, Papastamatiou YP, Katsufumi S, Meyer CG (2011) Yo-yo vertical movements suggest a foraging strategy for tiger sharks Galeocerdo cuvier. Mar Ecol Prog Ser 424:237–246
Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE (2008) A movement ecology paradigm for unifying organismal movement research. Proc Nat Acad 105:19052–19059. doi:10.1073/pnas.0800375105
Nielsen A, Bigelow KA, Musyl MK, Sibert JR (2006) Improving light-based geolocation by including sea surface temperature. Fish Ocean 15(4):314–325. doi:10.1111/j.1365-2419.2005.00401.x
Papastamatiou YP, Wetherbee BM, Lowe CG, Crow GL (2006) Distribution and diet of four species of carcharhinid shark in the Hawaiian Islands: evidence for resource partitioning and competitive exclusion. Mar Ecol Prog Ser 320:239–251
Papastamatiou YP, Cartamil DP, Lowe CG, Meyer CG, Wetherbee BM, Holland KN (2011) Scales of orientation, directed walks and movement path structure in sharks. J Anim Ecol 80:864–874. doi:10.1111/j.1365-2656.2011.01815.x
Papastamatiou YP, Meyer CG, Carvalho F, Dale JJ, Hutchinson MR, Holland KN (2013) Telemetry and random walk models reveal complex patterns of partial migration in a large marine predator. Ecol Soc Am 94:2595–2606. doi:10.1890/12-2014.1
Park T (2007) NSW gamefish tournament monitoring. Angling research tournament monitoring program. NSW Department of Primary Industries, Cronulla
Paterson RA (1990) Effects of long-term anti-shark measures on target and non-target species in Queensland, Australia. Biol Conserv 52:147–159
Pepperell JG (2008) Monitoring and research on landed fish at game fishing tournaments in NSW. Pepperell Research and Consulting Pty Ltd, Australia
Pratt HL, Carrier JC (2001) A review of elasmobranch reproductive behaviour with a case study on the nurse shark, Ginglymostoma cirratum. Environ Biol Fish 60(1–3):157–188
R Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN: 3-900051-07-0, URL http://www.R-project.org/
Reid DD, Krough M (1992) Assessment of catches from protective shark meshing off New South Wales beaches between 1950 and 1990. Aust J Mar Fres Res 43:283–296. doi:10.1071/MF9920283
Reid DD, Robbins WD, Peddemors VM (2011) Decadal trends in shark catches and effort from the New South Wales Shark Meshing Program 1950 to 2010. Mar Fresh Res 62:676–693
Ridgway KR, Godfrey JS (1997) Seasonal cycle of the East Australian Current. J Geophys Res 102(10):921–936
Rowling K, Hegarty A, Ives M (2010) Status of fisheries resources in NSW 2008/09. NSW Industry and Investment, Cronulla
Simpfendorfer C (1992) Biology of tiger sharks (Galeocerdo cuvier) caught in the Queensland shark meshing program off Townsville, Australia. Aust J Mar Fres Res 43:33–43. doi:10.1071/MF9920033
Simpfendorfer C (2009) Galeocerdo cuvier. The IUCN Red List of Threatened Species. Version 2014.2. www.iucnredlist.org
Sippel T, Holdsworth J, Dennis T, Montgomery J (2011) Investigating behaviour and population dynamics of striped marlin (Kajikia audax) from the southwest Pacific Ocean with satellite tags. PLoS One 6(6). doi:10.1371/journal.pone.0021087
Skov C, Aarestrup K, Baktoft H, Brodersen J, Brönmark C, Hansson L, Nielsen EE, Nielsen T, Nilsson PA (2010) Influences of environmental cues, migration history, and habitat familiarity on partial migration. Behav Ecol 21:1140–1146. doi:10.1093/beheco/arq121
Steinberg C (2007) Impacts of climate change on the physical oceanography of the Great Barrier Reef. In: Johnson JE, Marshall PA (eds) Climate change and the great barrier reef. Great Barrier Reef Marine Park Authority and Australian Greenhouse Office, Australia
Stevens JD (1984) Biological observations on sharks caught by sport fishermen off New South Wales. Aust J Mar Fres Res 35:573–590
Stevens JD, West GJ, McLoughlin KJ (2000) Movements, recapture patterns, and factors affecting the return rate of carcharhinid and other sharks tagged off northern Australia. Mar Fresh Res 51:127–141
Tavares R, Ortiz M, Arocha F (2012) Population structure, distribution and relative abundance of the blue shark (Prionace glauca) in the Caribbean Sea and adjacent waters of the North Atlantic. Fish Res 129–130:137–152. doi:10.1016/j.fishres.2012.06.018
Vaudo JJ, Wetherbee BM, Harbey G, Nemeth RS, Aming C, Burnie N, Howey-Jordan LA, Shivji MS (2014) Intraspecific variation in vertical habitat use by tiger sharks (Galeocerdo cuvier) in the western North Atlantic. Ecol Evol 4(10):1768–1786
Weng KC, Boustany AM, Pyle P, Anderson SD, Brown A, Block BA (2007) Migration and habitat of white sharks (Carcharhodon carcharias) in the eastern Pacific Ocean. Mar Biol 152(4):877–894. doi:10.1007/s00227-007-0739-4
Werry JM, Planes S, Berumen ML, Lee KA, Braun CD, Clua E (2014) Reef-fidelity and migration of tiger sharks, Galeocerdo cuvier, across the Coral Sea. PLoS One 9(1):e83249. doi:10.1371/journal.pone.0083249
Williams LE (2002) Queensland’s fisheries resources: current conditions and recent trends 1988–2000. Department of Primary Industries, Queensland
Wirsing AJ, Heithaus MR, Dill LM (2006) Tiger shark (Galeocerdo cuvier) abundance and growth in a subtropical embayment: evidence from 7 years of standardized fishing effort. Mar Biol 149:961–968
Zischke MT, Griffiths SP, Tibbetts IR (2012) Catch and effort from a specialised recreational pelagic sport fishery off eastern Australia. Fish Res 127–128:61–72
Acknowledgments
We wish to acknowledge the contribution of those who donated their time, effort and funds in support of the tagging operations in this study, especially Greg Barea, Paddy Dimond, Mark Doohan, Tony Ham, Wayne Sumpton, Jeff Krause, Matt Ghosn, David Toohey, Jamie Ward, Anthony Sawa, Mark Cawthray, Michael Swindells, and Drs. Adrian Gutteridge, Lindsay Marshall and Jonathan Werry. We also give our deepest gratitude to the numerous volunteers that helped with fieldwork, especially Aaron Davison and Simon Harmon. Thanks also to Tim Lam, Tu Nguyen, Robert Campbell and Nadia Engstrom for help with tag analysis, mapping and commercial fishing information. We would like to acknowledge the research sponsors Fellowship Fund Incorporated, F.G. Wilson Pty. Ltd., RipCom Telecommunications, Elanora State School, Mohammed Bin Zayed Species Conservation Fund, Discovery Channel, Wildlife Preservation Society of Queensland, Fisheries Queensland, New South Wales Recreational Fishing Trust and the New South Wales Game Fishing Association. In-kind support was gratefully received from the Moreton Bay Research Station, Tangalooma Wild Dolphin Resort and the Fox Research Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Righton.
Rights and permissions
About this article
Cite this article
Holmes, B.J., Pepperell, J.G., Griffiths, S.P. et al. Tiger shark (Galeocerdo cuvier) movement patterns and habitat use determined by satellite tagging in eastern Australian waters. Mar Biol 161, 2645–2658 (2014). https://doi.org/10.1007/s00227-014-2536-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2536-1