Abstract
The combined effects of exposure to copper and temperature were investigated in adult specimens and germlings of the canopy-forming brown alga Fucus serratus. A matrix of four temperatures, 6, 12, 17 and 22 °C, and three concentrations of copper, 0, 100 and 1,000 nM total copper were used. Measured endpoints were growth rate, chlorophyll fluorescence parameters and for germlings also survival. The growth rate of adult specimens of F. serratus changed with increasing temperature. Growth tended to be negatively affected by high concentrations of copper when exposed to heat (22 °C) though not significantly so. The photosynthetic performance (i.e., chlorophyll fluorescence parameters: F v/F m, maximum electron transport rate (ETRmax) and maximum non-photosynthetic quenching (NPQmax) of adults was largely unaffected by both copper and temperature. Germling survival, growth rate and chlorophyll fluorescence parameters were affected by the combination of copper concentration and temperature. Increasing temperature led to reduced survival, increased rhizoid growth and higher F v/F m and ETRmax, whereas high copper concentration had a negative effect on the latter three endpoints. The negative effect of high copper concentration was amplified by high temperature. We conclude that juveniles of F. serratus are more susceptible to environmental stressors than adult specimens and recommend therefore including early life stages when assessing the risk of exposure to toxic compounds. Considering the response of adult specimens only may lead to false conclusions regarding the ecological impact of environmental stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brown macroalgae, belonging to the genus Fucus, form extensive populations and constitute the foundation species of intertidal and subtidal communities on temperate rocky shores. These macrophyte assemblages support marine ecosystems by providing food and shelter for various marine invertebrates and fish, as well as substratum for epiphytic algae (Begin et al. 2004). While brown macroalgae may resist some degree of environmental stress, their long-term persistence depends on their reproductive ability as well as the survival and growth of early life history stages (germlings) that are generally more susceptible to natural and anthropogenic stressors than adults (Andersson and Kautsky 1996; Steen 2004a; Fredersdorf et al. 2009). There is a definite risk that fucoids are threatened as a result of increased exposure to natural and human-induced stressors such as extreme temperatures, excess nutrients and toxic compounds. Brown algal habitats are already under threat from anthropogenic pollution (Kevekordes 2000), and effects of pollution are likely to act on both adult and germlings performance (Kevekordes and Clayton 2000; Nygard and Dring 2008). The range distribution of marine algae is largely determined by water temperature (Lüning 1984), and ongoing changes in sea water temperature are therefore expected to cause changes in the north–south (latitudinal) distribution of many marine algae (Adey and Steneck 2001; Muller et al. 2009; Harley et al. 2012). Such changes have already been documented for inter-tidal seaweeds, such as fucoids (Wernberg et al. 2011; Diez et al. 2012). Variations in temperature may be caused by local or large-scale fluctuations or by climate change. A study on the effects of human-induced stress on the survival of brown macroalgae should therefore evolve around synergistic effects of temperature and pollution agents on germling performance in addition to adult brown algae performance. Most fucoids are considered to be cold-temperate species (Lüning 1984), and temperatures above 20 °C are generally considered unsuitable for these algae (Wiencke et al. 1994; Zou et al. 2012), although exceptions from this pattern are known (Nygard and Dring 2008).
It is well known that exposure to contaminants, especially heavy metals, may reduce the growth and photosynthesis of brown macroalgae (Connan and Stengel 2011). Recent studies showed, in contrast, that adult specimens of Fucus serratus seem rather tolerant to quite high concentrations of copper (Eklund and Kautsky 2003; Nielsen and Nielsen 2010). A few studies have shown that germlings are more susceptible to metal-induced stress than adult specimens (Andersson and Kautsky 1996; Bond et al. 1999). The performance of adult brown algae is often assessed as growth, while that of germlings is assessed by measuring germination success and rhizoid elongation. However, previous research (Nielsen et al. 2003a; Nielsen and Nielsen 2005) has indicated that there are at least two distinctive targets for environmental stress in brown algae: photosynthesis on the one hand and growth and rhizoid germination and elongation on the other.
The effect of high temperature stress on photosynthesis in brown algae is related to inactivation of enzymes and the induction of reactive oxygen species (ROS), leading to photoinhibition (Suzuki and Mittler 2006). Induction of ROS results in cellular damage and may also be induced by pollutant exposure (Nielsen et al. 2003a) in brown algae. Photoinhibition may thus be induced both as a result of temperature stress and as a result of exposure to contaminants, e.g., heavy metals, and may cause reduced energy fixation and growth. Growth rates of adult brown macroalgae may finally be affected by temperature through the increase in metabolic rates (Nygard and Dring 2008). Heavy metals have a direct adverse effect on growth of algae in addition to the inhibitory effects on photosynthesis through effects on cell wall loosening and cell division (Xylander et al. 1998; La Rocca et al. 2009).
The toxicity mechanism of anthropogenic pollution in germling development is well documented (Bond et al. 1999; Nielsen et al. 2003a). Germination and rhizoid elongation occur by tip growth where apical expansion of the rhizoid is directed by a Ca2+ gradient at the rhizoid apex (Roberts et al. 1993), and it has previously been shown that heavy metals inhibit rhizoid germination and elongation by selectively attenuating the growth-conducting Ca2+ signal at the rhizoid apex, presumably by inhibiting the entry of external Ca2+ into the cell (Nielsen et al. 2003a). Information on specific targets for temperature stress on germling development is more sparse (Steen 2004b; Steen and Rueness 2004; Steen and Scrosati 2004), but most likely involves the metabolic enzymatic processes, supporting rhizoid germination and elongation, being slowed down as a result of temperature stress.
There are thus at least two distinctive targets for environmental stress in brown algae. Inhibition of photosynthesis may represent a general target for environmental stress that is different from the processes involved in growth and rhizoid development that are inhibited directly by heavy metal pollution. While algal performance is normally determined by measuring growth in adults and germination success and rhizoid elongation in germlings, this approach alone is not sufficient to assess stress responses. Parallel studies of the stress response of the photosynthetic apparatus must be carried out to complement the assessment of growth and rhizoid development and provide more complete information of algal responses to environmental stress. Information on synergistic effects of pollutants and temperature or other stressors on brown algae germlings must be collected and compared with effects on adult specimens in order to fully assess the potential threat from the combined effects of high temperature and anthropogenic pollution.
In this study, we test the effects of temperature and copper on growth, survival and photosynthetic parameters in adult specimens and germlings of the fucoid brown algae F. serratus. Adult specimens and germlings were grown in a matrix of four different temperatures and three different concentrations of copper. We chose to test temperatures that are both higher and lower than present day summer means in the North Sea region. As F. serratus releases its gametes from July to September (Knight and Parke 1950; Malm et al. 2001), the two intermediate temperatures were chosen to bracket the diurnal mean temperatures during the warmest month of the year in northern Europe (around 15 °C). We hypothesized that lower and higher temperatures (here represented by 6 and 22 °C, respectively) would constitute environmental stress, defined as a significant deviation from the optimal condition of life (Larcher 2001), and that these effects would be exacerbated by the presence of a pollutant, in this case copper. The copper concentrations are selected so that the intermediate and the high concentration are representative of concentrations in the Baltic straits open water and in polluted harbor waters, respectively (Pohl et al. 1993; Pohl and Hennings 1999).
Materials and methods
Adults
Collection and culturing
Adult specimens of F. serratus were collected at low tide from Belhaven Bay on the SE of the Firth of Forth, Scotland, UK, in March 2008 at a water temperature of 5–6 °C. Samples were transported to the laboratory on ice within 1.5 h of collection. In the laboratory, vegetative apical frond tips of approximately 3 cm length were allowed to recover from cutting for 5 days in aerated filtered ultraviolet (UV)-treated seawater at 15 °C (Nielsen and Nielsen 2005, 2010). The fronds were then placed in 150 ml incubation medium in individual aerated beakers placed in temperature-adjusted water baths at 6, 12, 17 and 22 °C for 18 days. The incubation medium was made from artificial seawater (ASW) prepared using Aquil (Morel et al. 1979). This medium contains macro- and micronutrients, essential trace metals as well as vitamins. Copper was added as CuSO4 from the beginning of the 18-day period in the following concentrations: 0, 100 and 1,000 nM total copper (Cu T ), corresponding to 0, 42 and 422 nM free Cu2+. The medium was changed 3 times per week. An irradiance of 400 μmol m−2 s−1 was provided on a 16:8-h light/dark cycle during both the 5-day recovery period and during the following experimental incubation, using white (daylight spectrum) metal–halide lamps. Five individual fronds from different adult individuals in separate beakers were subjected to each treatment (n = 5). The following endpoints were measured:
Growth
Relative growth rates were based on the change in fresh weight. The fronds were carefully wiped with soft tissue paper to remove excess water from the surface before being weighted. Relative growth rate was calculated as \(\frac{{\varvec{In }W_{t} - \varvec{In }W_{o} }}{t}\), where W t is the fresh weight at day t, W 0 is the fresh weight at day 0 and t is the number of days (18 days) between the two measurements.
Chlorophyll fluorescence parameters
Chlorophyll fluorescence parameters were measured at the same temperatures that the fronds were incubated at on a Hansatech FMS 1 PAM (Hansatech, King’s Lynn, UK), using default factory settings. Fronds were dark adapted for 15 min, and F 0 and F m were determined prior to each series of measurements. Subsequently, F m and F t were determined using a light curve with 8 different irradiances gradually increasing from 0 to 1,200 μmol m−2 s−1, each irradiance lasting 60 s (Nielsen and Nielsen 2008). The light source in this PAM is a broad-spectrum halogen lamp that provides both the actinic light and the saturation pulses. During the measurements, the fronds were placed in petri dishes with ASW. The optical fiber was kept at a fixed working distance of 4 mm from the frond surface, kept in place by the provided Hansatech leaf-clips. Thallus light absorbance for calculation of relative electron transport rate (rETR) was based on transmission measurements using a Hansatech QRT1 light meter (Hansatech, King’s Lynn, UK) and found to average 0.912 ± 0.019. The average was used for calculations of ETR values. Chlorophyll fluorescence parameters (F v, F m, ETR and NPQ) were calculated according to Maxwell and Johnson (2000).
Germlings
Gamete acquisition and culture of zygotes
Adult F. serratus were collected and transported to the laboratory as described above. Mature receptacles were cut from male and female algae, blotted dry and stored in the dark at 3–5 °C for up to 2 weeks. Gamete release was stimulated by rinsing receptacles in tap water and exposing them to natural day light for 45 min. Transfer of receptacles to the same ASW as described above (Morel et al. 1979) induced gamete release (Nielsen et al. 2003a). The concentration of gametes was adjusted by measuring the absorbance of gametes in ASW in a 1-ml cuvette on a spectrophotometer. The sperm concentration was kept constant at approximately 4.25 × 106 cells ml−1, which was equivalent to an absorbance of 0.2 at a wavelength of 480 nm, the primary absorption wavelength of carotenoids. The egg concentration was kept constant at approximately 200 ml−1, which was equivalent to an absorbance of 0.1 at a wavelength of 450 nm, the primary absorption wavelength of chlorophyll. Mixing of male and female gametes in ASW induced fertilization. The resulting zygotes were filtered through a 100-μm mesh into fresh ASW (Nielsen et al. 2003a). The zygotes were cultured in small petri dishes fitted with cover slip bases onto which the zygotes were sown at a density of approximately 200 per dish. After settlement and initial attachment of zygotes, the dishes were filled with 5 ml ASW. Germlings were transferred to the following copper concentrations: 0, 100 and 1,000 nM total copper (Cu T ), corresponding to 0, 42 and 422 nM free Cu2+ (Nielsen et al. 2003a, b, 2005) 24 h after fertilization. The medium was changed at 2, 4 and 6 days after fertilization. To determine survival, growth rate, measured as rhizoid elongation, and photosynthesis, germlings were plated in petri dishes in ASW at the same copper concentrations at an approximate density of 100 cm−2.
Petri dishes and beakers were placed in incubators at 6, 12, 17 and 22 °C at an irradiance of 100 μmol m−2 s−1 in a 16:8-h light/dark cycle. Germlings were cultured for a total of 7 days (until 7 days after fertilization), where the following endpoints were measured: germling survival, rhizoid length (as a proxy for growth rate) and chlorophyll fluorescence parameters. The whole experiment was repeated three times, each time with a different batch of germlings (n = 3). Batches were run in parallel to avoid any seasonal effects.
Germling survival
Germling survival was determined as the number of live germinated germlings out of a total of 80 germinated germlings for each treatment, evaluated under a microscope at 10× magnification. A live germinated germling is one that evinces cell division after germination (Brownlee and Bouget 1998).
Rhizoid length
Digital images were recorded of 20–40 germlings (unrelated to treatment combination) for each treatment at 10× magnification (Sony Cyber-Shot, 3.3 megapixels, Sony Corporation, Tokyo, Japan). The rhizoid length was defined as the distance from the wall dividing the thallus from the rhizoid to the rhizoid tip and was measured using the image analysis SOFTWARE SIGMA SCAN PRO v. 5.
Chlorophyll fluorescence parameters
The following parameters were recorded: maximum quantum yield of PSII (F v/F m), nonphotochemical quenching (NPQ) and ETR (Maxwell and Johnson 2000). All parameters were determined on a microscopy PAM (Walz, Effeltrich, Germany) using default factory settings at the respective treatment temperatures. Germlings were dark adapted for 15 min, and F 0 and F m were determined prior to each series of measurements. Subsequently, F m and F t were determined at eight different irradiances, gradually increasing from 0 to 520 μmol m−2 s−1. Each step lasted 30 s. The light source in this PAM is a blue light emitting diode (LED) (470 nm) that provides both the actinic light and the saturation pulses. A generic light absorbance of 84 % was used to calculate ETR values (Maxwell and Johnson 2000).
Statistical analysis
Adults
Adult response variables (growth and chlorophyll fluorescence parameters) were subjected to two-way ANOVA with temperature and Cu concentration as fixed factors. Tukey’s test was subsequently used to compare individual means across significantly different treatment levels. Data were tested for homogeneity of variance (Cochran’s test) and normal distribution (Kolmogorov–Smirnoff goodness of fit test) before being analyzed by ANOVA. All tests on data from adult specimens were carried out using SYSTAT v. 13 with α = 0.05.
Germlings
Randomized Block ANOVA was used to test the effects of temperature (4 levels fixed) and Cu (3 levels fixed) on germling survival, rhizoid length and fluorescence parameters (F v/F m, ETRmax and NPQmax). The germlings used in the experiment originated from three different batches, each being used for one set of replicated treatments, and these batches were therefore considered as “blocks” (random factor). We followed the recommendation by Quinn and Keough (2002) and used a nonadditive model and separate error terms to estimate appropriate F ratios and P values for the main effects and their interaction. As opposed to the traditional use of additive models for testing main effects in Randomized Block designs, this approach is not affected by the potential presence of Block × factor interactions, the downside being that there is no specific test for the effect of Block and no tests for the Block × factor interactions. F ratios were estimated as: F Temp = MSTemp/MSTemp×Block, F Cu = MSCu/MSCu×Block and F Temp×Block = MSTemp×Cu/MSTemp×Cu×Block (Table 10.6 in Quinn and Keough (2002)). All analyses were conducted using permutational ANOVA on PERMANOVA + for PRIMER. Analyses were executed using Type III sum of squares on geometric (Euclidean) distances and unrestricted permutation (9,999 times) of raw data (Anderson et al. 2008). All tests were carried out using α = 0.05.
Results
Adult Fucus
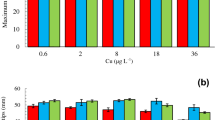
The relative growth rate of adult F. serratus (Fig. 1) ranged from 0.9 to 2.8 % d−1 across treatments and was only affected significantly by temperature, but not by copper concentration and not by the interaction between temperature and copper (Table 1). The mean growth rate (across levels of Cu) at low temperature (6 °C) was 25–50 % lower than those at higher temperatures (12–22 °C). Exposure to the highest concentration of Cu had a marked (albeit the interaction was not significantly affected; p = 0.088) effect at 22 °C, where the mean growth rate was reduced by ca. 50 % relative to that in the control treatment (0 nmol Cu 22 °C). Maximum quantum yield (F v/F m) of adult Fucus averaged 0.694 ± 0.063 (mean ± SD) across all treatments (Fig. 2) and was only affected by temperature, but neither by copper nor by the Temperature × Cu interaction (Table 1). ETRmax and NPQmax averaged 7.38 ± 3.14 (mean ± SD) and 86.38 ± 23.59, respectively, across all treatments (Fig. 2), but none of these response variables were affected significantly by any of the treatments (Table 1).
Relative growth rates (% d−1) of adult F. serratus specimens as a function of the various temperatures (°C) and copper concentrations employed in this work. Open bars (left) total copper concentration (Cu T ) 0 nM, hatched bars (center) total copper concentration 100 nM, cross-hatched bars (right) total copper concentration 1,000 nM. Mean values ± 1 SE (n = 5) are shown. The total copper concentrations correspond to 0, 42 and 422 nM free Cu2+, respectively
Chlorophyll fluorescence parameters for adult F. serratus kept at the various temperatures (°C) and copper concentrations. Top panel shows maximum quantum efficiency (F v/F m); mid panel shows maximum electron transport rate (ETRmax); bottom panel shows maximum nonphotosynthetic quenching (NPQmax). Open bars (left) total copper concentration (Cu T ) 0 nM, hatched bars (center) total copper concentration 100 nM, cross-hatched bars (right) total copper concentration 1,000 nM. Mean values ± 1 SE (n = 5) are shown. The total copper concentrations correspond to 0, 42 and 422 nM free Cu2+, respectively
Fucus germlings
Germling survival ranged from 100 to 82 % depending on treatment (Fig. 3) and was affected by both temperature and copper (Table 2). Survival decreased with increasing temperature as well as with increasing copper concentration. Although the negative effect of copper seemed stronger at higher temperatures (i.e., 17 and 22 °C), there was no significant interaction between the two factors.
Relative germling survival at the various temperatures (°C) and copper concentrations, employed in this work. Open bars (left) total copper concentration (Cu T ) 0 nM, hatched bars (center) total copper concentration 100 nM, cross-hatched bars (right) total copper concentration 1,000 nM. Mean values ± 1 SE (n = 3) are shown. The total copper concentrations correspond to 0, 42 and 422 nM free Cu2+, respectively
Germling growth rate (rhizoid elongation; Fig. 4) ranged from 170 to 710 μm (Table 2). Increasing temperature had a significantly positive effect on growth (Table 2), which increased almost threefold (at 0 nM Cu) from 236 µm at 6 °C to 694 µm at 22 °C. At the same time, germling growth decreased significantly with increasing copper exposure, so that germlings exposed to 1,000 nM total copper grew markedly slower than germlings exposed to no or low concentrations of copper. The negative effect of Cu became more evident with increasing temperature.
Germling rhizoid elongation in µm at the various temperatures (°C) and copper concentrations employed in this work. Open bars (left) total copper concentration (Cu T ) 0 nM, hatched bars (center) total copper concentration 100 nM, cross-hatched bars (right) total copper concentration 1,000 nM. Mean values ± 1 SE (n = 3) are shown. The total copper concentrations correspond to 0, 42 and 422 nM free Cu2+, respectively
All chlorophyll fluorescence parameters (i.e., F v/F m, ETRmax and NPQmax; Fig. 5) were affected by the interaction between temperature and copper (Table 2). F v/F m increased from 0.45 at 6 to 0.63 at 22 °C, indicating that increasing temperature alleviated photostress in the germlings. Exposure to copper had no effect on F v/F m at 6 °C, but lowered F v/F m at 12, 17 and 22 °C, respectively. ETRmax was low at 6 °C (3.21 ± 0.27) and increased significantly from 6 to 12 °C (14.40 ± 1.95) at 0 nM Cu (Fig. 5; Table 2), while there was no further increase in ETRmax at higher temperatures. Exposure to copper had no effect on ETR at 6 °C, but it had a strong negative effect at the three higher temperatures. NPQmax showed the exact opposite trend of ETRmax (Fig. 5). NPQmax was highest at 6 °C (5.69 ± 0.79) and significantly lower at 12 °C (2.93 ± 0.10) at 0 nM Cu, but did not decrease any further with increasing temperatures above 12 °C. As for ETRmax, NPQmax was affected by exposure to copper at the three highest temperatures, but not at 6 °C, so that NPQmax was higher at 1,000 nM copper than at 0 or 100 nM copper.
Chlorophyll fluorescence parameters for F. serratus germlings at the various temperatures (°C) and copper concentrations. Top panel shows maximum quantum efficiency (F v/F m); mid panel shows maximum electron transport rate (ETRmax); bottom panel shows maximum nonphotosynthetic quenching (NPQmax). Open bars (left) total copper concentration (Cu T ) 0 nM, hatched bars (center) total copper concentration 100 nM, cross-hatched bars (right) total copper concentration 1,000 nM. Mean values ± 1 SE (n = 3) are shown. The total copper concentrations correspond to 0, 42 and 422 nM free Cu2+, respectively
Discussion
The growth rate of adult F. serratus responded negatively to low temperature (6 °C), but was not affected negatively by the highest temperature used in the present experiment (22 °C). The negative effect of heat stress on algal growth that has been reported for many brown algal species (e.g., Wiencke et al. 1994) was thus not seen in F. serratus. This may seem surprising, given that temperature effects on fucoid algae have been documented in other studies (Nygard and Dring 2008; Dethier and Williams 2009; Martinez et al. 2012), but these studies, in addition to interactions with other stressors, also indicated seasonal variation in temperature responses, a factor not included in the present study. The slow growth observed at 6 °C must have been due to a general decline in metabolic processes at low temperature, but it was not related to a decrease in photosynthetic performance, measured as chlorophyll fluorescence, since the chlorophyll fluorescence data were largely unaffected by temperature. This is not in accordance with other studies showing temperature effects on chlorophyll fluorescence parameters in fucoids as well as in other macroalgae (e.g., Martinez et al. 2012; Andersen et al. 2013), but may be related to seasonal or genotypic variations. Exposure to the highest concentration of copper (1,000 nM) had a marked negative effect on growth (50 % reduction) at 22 °C, although this effect was only marginally significant (p = 0.088). The highest copper concentration used in the present study corresponds to that found in the most polluted harbors in the Baltic Sea area (Pohl et al. 1993; Pohl and Hennings 1999), and it is offsetting the positive effect of high temperature. In contrast, copper had no apparent effect on F. serratus growth at lower temperatures. This finding corresponds to previous findings that adult F. serratus seem to have a relatively high tolerance to heavy metal exposure (Nielsen and Nielsen 2010).
A different pattern became, however, evident when we tested how Fucus germlings responded to temperature and exposure to copper. Germling survival was negatively affected by the highest temperature, although the survival rate only declined by 8–12 % compared with the highest survival, which was found at 6 °C. Survival rate was also significantly affected by the presence of copper, but only at the highest concentration. This supports previous findings that the effect of environmental stressors on Fucus germlings is temperature dependent (Altamirano et al. 2003). Although the survival rate declined with increasing temperatures, the growth rate (rhizoid elongation) of the surviving germlings increased threefold as the temperature was raised from 6 to 22 °C. As previously documented in the literature (Andersson and Kautsky 1996; Steen and Rueness 2004; Fredersdorf et al. 2009), germlings were more sensitive to heavy metal exposure than adult specimens of F. serratus, although they were only significantly affected by the highest copper concentration used. While growth rates increased by almost 300 % with increasing temperature in the copper-free control, they did not vary significantly with temperature at the highest copper concentration. The negative effect of copper on germling growth observed in this study is undoubtedly due to the direct negative effect that copper has on cell division and elongation (Bond et al. 1999), but our data also indicate that copper had a negative effect on the photosynthetic performance of the germlings (Fig. 5). The chlorophyll fluorescence data showed patterns consistent with the observed variations in growth rates of Fucus germlings; the maximum quantum yield was positively correlated to temperature, indicating that low temperatures induce photostress in the germlings, unlike what was found for adult individuals. Values for F v/F m remained lower than for the adults even at the highest temperature, which was also the case for ETRmax, indicating that the photosynthetic machinery was not being fully developed in germlings. F v/F m and ETRmax decreased, indicating induction of photostress, and NPQmax increased with increasing copper concentrations, which may have resulted from the generation of ROS (Nielsen et al. 2003a). The reason we find a coupling between photostress and reduced growth in germlings, but not in adults, is probably that germlings have thin thalli, one cell layer thick, while the adults have a thick thallus. It has previously been shown (Nielsen and Nielsen 2005, 2008) that the thick thallus in fucoid algae compensates for photostress in the uppermost cell layers, where chlorophyll fluorescence is measured, as cells deeper in the thallus increase their photosynthetic rate when photosynthesis is reduced in the outmost cell layers.
It is clear that the highest copper concentration used here does have negative effects on survival, growth and photosynthetic performance in F. serratus germlings and that these negative effects are exacerbated with increasing temperatures. However, it should also be kept in mind that both growth rate and photosynthetic rates increased with increasing temperatures even in germlings stressed most by high copper concentration. This indicates that the increasing temperature, at least partly, offsets the negative effects of high copper concentrations. We hypothesize that the high metabolic rate, associated with higher temperatures, enhances detoxifying mechanisms and/or regeneration of the photosynthetic system.
Our results show that early life history stages of F. serratus are more susceptible to environmental stressors than adult specimens. This is probably a general pattern, not only among fucoid algae, but in macroalgae in general (Steen 2004a; Fredersdorf et al. 2009). Adult specimens were not affected by exposure to copper (within the ranges used in this experiment) and only slightly affected by the temperatures that we exposed them to. This could lead to the conclusion that F. serratus is not affected seriously by anthropogenic pollution and ongoing climate changes. A different picture emerged when germlings were considered; these were more susceptible to contaminants and increasing temperatures than the adult specimens. It is therefore necessary to include early life stage responses in the assessment of effects of environmental changes on fucoid algae—and probably also other macroalgae—as only considering fully developed adults specimens may lead to false conclusions. We argue that growth and survival are meaningful parameters for the assessment of effects of environmental stress on organisms, but also that chlorophyll fluorescence may be used as a sensitive, early warning, tool indicating the onset of environmental stress on a physiological level (Baumann et al. 2009) and should therefore be included in studies on the effects of environmental stressors on photosynthetic organisms.
References
Adey WH, Steneck RS (2001) Thermogeography over time creates biogeographic regions: a temperature/space/time-integrated model and an abundance-weighted test for benthic marine algae. J Phycol 37:677–698
Altamirano M, Flores-Moya A, Figueroa FL (2003) Effects of UV radiation and temperature on growth of germlings of three species of Fucus (Phaeophyceae). Aquat Bot 75:9–20
Andersen GS, Pedersen MF, Nielsen SL (2013) Temperature acclimation and heat tolerance of photosynthesis in Norwegian Saccharina latissima (Laminariales, Phaeophyceae). J Phycol 49:689–700
Anderson WH, Gorley RN, Clarke KR (2008) Permanova + for Primer. Guide to software and statistical methods. PRIMER-E Ltd., Plymouth
Andersson S, Kautsky L (1996) Copper effects on reproductive stages of Baltic Sea fucus vesiculosus. Mar Biol 125:171–176
Baumann HA, Morrison L, Stengel DB (2009) Metal accumulation and toxicity measured by PAM-chlorophyll fluorescence in seven species of marine macroalgae. Ecotoxicol Environ Saf 72:1063–1075
Begin C, Johnson LE, Himmelman JH (2004) Macroalgal canopies: distribution and diversity of associated invertebrates and effects on the recruitment and growth of mussels. Mar Ecol Prog Ser 271:121–132
Bond PR, Brown MT, Moate RM, Gledhill M, Hill SJ, Nimmo M (1999) Arrested development in Fucus spiralis (Phaeophyceae) germlings exposed to copper. Eur J Phycol 34:513–521
Brownlee C, Bouget FY (1998) Polarity determination in fucus: from zygote to multicellular embryo. Semin Cell Dev Biol 9:179–185
Connan S, Stengel DB (2011) Impacts of ambient salinity and copper on brown algae: 1. Interactive effects on photosynthesis, growth, and copper accumulation. Aquat Toxicol 104:94–107
Dethier MN, Williams SL (2009) Seasonal stresses shift optimal intertidal algal habitats. Mar Biol 156:555–567
Diez I, Muguerza N, Santolaria A, Ganzedo U, Gorostiaga JM (2012) Seaweed assemblage changes in the eastern Cantabrian Sea and their potential relationship to climate change. Estuar Coast Shelf Sci 99:108–120
Eklund BT, Kautsky L (2003) Review on toxicity testing with marine macroalgae and the need for method standardization—exemplified with copper and phenol. Mar Pollut Bull 46:171–181
Fredersdorf J, Muller R, Becker S, Wiencke C, Bischof K (2009) Interactive effects of radiation, temperature and salinity on different life history stages of the Arctic kelp Alaria esculenta (Phaeophyceae). Oecologia 160:483–492
Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA, Graham MH (2012) Effects of climate change on global seaweed communities. J Phycol 48:1064–1078
Kevekordes K (2000) The effects of secondary-treated sewage effluent and reduced salinity on specific events in the early life stages of Hormosira banksii (Phaeophyceae). Eur J Phycol 35:365–371
Kevekordes K, Clayton MN (2000) Development of Hormosira banksii (Phaeophyceae) embryos in selected components of secondarily-treated sewage effluent. J Phycol 36:25–32
Knight M, Parke M (1950) A biological study of Fucus vesiculosus and F. serratus. J Mar Biol Assoc UK 29:87–90
La Rocca N, Andreoli C, Giacometti GM, Rascio N, Moro I (2009) Responses of the Antarctic microalga Koliella antarctica (Trebouxiophyceae, Chlorophyta) to cadmium contamination. Photosynthetica 47:471–479
Larcher W (2001) Physiological plant ecology. Ecophysiology and stress physiology of functional groups. Springer, Berlin
Lüning K (1984) Temperature tolerance and biogeography of seaweeds—the marine algal flora of Helgoland (North Sea) as an example. Helgolander Meeresuntersuchungen 38:305–317
Malm T, Kautsky L, Engkvist R (2001) Reproduction, recruitment and geographical distribution of Fucus serratus L. in the Baltic Sea. Bot Mar 44:101–108
Martinez B, Arenas F, Rubal M, Burgues S, Esteban R, Garcia-Plazaola I, Figueroa FL, Pereira R, Saldana L, Sousa-Pinto I, Trilla A, Viejo RM (2012) Physical factors driving intertidal macroalgae distribution: physiological stress of a dominant fucoid at its southern limit. Oecologia 170:341–353
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Morel FMM, Rueter JG, Anderson DM, Guillard RRL (1979) Aquil: a chemically defined phytoplankton culture medium for trace metal studies. J Phycol 15:135–141
Muller R, Laepple T, Bartsch I, Wiencke C (2009) Impact of oceanic warming on the distribution of seaweeds in polar and cold-temperate waters. Bot Mar 52:617–638
Nielsen HD, Nielsen SL (2005) Photosynthetic responses to Cu2+ exposure are independent of light acclimation and uncoupled from growth inhibition in Fucus serratus (Phaeophyceae). Mar Pollut Bull 51:715–721
Nielsen HD, Nielsen SL (2008) Evaluation of imaging and conventional PAM as a measure of photosynthesis in thin- and thick-leaved marine macroalgae. Aquat Biol 3:121–131
Nielsen HD, Nielsen SL (2010) Adaptation to high light irradiances enhances the photosynthetic Cu2+ resistance in Cu2+ tolerant and non-tolerant populations of the brown macroalgae Fucus serratus. Mar Pollut Bull 60:710–717
Nielsen HD, Brown MT, Brownlee C (2003a) Cellular responses of developing Fucus serratus embryos exposed to elevated concentrations of Cu2+. Plant Cell Environ 26:1737–1747
Nielsen HD, Brownlee C, Coelho SM, Brown MT (2003b) Inter-population differences in inherited copper tolerance involve photosynthetic adaptation and exclusion mechanisms in Fucus serratus. New Phytol 160:157–165
Nielsen HD, Burridge TR, Brownlee C, Brown MT (2005) Prior exposure to Cu contamination influences the outcome of toxicological testing of Fucus serratus embryos. Mar Pollut Bull 50:1675–1680
Nygard CA, Dring MJ (2008) Influence of salinity, temperature, dissolved inorganic carbon and nutrient concentration on the photosynthesis and growth of Fucus vesiculosus from the Baltic and Irish Seas. Eur J Phycol 43:253–262
Pohl C, Hennings U (1999) Bericht zum Ostsee-Monitoring: die Schwermetall-Situation in der Ostsee im Jahre 1999. Institut für Ostseeforschung
Pohl C, Kattner G, Schulzbaldes M (1993) Cadmium, copper, lead and zinc on transects through arctic and eastern Atlantic surface and deep waters. J Mar Syst 4:17–29
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Roberts SK, Berger F, Brownlee C (1993) The role of Ca2+ in signal-transduction following fertilization in Fucus serratus. J Exp Biol 184:197–212
Steen H (2004a) Effects of reduced salinity on reproduction and germling development in Sargassum muticum (Phaeophyceae, Fucales). Eur J Phycol 39:293–299
Steen H (2004b) Interspecific competition between Enteromorpha (Ulvales: chlorophyceae) and Fucus (Fucales : phaeophyceae) germlings: effects of nutrient concentration, temperature, and settlement density. Mar Ecol Prog Ser 278:89–101
Steen H, Rueness J (2004) Comparison of survival and growth in germlings of six fucoid species (fucales, Phaeophyceae) at two different temperature and nutrient levels. Sarsia 89:175–183
Steen H, Scrosati R (2004) Intraspecific competition in Fucus serratus and F. evanescens (phaeophyceae : fucales) germlings: effects of settlement density, nutrient concentration, and temperature. Mar Biol 144:61–70
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126:45–51
Wernberg T, Russell BD, Thomsen MS, Gurgel CFD, Bradshaw CJA, Poloczanska ES, Connell SD (2011) Seaweed communities in retreat from Ocean warming. Curr Biol 21:1828–1832
Wiencke C, Bartsch I, Bischoff B, Peters AF, Breeman AM (1994) Temperature requirements and biogeography of antarctic, arctic and amphiequatorial seaweeds. Bot Mar 37:247–259
Xylander M, Fischer W, Braune W (1998) Influence of mercury on the green alga Haemotococcus lacustris. Inhibition effects and recovery of impact. Botanica Acta 111:467–473
Zou DH, Liu SX, Du H, Xu JT (2012) Growth and photosynthesis in seedlings of Hizikia fusiformis (Harvey) Okamura (Sargassaceae, Phaeophyta) cultured at two different temperatures. J Appl Phycol 24:1321–1327
Acknowledgments
HDN was supported by a grant from the Danish Natural Science Research Council. The council had no involvement in study design, data collection and interpretation, or writing and submitting the paper. We thank Theresa Fernandes and Paul Tett at Napier University, Edinburgh, for housing and supporting HDN during work on this project. We thank Dr. Gary T. Banta for advice on statistical methods.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Weinberger.
Rights and permissions
About this article
Cite this article
Nielsen, S.L., Nielsen, H.D. & Pedersen, M.F. Juvenile life stages of the brown alga Fucus serratus L. are more sensitive to combined stress from high copper concentration and temperature than adults. Mar Biol 161, 1895–1904 (2014). https://doi.org/10.1007/s00227-014-2471-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2471-1