Abstract
Body size during larval development is one of the most important attributes of aquatic animals. The optimal size for changing form or habitat may vary according to ecological traits of species, while phylogenetic constraints also play a significant role. The major goal of this study is to reveal the patterns in the settlement size of planktotrophic larvae in an archaic gastropod superorder Neritimorpha. We take advantage of the fact that size at various ontogenetic stages of neritimorphs can be rigorously estimated by measuring features of the adult opercula. This unique feature of neritimorphs has allowed us to generate the largest data set so far on larval settlement sizes within a group of marine invertebrates that recruit into very different post-metamorphic habitats. Eighty-eight species that represent most extant genera from rocky shores, seagrass beds, mangroves, estuaries, streams, submarine caves, deep-sea vents or seeps showed negligible intraspecific variation and considerable interspecific differences in settlement size, particularly between genera or families. Settlement size is determined primarily by phylogenetic constraints, while parallel evolution toward smaller sizes was shown to occur exclusively in four independent clades (two living and two extinct) of amphidromous snails with a marine larval period followed by a limnic adult phase. The smaller settlement size may possibly reduce the risk of being wafted away from the estuaries of their natal streams through less time achieving metamorphic competence, while ability to make occasional long-distance trips is retained by the presence of a sufficiently long delay period. This delay period also seems to obscure the possible correlation between settlement size and geographic distribution range of neritimorph species, both fully marine and amphidromous.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Body size during larval development is one of the most important attributes of aquatic invertebrates with complex life cycles from both an ecological and evolutionary standpoint (Marshall and Keough 2007). In particular, special attention has been paid to the size at metamorphosis, which can be affected by selection on the initial size of offspring as well as the optimal size for changing form or habitat (Strathmann 1993). Intraspecific variation of settlement size is associated not only with maternal effects, but also influences post-settlement mortality and growth rate in planktotrophic species (Phillips 2002; Giménes 2010). Larger size at metamorphosis enables adaptation to harsh environmental conditions, including exposure to desiccation, predation and starvation (Spight 1976; Moran and Emlet 2001). On the other hand, interspecific variation of settlement size has been often attributed to the ecological characteristics of the species. For example, predatory caenogastropods that feed on active prey after metamorphosis tend to have larger settlement sizes than grazing herbivores or carnivores feeding on sessile animals (Lesoway and Page 2008). Settlement size may be related to the sediment characteristics of settling areas in bivalves, possibly because their fragile larvae need to be larger than sediment grains (Cardoso et al. 2006). However, phylogenetic constraints and ancestral conditions have rarely been taken into consideration when discussing size differences with respect to various ecological traits (Hadfield and Switzer-Dunlap 1984; Kohn and Perron 1994; Levitan 2000; Collin 2003). Settlement size is often used in taxonomic studies to diagnose genera and families that may contain species with different diets and habitats (e.g., Kano and Kase 2002; Knowlton and Vargo 2004).
Shell-bearing gastropods are ideal subjects for the interspecific comparison of settlement size, because they flourish in almost all aquatic environments from intertidal to hadal waters as well as in freshwater ponds and streams (Kano et al. 2002), and because a calcified shell is a reliable indicator of overall body size and its rigidity allows accurate measurement (Lesoway and Page 2008). The metamorphosis of their planktotrophic larvae, which often become competent to metamorphose at some earlier point in their development, is generally instigated by particular chemical or physical cues (Pechenik 1990). Larvae of some species continue to grow when lack of environmental induction forces a delay of metamorphosis, resulting in a high level of intraspecific size variation at settlement (Pechenik 1980; Lesoway and Page 2008). In contrast, most gastropod species arrest growth during the competent period and as a consequence show small intraspecific variation of body size at settlement (see Lesoway and Page 2008 for review). This uniformity of settlement size is advantageous for studying the evolution of selection on an optimal size at settlement in aquatic gastropods.
Another and even more important advantage of shell-bearing gastropods is that the accretionary growth of the shell throughout their ontogeny leaves the larval shell at the apex of the adult shell as the protoconch (Jablonski and Lutz 1983). Instead of the direct observation of larvae, settlement size has been studied through the measurement of the protoconch on adult shells in both recent and fossil species (e.g., Shuto 1974; Rex and Etter 1998). The presence or absence of feeding (planktotrophy) can also be inferred from the protoconch morphology. The protoconch consists of both embryonic and larval shells in species with planktotrophic development; in non-planktotrophic species, on the other hand, there is no larval shell, and the protoconch consists exclusively of a relatively large embryonic shell formed prior to hatching. The protoconch as a whole is accordingly multispiral in the former species and paucispiral in the latter species (e.g., Bouchet and Warén 1979; Jablonski and Lutz 1983; Lima and Lutz 1990). However, the apex of a gastropod shell is often worn and eroded. Microorganisms that bore into calcium carbonate as well as larger invertebrates do extensive damage to the shells of marine mollusks; abiotic agents also influence erosion, especially in animals inhabiting environments that are harsh physically or chemically, including rocky shores with strong surf, acidic freshwater streams, mangrove swamps, and deep-sea hydrothermal vents and seeps (Kano 2006). The original shape of the protoconch in those taxa remains intact for only a short period after metamorphosis, so it can be extremely difficult to infer the developmental mode by examining the protoconch on juvenile or adult shells.
Neritimorpha (=Neritopsina), a gastropod superorder, comprises several hundred living species in four aquatic families (Neritopsidae, Neritiliidae, Neritidae and Phenacolepadidae; Kano et al. 2002) and four terrestrial ones (Bouchet and Rocroi 2005). This group has undergone a major adaptive radiation and currently occupies a great variety of habitats, including rocky shores, seagrass beds, submarine caves, mangrove swamps, freshwater streams, subterranean waters, and deep-sea vents and seeps, in addition to terrestrial and arboreal ecosystems (Ponder and Lindberg 1997; Kano et al. 2002). Aquatic neritimorphs often have a prolonged planktotrophic larval phase of a few or several months (Scheltema 1971; Holthuis 1995; Kano 2006; Lesoway and Page 2008). Their larvae are characterized by spherical, strongly convoluted shells, which make them easily distinguishable from other gastropod larvae (e.g., Scheltema 1971; Page and Ferguson 2013). However, the protoconch is eroded in most metamorphosed individuals except those in shallow subtidal waters due to the unfavorable conditions as mentioned above.
Meanwhile, this snail group provides a rare opportunity to assess the interspecific variation and adaptive significance of settlement size with unique morphological characteristics. Kano (2006) has shown that the operculum of the larval shell remains as the opercular nucleus in almost all adult opercula of neritimorphs. The form of the nucleus reflects the type of larval development as the protoconch does, while the organic composition of the nucleus makes it tolerant to erosion and thus advantageous, compared to the protoconch, in this ecologically diverse group. Species with planktotrophic larvae are characterized by the paucispiral nucleus with a small initial region or embryonic operculum (see Kano 2006, fig. 4, nucleus type A). Non-planktotrophic species have three types of the opercular nucleus: paucispiral with a large initial region (type B), paucispiral without a distinct initial region (type C) and concentric without conspicuous growth lines (type D). This method is applicable to almost all species and individuals in the Neritimorpha except less than two dozen species in three genera, namely Neritopsis, Titiscania and Neritodryas, due to the erosion of the opercular nucleus, total absence of the adult operculum and methodological difficulty in peeling off the calcareous layer overlying the nucleus, respectively (Kano 2006). The measurements of the opercular nucleus may also be a useful estimate of settlement size. The neritimorph protoconch is extremely uniform in shape and sculpture, and the operculum fits closely into the shell aperture in all aquatic species of the superorder (Bandel 1982; Kano 2006; Page and Ferguson 2013). Thus, settlement size may be potentially correlated with, and inferred from, the size of the nucleus, which is retained in the adult operculum.
The major goal of the present study is to reveal the phylogenetic and ecological patterns of the interspecific variation of settlement size in the gastropod superorder Neritimorpha through the measurement of adult opercula. By taking advantage of this snail lineage, we aim to provide the most comprehensive data on the settlement size of marine invertebrates in terms of taxonomic sampling and coverage of different habitats. We selected 88 planktotrophic species from almost all extant genera and measured the diameter of the larval operculum retained as the nucleus of the adult operculum. The diameters of the protoconch and operculum in post-settlement juveniles were also measured to investigate whether the two measurements are correlated and whether the latter can be used as a reliable indicator of settlement size across the superorder. Finally, the adaptive significance of the various settlement sizes in this clade is discussed in phylogenetic and ecological contexts. The usefulness of the opercular nucleus and protoconch for identifying species and phylogenetic lineages is also illustrated for the taxonomy and paleontology of neritimorph gastropods as well as ecological studies on their larval dispersal and recruitment.
Materials and methods
Selection of study taxa
In this study, we selected specimens of 88 planktotrophic species belonging to 17 genera that represent all four families of the recent aquatic Neritimorpha (Table 1). The species were collected from rocky shores, seagrass beds, sand flats, mangrove swamps, estuaries, freshwater streams, submarine caves, deep-sea hydrothermal vents and oil seeps. Familial and generic assignments follow Holthuis (1995), Kano et al. (2002) and Frey (2010). Species identification was confidently made based on our unpublished molecular and morphological data, while scientific names used for the Neritidae are provisional as the nomenclature of this large group requires a major revision.
Measurement of opercular nucleus
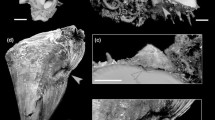
Up to 23 (an average of 5.2) opercular nuclei were observed and measured for each species. Conspecific individuals from multiple localities worldwide were also selected when samples were available. The diameter of the opercular nucleus (Fig. 1, nd; see Kano 2006, fig. 1) was measured to 5-μm precision by tracing the outline of horizontally placed nuclei using a stereomicroscope (Nikon SMZ1500) equipped with a drawing tube. For Nerita specimens, we used forceps and needles to peel off the outer calcareous layer to reveal the opercular nuclei as described in Kano (2006). We gathered the nucleus sizes of 42 individuals from previous studies (Kano 2006, Table 2; Kano 2009, online supplementary fig. 1) for seven species in four genera in addition to the measured values in the present study. For the rare planktotrophic Neritopsis, the apertural size of the protoconch was measured in a juvenile shell of an unidentified species through an application of micro-CT techniques (Kano et al., in preparation) and was used instead of the measurement of the opercular nucleus.
a Newly settled juvenile of Shinkailepas kaikatensis, Myojin Knoll, Izu-Ogasawara Arc, Pacific. b SEM image of adult operculum of Smaragdia souverbiana (modified from Kano 2006, fig. 1A). c Close-up of nucleus in (b). eo embryonic operculum or initial region of opercular nucleus, nd diameter of larval operculum, nu opercular nucleus or larval operculum, op adult operculum, pd diameter of protoconch or settlement size
Comparison between sizes of opercular nucleus and protoconch
Forty-five post-settlement juveniles were used to test the correlation between the larval shell size at settlement and the diameter of the opercular nucleus. We used only small juveniles that had less than 0.5 volution of the teleoconch for precise measurement of the protoconch (Fig. 1, pd). The protoconch was measured to 5-μm precision in the same way as for the opercular nucleus in adult specimens; the juvenile shells were placed in a small hole made in a rubber plate, except the single shell of Neritopsis sp. that was measured by micro-CT scanning.
Comparison of settlement size distribution among lineages and habitats
Phylogenetic trends of settlement size in the Neritimorpha were examined by a size comparison among six lineages (Neritopsidae, Neritiliidae, Phenacolepadidae, Neritinae, Theodoxinae and Smaragdiinae). Each lineage represents a monophyletic clade (Fig. 2) that occupies a wide range of different habitats (Kano et al. 2002). These habitats can be divided into three groups, i.e., marine, brackish and freshwater, for the comparison of nucleus diameter among habitats. (1) The marine group refers to species from (near) euhaline habitats including the rocky shore, sand flat, mangrove swamp, seagrass bed, submarine cave, deep-sea hydrothermal vent and cold seep. (2) The brackish group includes species living in the mixohaline water of the estuary and stream mouth. Members of this group may be able to tolerate occasional exposure to both fully marine or freshwater conditions. (3) Freshwater group refers to limnic species that mainly occur in the upper and middle reaches of the stream and river, while some show tolerance to low-salinity brackish water. Opercular size distribution was compared among the six lineages or three habitat groups. The mean and standard deviation of the nucleus size for each group was calculated by averaging the mean size of species.
Relationship between settlement size and geographic range
The relationship between settlement size and geographic distribution area was examined for 60 representative species to assess the effect of settlement size in determining the distribution in the sea, i.e., whether a larger larva results in a wider geographic range through a presumably longer planktonic period. The 60 species were selected from the 88 study species for the nucleus measurements based on the availability of the information on the distribution range and nucleotide sequences in previous literature or our unpublished data library. Morphological species with multiple evolutionarily significant units (ESUs) were excluded, or only one ESU from such morphospecies was selected to include the specimens used for the measurement of the nucleus and protoconch. The distribution range was represented by the distance between two remotest known occurrences of the species via a straight line at a 50-km level of precision. The size of the larval shell at settlement was estimated from that of the opercular nucleus when an adequate protoconch was not available for measurement of each species (see Results).
Results
Size of opercular nucleus
Table 1 summarizes the diameter of the opercular nucleus in the study species of planktotrophic neritimorphs. The diameter of the nucleus revealed a broad size range (nd: 175–570 μm). Contrary to the small intraspecific variation, there was considerable interspecific variation in the diameter, as previously shown in Kano (2006) with a smaller data set. Among the study species, Neritina petitii was found to have the widest intraspecific variation with its range corresponding to 10.7 % of the mean, and only four species (Nerita insculpta, Clithon bicolor, Neritina delestennei, Neritina petitii) showed intraspecific variation exceeding 10 %. Overall, the average of intraspecific variation was 5.2 % of the mean for each species. The diameter often differed considerably among species within the same genus, without an overlap of size ranges (Table 1).
The dimensions of the opercular nucleus differed among six lineages. Smaragdiinae represent the largest average diameter (482 ± 59; range 405–570 μm) and include the species with the largest opercular nucleus in Neritimorpha (Smaragdia rangiana: up to 570 μm). Phenacolepadidae are the second largest (452 ± 51; 340–520 μm). Within this family, the reciprocal sister clades Phenacolepadinae and Shinkailepadinae showed different ranges of the nucleus size. The former shallow water group, here represented by Phenacolepas and Cinnalepeta, had smaller nuclei (405 ± 36; 340–445 μm) than those of Shinkailepas, Olgasolaris and Bathynerita from deep-sea chemosynthetic environments (489 ± 16; 460–520 μm). Neritopsidae and Neritinae showed moderate sizes. Neritopsis from a submarine cave had a nucleus of 350 μm wide. Neritinae, which comprise the monotypic genus Nerita from intertidal rocky shores and mangrove swamps, showed a size range of 300–450 μm (375 ± 39 μm); the smallest nucleus was found in Nerita histrio and the largest in Nerita melanotragus.
Theodoxinae had smaller nuclei than the above four groups (298 ± 26; 235–360 μm). Among seven theodoxine genera, exclusively marine or brackish water taxa (Puperita and Neripteron) tend to have slightly larger nuclei than freshwater (Neritina, Vitta) or fresh/brackish water genera (Clithon, Vittina and Septaria). The monotypic species of the fully marine genus Puperita had a nucleus diameter of 310 ± 5 (305–315 μm); 10 species of the brackish water genus Neripteron showed a similar range (305 ± 27; 255–360 μm). There was no difference between the exclusively freshwater Neritina and Vitta and the fresh/brackish water Clithon, Vittina and Septaria. Neritina and Vitta showed the respective sizes of 295 ± 13 (279–310 μm) and 261 ± 17 (245–280 μm); Clithon, Vittina and Septaria were 301 ± 28 (260–345 μm), 313 ± 8 (295–330 μm) and 261 ± 22 (235–290 μm), respectively. At the species level, the largest nucleus for Theodoxinae was found in Neripteron reticulatum (360 μm) and the smallest in Septaria tessellata (235 μm), both of which are brackish dwellers.
Neritiliidae had the smallest opercular nuclei (194 ± 16; 175–230 μm) among neritimorph gastropods. The nuclei were larger in the submarine-cave genus Pisulina (217 ± 8; 210–230 μm) than those in freshwater Platynerita (185–200; 193 ± 6 μm) and Neritilia (184 ± 1; 175–190 μm).
Size of protoconch
We measured the diameter of 45 protoconchs in post-settlement juveniles. The specimens belonged to five families or subfamilies and at least nine genera: Neritopsidae (Neritopsis), Neritiliidae (Neritilia), Phenacolepadidae (Shinkailepas), Smaragdiinae (Smaragdia), Theodoxinae (Clithon, Neritina, Vittina, Neripteron and Septaria). Of these, 28 specimens were identified at the species level based on the teleoconch morphology; ten live-caught juveniles of six species (Table 2) enabled us to also measure the size of the in situ operculum. The remaining 18 empty shells were identified to five species (Table 2), and the diameters of their larval opercula were extrapolated from the mean diameters of opercular nuclei for each species obtained in the above measurement of adult specimens. Measurements were also taken for the protoconch and larval operculum for 16 live-caught specimens that were identified only at the generic or higher level for the inclusion in the comparisons of the two sizes (results shown in Fig. 3 but not in Table 2).
The diameter of the protoconch varied greatly among neritimorph species, ranging from 345 μm in Neritilia vulgaris to 855 μm in an unidentified neritoid species from submarine caves in Palau and Yap, western Pacific. The intraspecific variation was small (<5 % in up to 6 individuals for each species), conforming to Kano (2006) and Lesoway and Page (2008). There is noticeable phylogenetic variation: Smaragdiinae and Phenacolepadidae had the largest protoconchs (675–815 and 675–715 μm, respectively), Neritopsidae and Theodoxinae were intermediate (580 and 415–560 μm, respectively) and Neritiliidae represent the smallest size class (345–360 μm). This phylogenetic pattern of the protoconch size agrees closely with that of the size of the opercular nucleus (=larval operculum) described above. The comparison of the two sizes in each species showed a strong correlation (Pearson’s correlation test: r = 0.982, P < 0.00001), which suggests nearly uniformly shaped larval shells and opercula at metamorphic competence across the planktotrophic Neritimorpha. The approximate size of the protoconch (y) can therefore be estimated from the size of the nucleus (x) with a formula 1.29x + 80.55 (Fig. 3).
Comparison of settlement size distribution among lineages and habitats
The diameter of the opercular nucleus, hence the size of the larval shell at settlement, differed significantly among families or subfamilies (analysis of variance: P < 0.00001; Fig. 4), with the exception of Neritopsidae, which was represented by a single specimen and therefore was excluded from the analysis. Tukey–Kramer test detected significant differences (P < 0.0001) between all groups except between Phenacolepadidae and Smaragdiinae (P = 0.419).
Moreover, settlement size differed significantly between the marine group and freshwater or brackish group (Fig. 5; Tukey–Kramer test: P < 0.000001). No significant difference was detected between the freshwater and brackish water groups (P = 0.336), nor among the three habitats in an omnibus test (analysis of variance: P = 0.13).
Comparison of settlement size and geographic range
There was no correlation between settlement size and geographic distribution area of species among all study taxa or within any of the six phylogenetic groups (Fig. 6; Supplementary Table 1). Distances between the two remotest known occurrences of species in each group were: 700–18,950 km for Neritiliidae (n = 4), 2,250–17,150 km for Phenacolepadidae (n = 7), 1,550–19,950 km for Neritinae (n = 19), 6,600–16,500 km for Smaragdiinae (n = 5) and 1,600–15,250 km for Theodoxinae (n = 24). Species that have either small or large settlement size can have narrow-to-wide distribution ranges. Pearson’s correlation tests did not result in a significant P value for all species or species within each phylogenetic group (P > 0.2).
Discussion
Inference of settlement size from measurement of adult operculum
In this study, we demonstrate that settlement size can be precisely estimated by measuring the diameter of the opercular nucleus in the planktotrophic species of the gastropod superorder Neritimorpha. There is a strong correlation between settlement size and diameter of the nucleus (Fig. 3), confirming nearly uniformly shaped protoconchs and larval opercula across the group (Bandel 1982; Kano 2006; Page and Ferguson 2013). The opercular nucleus retains its original shape in nearly all fully grown specimens, while the protoconch is worn and eroded in the majority of metamorphosed individuals (Kano 2006). Moreover, the flat opercular nucleus is easier to measure than that of a globular protoconch, where the overlapping growth of the teleoconch makes the measurement even more difficult. This finding enables us to generate the largest data set so far on larval settlement sizes within a group of marine invertebrates that recruit into very different post-metamorphic habitats.
The size of the opercular nucleus is fairly constant within a species, with a range of intraspecific variation less than 10.7 % of the average diameter in each species, in agreement with preliminary results by Kano (2006). Observations during rearing of planktotrophic larvae have shown determinate growth in Nerita melanotragus (as N. atramentosa): their shell growth is arrested during the delayed period unlike some caenogastropods that continue to grow when lack of environmental induction forces a delay of metamorphosis (Lesoway and Page 2008). The constant intraspecific sizes at settlement obtained in the present study strongly suggest the determinate larval growth in all planktotrophic species of the superorder. Our results support the evolutionary hypothesis by Lesoway and Page (2008) that the capacity for continued growth during the delay period, as exhibited by some caenogastropods, is a derived innovation among feeding gastropod larvae.
Interestingly, Przeslawski (2011) has shown that the egg capsule of N. melanotragus sometimes persists for much longer than would be expected with strict planktotrophy. Their larvae occasionally hatch as crawling juveniles with smaller body sizes than the settlement size of feeding larvae (Przeslawski 2011), possibly representing the first reported case of gastropod poecilogony outside the Heterobranchia (see Bouchet 1989). In the present study, however, the opercular nucleus and estimated settlement size were fairly constant among the three specimens of this species and only negligible intraspecific variation (<2.2 %) was detected among 119 specimens in the genus Nerita (Table 1). Possible explanations for the incongruence between the direct observation and inference from the operculum include a higher mortality for the smaller metamorphosed hatchlings, and poecilogony as the species-specific character of N. melanotragus. The proportion of adults that hatched as crawling juveniles can be estimated by measuring a sufficient number of nuclei in the fully grown opercula of the species (Fukumori et al. in preparation).
Settlement size as an identification trait for juveniles and larvae
The diameter of the opercular nucleus often differs substantially among species, in contrast to the small intraspecific variation (Table 1). The presence of significant interspecific variation of settlement size makes the protoconch and opercular nucleus very useful for identifying juveniles, which are typically nondescript with few suitable taxonomic characters, to genus or species. This is particularly the case in the tropical West Pacific islands where the species diversity of neritids is the highest (Frey and Vermeij 2008; Kano et al. 2011).
Settlement size can also be used as an identification trait for neritimorph larvae. Larval shells of Neritimorpha that reached their final size are distinguished from immature larvae by the flared outer lip of the aperture (Bandel 1982; Kano 2006; Page and Ferguson 2013). The usefulness of this character is enhanced by the proportionally long delay period in their entire larval life. For example, the larvae of Nerita melanotragus tripled in shell length 45 days after hatching, but showed no further growth until the end of culture period of 69 days (Lesoway and Page 2008). While the exact duration of the delay period is not known for this and other neritimorph species, Underwood (1974) found the first newly settled juveniles nearly half a year after the first sighting of egg capsules of N. melanotragus at his study site, suggesting roughly four to five months of pelagic life including three to four months of the delay period. Likewise, the hatched planktotrophic larvae of the Caribbean species Smaragdia viridis were estimated to require about 25 days of the onset of metamorphic competence, and the fully grown larvae were kept at least 30 days in laboratory culture (Scheltema 1971). The delay period of this species may be much longer as the larvae were collected west of Azores Islands, to which the journey may take several months in the North Atlantic Drift (Scheltema 1971).
Taxonomic identification of the juveniles and larvae can be more effectively practiced in combination with information from other morphological characteristics as well as molecular data. Besides the unique, uniformly multispiral and globose shape, neritimorph larvae sometimes have distinct coloration. Certain species of Smaragdia have a bright green shell and soft tissue before and after metamorphosis (Scheltema 1971). Shinkailepas species consistently have a grayish-purple protoconch (Fig. 1; Beck 1992). Settlement size and other morphological criteria are indispensable for the identification of living neritimorph larvae for ecological and behavioral studies, while such characters can also facilitate the screening of specimens for DNA barcoding (see Garland and Zimmer 2002; Barber and Boyce 2006). A promising application of the present finding is to study the larval behavior and dispersal of limnic (amphidromous) and deep-sea hydrothermal vent species in the field, which to date has been inferred from larval rearing, comparison of spawning times and settlement dates, and genetic population analyses (e.g., Holthuis 1995; Crandall et al. 2010; Young et al. 2012).
Phylogenetic constraints on settlement size for neritimorph subclades
The present study reveals that the settlement size of the planktotrophic Neritimorpha primarily reflects phylogenetic constraints rather than adaptive consequences of ecological radiation within each lineage. The Neritiliidae have the smallest settlement sizes among the Neritimorpha regardless of different habitats they occupy (Fig. 4). Within the Neritidae, the species of Theodoxinae are smaller than the Smaragdiinae at settlement, and the Neritinae show intermediate sizes. Members of its sister family Phenacolepadidae (Fig. 2) have the second largest average size at settlement among the six lineages, next to the Smaragdiinae. The planktotrophic larva of the archetypal family Neritopsidae (Kano et al. 2002) is moderate in size (580 μm) and possibly represents the ancestral condition for the extant Neritimorpha. While radically different larval shell morphologies in other gastropod clades (Scheltema 1971; Bandel 1982) prevent outgroup comparison for this character, early neritimorphs from the fossil record show that moderate protoconch sizes were common (e.g., Bandel and Frýda 1999; Bandel and Kiel 2003). Assuming the condition in Neritopsis is the shared ancestral state, body size at metamorphosis may have decreased twice in the lineages leading to the Neritiliidae and Theodoxinae and increased twice in the clades of Phenacolepadidae and Smaragdiinae. The small settlement size as derived conditions is also favored by parsimony, as the large and moderate-sized larvae distribute polyphyletically in the evolutionary tree of the extant Neritimorpha (see Figs. 2, 4).
Interspecific variation in settlement size among planktotrophic species has been addressed by a limited number of studies on echinoid echinoderms and gastropod and bivalve mollusks (e.g., Levitan 2000; Collin 2003). Many of these studies focused on the post-metamorphic effects of maternal provisioning and found no relationship between egg size and settlement size (Hadfield and Switzer-Dunlap 1984; Kohn and Perron 1994; Levitan 2000; but see Marshall and Keough 2007). In particular, there has been almost no research of how environmental conditions affect settlement sizes of different planktotrophic species. Podolsky and Moran (2006) provided a rare but very interesting empirical account by examining six geminate pairs of bivalve species in the eastern Pacific and western Atlantic, where settlement size tends to be larger in the former ocean with higher productivity. This paucity of data reflects difficulties not only in measuring settlement size for a sufficient number of species, but also in obtaining reliable phylogenetic hypotheses. Inherited developmental programs may impose limits on realized growth and differentiation of larvae (Hadfield and Miller 1987). Therefore, the question about selection on settlement size can be addressed only when the influence of ancestry on observed differences is clarified (Levitan 2000; Marshall and Keough 2007; Lesoway and Page 2008). The present results that show ancestry is a major determinant at family, and subfamily levels in Neritimorpha further emphasizes the importance of phylogenies in understanding current selection for optimal size for changing form and habitat.
The more or less defined settlement size (hence the protoconch size) for a given clade enables us to infer the phylogenetic position of many neritimorph fossils. The fossil record of the superorder extends back at least to the middle Devonian and possibly as early as the Ordovician (Bandel and Frýda 1999). The first divergence among the extant families may have occurred in the late Paleozoic (Kano et al. 2002), so that the protoconch size may be used for the phylogenetic inference of Mesozoic and Cenozoic fossils. The familial or subfamilial positions of many Cretaceous and Paleogene taxa have not been conclusively determined by the traditional teleoconch characters. On the other hand, the preservation condition of such fossils is often complete enough to allow the examination of the protoconch (e.g., Bandel and Kiel 2003; Lozouet 2004), probably in part because many of the pre-Neogene species inhabited shallow subtidal waters, where physical and chemical erosion has been less extensive than in intertidal or limnic waters (Kano 2006). Future studies on the fossil material would shed new light on the evolutionary history of Neritimorpha by referring to the present data on settlement size.
Adaptive significance of smaller settlement size for amphidromous taxa
Besides the phylogenetic constraints, difference in the habitats of the adult individuals seemingly influences settlement size in Neritimorpha, particularly among different families and subfamilies. The freshwater and brackish species have significantly smaller sizes at metamorphosis than those of marine species (Fig. 5), while this may reflect a phylogenetic bias as two subclades, Neritiliidae and Theodoxinae, represent all freshwater species and most brackish ones among the living Neritimorpha (Table 1). We propose that the acquisition of small settlement size is an adaptive consequence of ecological radiation to limnic habitats in each lineage.
All limnic species of Neritiliidae and Theodoxinae except a few direct developers have an amphidromous life cycle (Kano 2006; Kano et al. 2011). Amphidromy is a strategy involving migration of juveniles from the sea into freshwater, where growth from juvenile to adult, attainment of sexual maturity and spawning all occur (McDowall 2007). The neritimorph larvae with this life cycle apparently spend a few months in the ocean as their marine relatives do, resulting in the widespread geographic distribution of the species regardless of the exclusively limnic nature in the following ontogenetic stage (Kano 2006; Crandall et al. 2010). However, the longer larval life as marine plankton may increase the risk of expatrial dispersal far from the mouth of the natal river or any other estuarine environment that is suitable for settlement: if the growth period of larvae is shortened, many individuals are likely to remain near the natal river (McDowall 2010). Given the proportional growth of the larval shell, the smaller settlement size of the amphidromous neritimorphs (Fig. 5) may reflect the shorter growth period from hatching to metamorphic competence in comparison with marine taxa with larger settlement sizes. On the other hand, the presence of the considerably long delay period (see above) may still allow the larvae to disperse over a long distance if needed and to colonize new habitats on remote islands on an evolutionary timescale (e.g., Crandall et al. 2010). The smaller settlement size of amphidromous species than that of fully marine relatives has not been reported in other animal groups such as prawns (Knowlton and Vargo 2004), possibly due to the absence or insufficiently long delay period. However, ontogenetic data are obviously too scarce for further consideration outside Neritimorpha.
Further support for the smaller settlement size in amphidromous taxa is given by the fossil record of two additional lineages of the limnic Neritimorpha bearing small protoconchs. Bandel and Riedel (1994) studied the Late Cretaceous fauna of Ajka in Hungary and concluded that this fauna flourished in freshwater to more or less brackish estuarine paleoenvironments. This fauna includes five neritimorph species, one of which (Schwardtina cretacea) belongs to a lineage close to the Recent terrestrial family Hydrocenidae, while three others in the genus Deianira (family Deianiridae) are morphologically similar enough to presume a phylogenetic relationship to another living terrestrial family Helicinidae (Bandel and Riedel 1994; Kano et al. 2002). The last species, Mesoneritina ajkaensis, shares the typical globose shell shape of Neritidae, but it probably represents an independent invasion into the limnic habitat prior to the Eocene radiation of extant amphidromous neritids in the subfamily Theodoxinae (Kano et al. 2002; Bandel and Kiel 2003; Symonds 2006). Notably, Schwardtina and Deianira bear among the smallest multispiral protoconchs (=planktotrophic larval shells) in fossil neritimorphs investigated so far, while the size is unknown in Mesoneritina. The maximum diameters of the protoconchs are 280 and 300–350 μm in S. cretacea and Deianira species, respectively (Bandel and Riedel 1994). Comparable protoconch sizes (pd) can be found only in the two amphidromous lineages among the extant species (Neritiliidae and Theodoxinae; Figs. 3, 4) and none of the other extinct lineages. Planktotrophy of riverine gastropod larvae is directly associated with an amphidromous life cycle as a result of downstream transport and scarcity of planktonic food in the running freshwater ecosystem (Holthuis 1995). Thus, the consistently small protoconchs in the four amphidromous lineages (two recent and two extinct) but nearly none in living and fossil marine taxa suggest the presence of strong evolutionary constraints that led to a hypothesized decreased settlement size for neritimorph species with this life strategy. Such constraints may have resulted from a reduced risk of being wafted away from the estuaries of their natal streams, given that small metamorphs spend less time achieving metamorphic competence.
Submarine-cave species of Neritiliidae represent the only few marine taxa with settlement sizes that are comparable to those of amphidromous species. In addition to Pisulina adamsiana investigated herein, a few more cave-dwelling neritiliids that have been represented exclusively by dead shells bear small protoconchs with diameters ranging from 360 μm (Laddia traceyi) to 530 μm (Siaesella fragilis; Kano and Kase 2008). The phylogenetic relationships among the neritiliid taxa and evolutionary transition between the two apparently contrasting habitats remain speculative due to the lack of material for anatomical and molecular analyses (Kano and Kase 2002, 2008). The small settlement size of the cave species may represent retention of the character state of the common ancestral species with an amphidromous life cycle, possibly in the underground water system (Kano and Kase 2004).
The larger sizes at metamorphosis in Smaragdiinae and Phenacolepadidae than in other lineages (Fig. 4) are more difficult to attribute to evolutionary consequences of adaptive differentiation. It is known that juveniles of benthic marine invertebrates are highly vulnerable while a larger body size provides a better survival rate against changing physiological and ecological pressures (e.g., Spight 1976; Gosselin and Qian 1997). One possible cause for the larger settlement size of Smaragdia is generally higher predation pressure in subtidal waters than in intertidal or limnic habitats (Vermeij 1993). Smaragdia species are specialized marine herbivores that utilize seagrasses as both food and habitat (Rueda et al. 2011; Unabia 2011). Another possibility is individuals that are too small may have difficulty in breaking the tough cell wall of seagrass leaves, regardless of their modified radular teeth for this feeding habit (e.g., Rueda et al. 2011). The Phenacolepadidae have acquired erythrocytes to increase the capacity of blood to transport oxygen in highly reducing environments, such as the underside of deep-buried stones in tidal flats and deep-sea hydrothermal vents, gas seeps and sunken-wood communities (Kano et al. 2002; Kano and Haga 2011; Young et al. 2012). The reduced dissolved oxygen and increased concentration of hydrogen sulfide and other toxic compounds might have favored larger settlers with more tolerance to harsh environmental conditions.
Settlement size and geographic distribution range
In general, the duration of the planktonic period is positively related to the larval dispersal distance of marine benthos (e.g., Todd et al. 1998; Shanks et al. 2003; Siegel et al. 2003; Shanks 2009) and consequently to their genetic homogeneity and geographic distribution range of species (Paulay and Meyer 2006; Weersing and Toonen 2009). The pelagic larval duration may theoretically be inferred from settlement size or the size of the protoconch in gastropods (Scheltema 1971; Hadfield and Switzer-Dunlap 1984; Kohn and Perron 1994). The simplest expectation would therefore be that species with a larger settlement size have a wider geographic distribution. However, no such correlation was found for the planktotrophic species of neritimorph gastropods, or any of the six subclades of the superorder that occupy different habitats as adults (Fig. 6).
When the neritimorph larvae reach the defined settlement size for each species (Table 1), they can be induced to metamorphose by certain external cues from their adult habitats, such as their food sources; without such cues, they remain as plankton (Lesoway and Page 2008). It is therefore probable that their long delay period up to a few or several months (see above) obscures the relationship between settlement size and distribution range. Another possibility for the absence of the correlation is that even the species with presumably shortest pelagic periods (e.g., neritiliids) may be good enough dispersers across oceanic basins (Kano and Kase 2003, 2008). Rafting on driftwood as adults has also been documented for a few estuarine species of the Neritidae (Kano et al. 2013). Interspecific variation in size at hatching may pose a further obstacle in estimating the dispersal ability of larvae from settlement size. The hatching size of neritimorphs tends to differ among species (Kano 2006) so that the duration of the growth period theoretically differs among species with the same settlement size. This possibility has not been explored in this or in previous studies. Future investigation on the diameter of the embryonic operculum in Neritimorpha may help to better understand the general relationship between the larval duration and biogeography of benthic animals. In conclusion, the inference of larval dispersal from the size at metamorphosis may be justified when various factors are considered, including the delay period and size at hatching, as well as other life history characteristics (e.g., larval behavior; Shanks 2009; see also Becker et al. 2007) and accurate species taxonomy (Paulay and Meyer 2006).
Concluding remarks
Gastropods offer many advantages for exploring hypotheses about phylogenetic and ecological patterns of the body size at ontogenetic life history transitions including larval metamorphosis and settlement. The calcified shell is a major advantage because it is usually a reliable indicator of overall body size, and its rigidity allows accurate measurement (Lesoway and Page 2008). Furthermore, all ontogenetic phases of shell secretion are retained in well-preserved adult shells of both extant and fossil gastropods (Bandel 1982; Jablonski and Lutz 1983; Lima and Lutz 1990). The present study revealed even more pronounced advantages in neritimorph gastropods thanks to the wide range of their habitat exploitation, resolved phylogeny and retention of the larval operculum in almost all adult individuals as a rigorous indicator of the size at metamorphosis.
The settlement size of the planktotrophic Neritimorpha primarily reflects phylogenetic constraints, while parallel acquisitions of small settlement sizes are also suggested in limnic habitats. The smaller size may possibly reduce the risk of being wafted away from the estuaries of their natal streams through less time achieving metamorphic competence, while the ability to make occasional long-distance trips is retained by the presence of a sufficiently long delay period. This delay period also seems to obscure the possible correlation between settlement size and geographic distribution range of neritimorph species, both marine and amphidromous. Interspecific variation in size at hatching may pose a further obstacle in estimating the dispersal ability of larvae from settlement size. Future investigation on the size at hatching using the opercula of neritimorphs and scanning electron microscopy may help to better understand the general relationship between the larval duration and biogeography of benthic animals. The same approach can be used to investigate the presence or absence of poecilogony in non-heterobranch mollusks and to assess the body size effect of hatchlings on the sizes at metamorphosis and maturity in a large number of species from different ecological and phylogenetic backgrounds.
References
Bandel K (1982) Morphologie und bildung der frühontogenetischen gehäuse bei conchiferen mollusken. Facies 7:1–198
Bandel K, Frýda J (1999) Notes on the evolution and higher classification of the subclass Neritimorpha (Gastropoda) with the description of some new taxa. Geol Palaeont 33:219–235
Bandel K, Kiel S (2003) Relationships of Cretaceous Neritimorpha (Gastropoda, Mollusca), with the description of seven species. Bull Naturhist Mus Wien A 96:1–65
Bandel K, Riedel F (1994) The late Cretaceous gastropod fauna from Ajka (Bakony Mountains, Hungary): a revision. Ann Naturhist Mus Wien A 96:1–65
Barber P, Boyce SL (2006) Estimating diversity of Indo-Pacific coral reef stomatopods through DNA barcoding of stomatopod larvae. Proc R Soc B 273:2053–2061
Beck LA (1992) Two new neritacean limpets (Gastropoda: Prosobranchia: Neritacea: Phenacolepadidae) from active hydrothermal vents at Hydrothermal Field 1 “Wienerwald” in the Manus Back-Arc Basin (Bismarck Sea, Papua-New Guinea). Ann Naturhist Mus Wien 93:259–275
Becker BJ, Levin LA, Fodrie FJ, McMillan PA (2007) Complex larval connectivity patterns among marine invertebrate populations. Proc Nat Acad Sci USA 104:3267–3272
Bouchet P (1989) A review of poecilogony in gastropods. J Moll Stud 55:67–78
Bouchet P, Rocroi JP (2005) Classification and nomenclator of gastropod families. Malacologia 47:1–397
Bouchet P, Warén A (1979) Planktotrophic larval development in deep-water gastropods. Sarsia 64:37–40
Cardoso JFMF, Van der Veer HW, Kooijman SALM (2006) Body-size scaling relationships in bivalve species: a comparison of field data with predictions by the dynamic energy budget (DEB) theory. J Sea Res 56:125–139
Collin R (2003) Worldwide patterns in mode of development in calyptraeid gastropods. Mar Ecol Prog Ser 247:103–122
Crandall ED, Taffel JR, Barber PH (2010) High gene flow due to pelagic larval dispersal among South Pacific archipelagos in two amphidromous gastropods (Neritimorpha: Neritidae). Heredity 104:563–572
Frey MA (2010) A revised classification of the gastropod genus Nerita. Veliger 51:1–7
Frey MA, Vermeij GJ (2008) Molecular phylogenies and historical biogeography of a circumtropical group of gastropods (Genus: Nerita): Implications for regional diversity patterns in the marine tropics. Mol Phylogenet Evol 48:1067–1086
Garland ED, Zimmer CA (2002) Techniques for the identification of bivalve larvae. Mar Ecol Prog Ser 225:299–310
Giménes L (2010) Relationships between habitat conditions, larval traits, and juvenile performance in a marine invertebrate. Ecology 91:1401–1413
Gosselin LA, Qian P-Y (1997) Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser 146:265–282
Hadfield MG, Miller SE (1987) On developmental patterns of opisthobranchs. Am Malacol Bull 5:197–214
Hadfield MG, Switzer-Dunlap M (1984) Opisthobranchs. In: Tompa AS, Verdonk NH, Van den Biggelaar JAM (eds) The Mollusca. Reproduction, vol 7. Academic Press, Orlando, pp 209–350
Holthuis B (1995) Evolution between marine and freshwater habits: a case study of the gastropod suborder Neritopsina. Dissertation, University of Washington
Jablonski D, Lutz RA (1983) Larval ecology of marine benthic invertebrates: paleobiological implications. Biol Rev Camb Philos Soc 58:21–89
Kano Y (2006) Usefulness of the opercular nucleus for inferring early development in neritimorph gastropods. J Morphol 267:1120–1136
Kano Y (2009) Hitchhiking behaviour in the obligatory upstream migration of amphidromous snails. Biol Lett 5:465–468
Kano Y, Haga T (2011) Sulphide rich environments. In: Bouchet P, Le Guyader H, Pascal O (eds) The Natural History of Santo. Patrimoines Naturels. vol. 69. Muséum National d’Histoire Naturelle, Paris, pp 373–375
Kano Y, Kase T (2002) Anatomy and systematics of the submarine-cave gastropod Pisulina (Neritopsina, Neritiliidae). J Moll Stud 68:365–384
Kano Y, Kase T (2003) Systematics of the Neritilia rubida complex (Gastropoda: Neritiliidae): three amphidromous species with overlapping distributions in the Indo-Pacific. J Moll Stud 69:273–284
Kano Y, Kase T (2004) Genetic exchange between anchialine-cave populations by means of larval dispersal: the case of a new gastropod species Neritilia cavernicola. Zool Scr 33:423–437
Kano Y, Kase T (2008) Diversity and distributions of the submarine-cave Neritiliidae in the Indo-Pacific (Gastropoda: Neritimorpha). Org Divers Evol 8:22–43
Kano Y, Chiba S, Kase T (2002) Major adaptive radiation in neritopsine gastropods estimated from 28S rRNA sequences and fossil records. Proc R Soc B 269:2457–2465
Kano Y, Strong EE, Fontaine B, Gargominy O, Glaubrecht M, Bouchet P (2011) Focus on freshwater snails. In: Bouchet P, Le Guyader H, Pascal O (eds) The Natural History of Santo. Patrimoines Naturels. vol. 69. Muséum National d’Histoire Naturelle, Paris, pp 257–264
Kano Y, Fukumori H, Brenzinger B, Warén A (2013) Driftwood as a vector for the oceanic dispersal of estuarine gastropods (neritidae) and an evolutionary pathway to the sunken-wood community. J Moll Stud 79 (in press)
Knowlton RE, Vargo CK (2004) The larval morphology of Palaemon floridanus Chace, 1942 (Decapoda, Palaemonidae) compared with other species of Palaemon and Palaemonetes. Crustaceana 77:683–715
Kohn AJ, Perron FE (1994) Life history and biogeography: Patterns in Conus. Oxford University Press, London
Lesoway MP, Page LR (2008) Growth and differentiation during delayed metamorphosis of feeding gastropod larvae: signatures of ancestry and innovation. Mar Biol 153:732–734
Levitan DR (2000) Optimal egg size in marine invertebrates: theory and phylogenetic analysis of the critical relationship between egg size and development time in echinoids. Am Nat 156:175–192
Lima GM, Lutz RA (1990) The relationship of larval shell morphology to mode of development in marine prosobranch gastropods. J Mar Biol Assoc UK 70:611–637
Lozouet P (2004) The European Tertiary Neritiliidae (Mollusca, Gastropoda, Neritopsina): indicators of tropical submarine cave environments and freshwater faunas. Zool J Linn Soc 140:447–467
Marshall DJ, Keough MJ (2007) The evolutionary ecology of offspring size in marine invertebrates. Adv Mar Biol 53:1–60
McDowall RM (2007) On amphidromy, a distinct form of diadromy in aquatic organisms. Fish Fish 8:1–13
McDowall RM (2010) Why be amphidromous: expatrial dispersal and the place of source and sink population dynamics? Rev Fish Biol Fisheries 20:87–100
Moran AL, Emlet RB (2001) Offspring size and performance in variable environment: field studies on marine snail. Ecology 82:1597–1612
Page LR, Ferguson SJ (2013) The other gastropod larvae: larval morphogenesis in a marine neritimorph. J Morphol 274:412–428
Paulay G, Meyer C (2006) Dispersal and divergence across the greatest ocean region: Do larvae matter? Integr Comp Biol 46:269–281
Pechenik JA (1980) Growth and energy balance during the larval lives of three prosobranch gastropods. J Exp Mar Biol Ecol 44:1–28
Pechenik JA (1990) Delayed metamorphosis by larvae of benthic marine invertebrates: does it occur? Is there a price to pay? Ophelia 32:63–94
Phillips NE (2002) Effects of nutrition mediated larval condition on juvenile performance in a marine mussel. Ecology 83:2562–2574
Podolsky RD, Moran AL (2006) Integrating function across marine life cycles. Integr Comp Biol 46:577–586
Ponder WF, Lindberg DR (1997) Towards a phylogeny of gastropod molluscs: an analysis using morphological characters. Zool J Linn Soc 119:83–265
Przeslawski R (2011) Notes on egg capsule and variable embryonic development of Nerita melanotragus (Gastropoda: Neritidae). Moll Res 31:152–158
Rex MA, Etter RJ (1998) Bathymetric patterns of body size: implications for deep-sea biodiversity. Deep Sea Res II 45:103–127
Rueda JL, Salas C, Gofas S (2011) Contrasting shell morphology, ingestion and grazing preferences in the neritid gastropod Smaragdia viridis (L.) on two seagrass species. J Sea Res 66:222–230
Scheltema RS (1971) Larval dispersal as a means of genetic exchange between geographically separated populations of shallow-water benthic marine gastropods. Biol Bull 140:284–322
Shanks AL (2009) Pelagic larval duration and dispersal distance revisited. Biol Bull 216:373–385
Shanks AL, Grantham BA, Carr MH (2003) Propagule dispersal distance and the size and spacing of marine reserves. Ecol Appl 13:S159–S169
Shuto T (1974) Larval ecology of prosobranch gastropods and its bearing on biogeography and paleontology. Lethaia 7:239–256
Siegel DA, Kinlan BP, Gaylord B, Gaines SD (2003) Lagrangian descriptions of marine larval dispersion. Mar Ecol Prog Ser 260:83–96
Spight TM (1976) Ecology of hatching size for marine snails. Oecologia 24:283–294
Strathmann RR (1993) Hypothesis on the origins of marine larvae. Annu Rev Ecol Syst 24:89–117
Symonds MF (2006) The Neritidae of the Solent Group (Late Eocene and Early Oligocene) of the Hampshire Basin. Cainozoic Res 4:27–39
Todd CD, Lambert WJ, Thorpe JP (1998) The genetic structure of intertidal populations of two species of nudibranch molluscs with planktotrophic and pelagic lecithotrophic larval stages: are pelagic larvae “for” dispersal? J Exp Mar Biol Ecol 228:1–28
Unabia CRC (2011) The snail Smaragdia bryanae (Neritopsina, Neritidae) is a specialist herbivore of the seagrass Halophila hawaiiana (Alismatidae, Hydrocharitaceae). Invertebr Biol 130:100–114
Underwood AJ (1974) The reproductive cycles and geographical distribution of some common eastern Australian prosobranchs (Mollusca: Gastropoda). Aust J Mar Freshwat Res 25:63–88
Vermeij GJ (1993) A natural history of shells. Princeton University Press, New Jersey
Weersing K, Toonen RJ (2009) Population genetics, larval dispersal, and connectivity in marine systems. Mar Ecol Prog Ser 393:1–12
Young CM, He R, Emlet RB, Li Y, Qian H, Arellano SM, Van Gaest A, Bennett KC, Wolf M, Smart TI, Rice ME (2012) Dispersal of deep-sea larvae from the intra-American seas: simulations of trajectories using ocean models. Integr Comp Biol 52:483–496
Acknowledgments
The authors thank P. Bouchet, M. Fuji, T. Haga, T. Kase, H. Kawaguchi, H. Kikuchi, S. Kimura, S. Kojima, J. Leqata, J. Letourneux, K. Noda, S. Panha, G. Paulay, Y. Sato, J. Slapcinsky, J. Starmer and A. Warén for their help in the acquisition or loan of specimens and literature. Material from Santo Island, Vanuatu, was collected during the Santo 2006 expedition organized by P. Bouchet and loaned from the Muséum national d’Histoire naturelle, Paris MNHN. Invaluable comments were provided by N. Mateer and two anonymous reviewers for the improvement of the manuscript. Thanks are also due to T. Kitahashi for assistance with data analysis. Financial support was provided by Grants from Research Institute of Marine Invertebrates Foundation, Tokyo, Grant-in-Aid for JSPS Fellows (No. 25-6758) and JSPS KAKENHI (Nos. 23370040 and 24770072).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fukumori, H., Kano, Y. Evolutionary ecology of settlement size in planktotrophic neritimorph gastropods. Mar Biol 161, 213–227 (2014). https://doi.org/10.1007/s00227-013-2330-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-013-2330-5