Abstract
Although the establishment and spread of non-indigenous species depends upon survival in the face of novel environmental conditions and novel biological interactions, relatively little attention has been focused on the specific role of native predators in limiting invasion success. The European common periwinkle, Littorina littorea, was recently introduced to the Pacific coast of Canada and provides a case study of an introduction into an area with an important predator guild (sea stars) that is functionally minor in the invader’s native habitat. Here, we assess the likelihood of establishment, spread, and negative ecological impact of this introduced gastropod, with an emphasis on the role of native sea stars as agents of biotic resistance. Size frequency distributions and local market availability suggest that L. littorea was most likely introduced via the live seafood trade. Non-native hitchhikers (e.g., the trematode Cryptocotyle lingua) were found on/in both market and field specimens. Laboratory studies and field observations confirmed that L. littorea can survive seasonal low salinity in Vancouver, British Columbia. Periwinkles also readily consumed native Ulva, suggesting that periwinkles could impact native communities via herbivory or resource competition. Unlike native gastropods, however, L. littorea lacked behavioural avoidance responses to Northeast Pacific predatory sea stars (Pisaster ochraceus and Pycnopodia helianthoides), and sea star predation rates on L. littorea were much higher than predation rates on native turban snails (Chlorostoma funebralis) in common garden experiments. We therefore expect periwinkle establishment in British Columbia to be limited to areas with low predator density, as is seen in its field distribution to date. We caution that this conclusion may understate the importance of the L. littorea introduction if it also serves as a vector for additional non-indigenous species such as C. lingua.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To establish and spread in a novel habitat, a non-indigenous species must survive several filters: conditions during transport, abiotic conditions in the new habitat, and biological interactions with novel competitors, consumers, and parasites (Elton 1958; Mack et al. 2000). The tendency for resident species to reduce invasion success—a phenomenon termed biotic resistance—has now been widely demonstrated, particularly in the plant literature with regard to competitors and herbivores (Levine et al. 2004). Less well understood is the importance of native predators, which may also be an important source of biotic resistance (Carlsson et al. 2009, 2011). This may be especially true in benthic marine habitats, where top-down control by predators is particularly strong (Shurin et al. 2002), and for which there is growing evidence that native predators can limit the success and therefore the ecological impacts of certain invasions (deRivera et al. 2005; Shinen et al. 2009; Cheng and Hovel 2010).
One recent introduction that may become ecologically important is that of the common periwinkle, Littorina littorea, to the Northeast Pacific. Native to Northern Europe, L. littorea is already well established along the east coast of North America where it was putatively introduced as a food source or in rock ballast (Chapman et al. 2007, 2008; Blakeslee et al. 2008). Within 50 years of its discovery in North America in 1840, L. littorea had spread from Nova Scotia to as far as southern New Jersey (Chapman et al. 2007). L. littorea is now one of the most abundant molluscs in its invaded range in the Northwest Atlantic (Carlton 1992), and densities of several hundred periwinkles per square meter are common (e.g., Petraitis 1987; Carlson et al. 2006). The ecological effects of L. littorea where it is native and introduced in the Atlantic Ocean are extensive; common periwinkles reduce recruitment of algae (Lotze and Worm 2002) and invertebrates (Buschbaum 2000), control rocky intertidal community structure (Petraitis 1987) and species diversity (Lubchenco 1978), and can even convert depositional salt marsh habitat into cobble shores devoid of fine sediments (Bertness 1984).
Subsequent to its invasion of the Atlantic shores of Canada and the United States, L. littorea has appeared in several locations on the Pacific coast of North America, including Deception Pass, Washington (Hanna 1966), Newport Bay, Oregon (Carlton 2007), San Francisco Bay, California (Carlton 1992; Chang et al. 2011), and Anaheim Bay, California (Chang et al. 2011). Anecdotally, L. littorea may have been seen in the Vancouver, BC, area as early as the 1960s (A. Lamb, pers. com.), although the identification was not confirmed at the time and no specimens were vouchered. Although L. littorea can no longer be found at several of these points of introduction (Carlton 1992; Chang et al. 2011) and current populations in California do not yet appear to be self-recruiting (Chang et al. 2011), repeated introductions and multi-year persistence of adult populations suggest that eventual establishment is a realistic possibility. Given the substantial ecological impacts of L. littorea in the Atlantic, determining the likelihood of establishment and the potential for detrimental effects of this species on the Pacific coast is a priority.
Invasion ecology may differ substantially between eastern and western ocean margins, as exemplified by comparisons between the Northeastern Pacific and the Northwestern Atlantic (Ruiz et al. 2000; Choi 2011). The thermal environment is generally more stable and therefore less likely to approach lethal extremes in the Northeast Pacific (Sorte et al. 2011), and environmental stability may favour the establishment of non-native species (Oliveira et al. 2011). On the other hand, several of the focal sites for marine introductions on the Pacific coast (e.g., San Francisco Bay) are in estuaries with highly variable salinity regimes, and salinity fluctuations could reduce the likelihood of some non-native species becoming established. Furthermore, biological diversity is higher in the Northeast Pacific than in the Northwest Atlantic, and high diversity communities are more likely to resist invasions via biotic resistance than low diversity communities (Levine et al. 2004). For benthic invertebrates such as L. littorea, the effects of predation may be particularly important, as predatory sea stars are a major community structuring influence in the intertidal zone in the Northeast Pacific (Paine 1966; Harley 2011) but are relatively rare or absent on North Atlantic intertidal shores (Lewis 1964).
Here, we provide the first confirmed documentation of L. littorea on the Pacific coast of Canada, in Vancouver, British Columbia. Our goals were to (1) establish the current distribution and impacts of the species in the area, (2) begin to assess potential future impacts, should common periwinkles become established, by comparing the grazing impacts of L. littorea and an ecologically similar native gastropod, (3) determine whether L. littorea can survive in the local environment over the long term, specifically with regard to seasonal variation in salinity, and (4) explore the potential for biotic resistance to this introduced species by examining the susceptibility of L. littorea to native predators.
Materials and methods
Field sampling
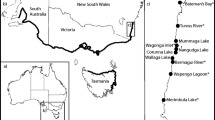
Littorina littorea was initially found on an undergraduate Marine Ecology course field trip to a boulder beach (49°18′08.3″N, 123°09′26.8″W) just north of Ferguson Point, Vancouver on 12 January 2010. Specimens were identified via shell and soft tissue (e.g., penis) morphology. A second population was subsequently located at Acadia Beach, Vancouver (49°16′48.0″N, 123°14′29.6″W) on 17 April 2011. Following initial detection, regular (approximately monthly) visits were made to both sites, and L. littorea were removed when found. No L. littorea have been seen at Ferguson Point or Acadia Beach since 12 March 2011 and 27 August 2011, respectively, despite multiple subsequent visits to each site over the following 6–12 months.
Numerous additional sites (see Fig. 1), which were being surveyed in conjunction with a separate project on a similarly sized intertidal gastropod (the dogwhelk Nucella lamellosa), were also checked for L. littorea in 2010 and 2011 (minimum search time: 1 person-hour per site). Although we did not specifically target sites of likely introduction, our surveys did include sites with very high human-visitation rates (e.g., popular public parks and shore trails in Vancouver, West Vancouver, and Victoria). With the exception of a single, hermit crab-occupied shell found at Tower Beach, Vancouver (49°16′24.2″N, 123°15′28.5″W), L. littorea was not found at other sites around Vancouver and in the southern Strait of Georgia (Fig. 1). However, owing to the difficulties associated with detecting rare species (Chapman 1999), we cannot exclude the possibility that L. littorea has been introduced elsewhere in the area and escaped detection.
To determine whether L. littorea may have already had some ecological effect by the time it was detected at Ferguson Point, the characteristics of the biological assemblage were measured at the introduction site and two neighbouring boulder fields on 4 March 2010. At each of these three sites, we collected data in 10–12 400 cm2 plots; this plot size was sufficiently large to encompass local-scale patchiness. Percent cover data were visually estimated for barnacles (primarily Balanus glandula), mussels (Mytilus trossulus), rockweed (Fucus gardneri), and ephemeral green algae (primarily Ulva sp.) with the aid of square quadrats subdivided into sixty-four 2.5 × 2.5 cm subsections (see Dethier et al. 1993 for rationale). The percent cover of bare space (rock or mud/shell hash) was also recorded. Counts were made of native limpets (Lottia spp.) and snails (Littorina plena plus L. scutulata, which are difficult to distinguish in the field).
Morphometric data
All field-collected L. littorea, along with empty shells and shells occupied by hermit crabs, were measured along with individuals from five market samples. Snail shell size was determined to the nearest 0.1 mm by measuring the length of the shell along the axis of coiling from the apex to the distal end of the body whorl. Shell damage in the form of chipped or chipped and repaired apertures was also recorded. Only chips likely to represent failed attacks by crabs—typically hemispherical chips with a radius >2 mm—were counted. The relationship between shell size and damage within field samples was determined by logistic regression.
Identification of potential hitchhikers
Wild-caught and store-purchased L. littorea were surveyed for macroscopic epibionts in the form of encrusting algae and sessile invertebrates. Because of the difficulty of identifying shell-encrusting algae, algae were identified to functional group. Presence and absence data were taken based on evidence of shell-boring parasites and barnacles. A small subset of wild-caught and store-purchased L. littorea were dissected to establish the presence of gut-inhabiting trematodes, which were identified based on morphology under a compound microscope. Our goal was simply to establish presence; no attempt was made to explicitly compare trematode prevalence in different samples.
Salinity tolerance
Although L. littorea is eurythermal and somewhat euryhaline, it is salinity that is most likely to be a limiting factor in the Vancouver area. Thermal tolerance in L. littorea is reported to range from −13.0 to 35.3 °C (Davenport and Davenport 2005), but because these limits were determined during 24-h exposures, thermal tolerance during shorter exposures (i.e., typical low tides) is likely to be broader. In Burrard Inlet, L. littorea may rarely, if ever, reach lethal body temperatures; water temperatures typically range from 5 to 20 °C, and air temperatures in Vancouver drop below −13 °C only exceptionally rarely and have never been recorded above 35 °C (temperature data for Burrard Inlet and Vancouver available from the Canadian Ministry of Environment). By contrast, the salinity tolerance of L. littorea could be exceeded during the summer freshet of the nearby Fraser River. L. littorea held at 15 °C can survive salinities as low as ~14 psu for extended periods of time, but complete mortality set in between 9 and 14 days at salinities below 13 psu (Todd 1964). Burrard Inlet can reach very low salinities (<10 psu) in the summer (Held and Harley 2009), and hyposmotic stress could conceivably prevent L. littorea from becoming established in and around Vancouver. Therefore, we investigated the salinity tolerance of field-collected L. littorea.
Snails were held fully submerged in 1-L bottles topped with a crumpled paper towel to prevent emergence and escape. Aeration was provided to each bottle by an aquarium air pump and an air stone. Bottles were filled with a mix of seawater and dechlorinated water at salinities of 5, 10, and 20, as measured with a hand-held refractometer. There were 4 L. littorea per bottle and three bottles per salinity treatment. Snails were observed after 1, 4 h, and then at approximately daily intervals after the initiation of the experiment. Snails were categorized as attached by the foot to the substratum or unattached; attachment is ecologically significant as it allows for locomotory behaviours including feeding and escape responses. Unattached snails were briefly removed from the water and tested for responsiveness by poking the operculum with a blunt metal wire. Snails that did not move following this stimulus were recorded as unresponsive, presumably as a result of sublethal or lethal physiological impairment. After 7 days, snails were returned to full strength seawater (salinity = 30) and allowed to recover for a further 2.5 days. Snails that had not emerged and reattached following the recovery period were declared dead. Variation in proportion responsive after 7 days and the proportion dead after the recovery period were assessed with one-way ANOVAs with bottle being the unit of replication (n = 3 per treatment).
Diet and feeding rates
To establish dietary overlap between introduced L. littorea and a common native gastropod, Chlorostoma (formerly Tegula) funebralis, we conducted feeding trials using these two herbivores and either F. gardneri or Ulva sp. as a food source. These two algal species were chosen because they are the two dominant mid-intertidal seaweeds in the Vancouver area and because they would provide some basic information on dietary breadth and overlap. L. littorea used in this experiment had been purchased from a local market, and the C. funebralis had been collected near the Bamfield Marine Sciences Centre. While C. funebralis is not found around Vancouver, it is the British Colombian regional native most similar to L. littorea in size and, potentially, diet. Should L. littorea successfully establish in Vancouver, it would likely spread to areas inhabited by C. funebralis.
Snails and algae were placed in perforated 473-mL plastic cups with mesh lids, and the cups were then submerged in a single recirculating seawater system. We used a fully factorial design with each replicate containing one herbivore (either L. littorea, mean mass ± SE = 4.7 ± 0.3 g, or C. funabralis, mean mass = 4.9 ± 0.3 g) or a no-herbivore control, and one piece of algae (either 0.518 ± 0.006 g Ulva or 0.521 ± 0.006 g Fucus). There were 13 replicates of the six treatment combinations. Snail and algal mass before and after the experiment were determined as a blotted mass where each was blotted dry using a paper towel until no more water was appearing on a dry paper towel. Mass consumed was calculated as the change in blotted algal tissue mass after 7 days of grazing. Data were analysed with a two-way ANOVA.
Vulnerability to predators
Three experiments were conducted to explore the responses to and vulnerability of L. littorea to native predators, with a particular emphasis on comparing L. littorea to the ecologically similar native gastropod, C. funebralis, in experiments two and three. The predators chosen for these experiments—the ochre star Pisaster ochraceus, the sunflower star Pycnopodia helianthoides, and the Dungeness crab Metacarcinus (formerly Cancer) magister—were chosen because they are the three most common large benthic predators in Burrard inlet, particularly in areas influenced by seasonal low salinity (CH, personal observation).
Experiment 1 examined behavioural responses to water-borne predator cue by measuring crawl-out responses (i.e., time taken to crawl out of the water), which is a useful metric of predator avoidance in L. littorea (e.g., Jacobsen and Stabell 1999). Two M. magister, seven P. ochraceus, and three P. helianthoides were separated by species and placed into 20-L tanks (one for each sea star species) or a 40-L tank (for the crabs). The crabs required a larger tank to allow movement of these larger organisms; however, the animal surface area to tank volume ratio of all organisms was similar. All tanks were semi-submerged in the water table to maintain temperature at 13 °C. Predator cue was allowed to develop within the tanks for 1 h. Store-bought and field-collected L. littorea were removed from their predator-free holding areas and acclimated for 1 h in freshly prepared artificial seawater (Instant Ocean) with a salinity of 30 to ensure a cue-free environment prior to the initiation of the trials.
For the trials, four treatments were established: Metacarcinus cue, Pycnopodia cue, Pisaster cue, and an artificial seawater control. Cue water was collected from the predator tanks immediately prior to each trial, and 400 mL of undiluted cue water (or control water) was placed into 1,200-mL clear polyurethane bottles. One snail, either market or field-collected, was placed aperture down in each bottle and observed for 25 min. The time it took for the snail to crawl out of the water was recorded. If the snail did not crawl out of the water, it was assigned a time of 25 min. A total of three replicates (market snails) or four replicates (field-collected snails) were run for each cue treatment. Data were analysed with a two-way ANOVA.
Given the lack of L. littorea escape responses when exposed to sea star cues in experiment 1 (see "Results"), we decided to further explore periwinkle responses to predatory sea stars in experiments 2 and 3. Gastropods often exhibit striking behavioural responses to sea star tactile cues (e.g., McClintock 1985). Therefore, experiment 2 determined the behavioural response of L. littorea and C. funebralis to direct contact with one of two native predatory sea stars: P. ochraceus and P. helianthoides. These two sea stars were of particular interest as they both readily consume snails in the genus Chlorostoma (Paine 1969; Thornber 2007) and may thus represent a predatory threat to similarly sized L. littorea. Individual snails were placed into aquaria with enough artificial seawater to fully submerge the snail. Upon emergence from the shell, the foot of the snail was exposed to one of three tactile stimuli or to a no-stimulus control. The three tactile stimuli were poking by the arm of either a live Pisaster or Pycnopodia, or poking by an abiotic control (glass thermometer). Snails were then allowed to move freely for 1 min, at which time we recorded the net translocation as the linear distance between point of stimulus and final location. Twelve to fourteen snails of each species were used in each of the four stimulus treatments. Data were analysed with a two-way ANOVA.
Experiment 3 assessed the vulnerability of non-native L. littorea and native C. funebralis to native asteroid predators. Six 20-L tanks were filled with seawater, provided with aeration, and covered with plexiglass lids in such a way that air bubbles did not build up below the lids to create a predator-free refuge above the waterline. These tanks were partially submersed in a recirculating seawater table to maintain tank temperatures at 13 °C. Five L. littorea (wet mass range 4.1–7.6 g) and five C. funebralis (3.1–8.2 g) were placed in each tank. Once the snails had all attached and begun crawling, two P. ochraceus (7–11 cm arm radius) were placed in each of three tanks, and one P. helianthoides (11–12 cm arm radius) was placed in each of the remaining three tanks. The trial ran for 3 days at which point 2 additional L. littorea were added to each tank to compensate for losses, and the sea stars were allowed to feed for another 4 days. Partial water changes were performed daily. Snails were categorized as consumed once an empty shell plus a detached operculum were noted; this avoided misidentification of live individuals that had withdrawn deeply into their shells. Mortality was confirmed by shell dissection at the end of the experiment. Data were analysed with paired t tests at day 4 and again at day 7.
Statistical analyses
Community assemblage analyses (PERMANOVA and nMDS ordination) comparing field sites with and without L. littorea were carried out using the vegan package in R (R Development Core Team 2004). After significant differences were found among the sites, three additional PERMANOVAs were run using each possible pair of sites in order to determine where differences were located. Community data were transformed using a modified Gower transformation to account for zero heavy data.
For analyses of variance, conformation to the assumptions of normality of the residuals and of homoscedasticity was tested by Shapiro–Wilk W tests and O’Brien tests, respectively. In the case of factorial experiments, homoscedasticity was tested by comparing all treatment levels against one another in a one-way design. A violation of assumptions in the tactile stimulus experiment was corrected with a log(x + 10) transformation. The assumption of homoscedasticity was violated and could not be corrected by data transformations in the among-sample comparison of mean shell length (where the assumption of normality was also violated) and in the herbivory trials. With regard to the shell length analysis, ANOVA is robust to departures from these assumptions when number of groups is 5 or more and n > 6 (Underwood 1997). Because we had 7 groups and >100 snails in each group, we proceeded with the ANOVA. With regard to the herbivory experiment, heteroscedasticity was driven by low variance in the no-herbivore controls for Fucus. Because ANOVAs are robust to violations of homoscedasticity in cases like this where the design is balanced, the treatments well replicated (n = 13), and one variance is smaller (rather than larger) than the others (Underwood 1997), we proceeded with the two-way ANOVA.
With the exception of community assemblage analyses (see above), all statistical analyses were conducted using JMP 9 (SAS Institute).
Results
Local distribution and community correlates
We found L. littorea at two sites in the Vancouver area: Ferguson Point and Acadia Beach (Fig. 1). Over the entire sampling period, a total of 158 periwinkles were found at Ferguson Point over an 80 × 15 m stretch of shore, and 196 were found at Acadia Beach within a 25 × 10 m area. Although a single L. littorea shell, occupied by a hermit crab, was located at a third site (Tower Beach), regular visits to that site failed to locate any live periwinkles. Native barnacles (B. glandula and Chthamalus dalli) and limpets (Lottia spp.) were abundant on L. littorea collected at Ferguson Point (totals of 481 and 32 individuals, respectively), but were only sparsely found on L. littorea collected at Acadia Beach (63 very recent barnacle recruits and 1 Lottia).
Because the abundance of native epibionts suggested a reasonably long period of L. littorea occupancy at Ferguson Point, we tested for potential ecological impacts at this site by comparing it with two neighbouring boulder fields that lacked L. littorea. The ecological community differed significantly among boulder fields (PERMANOVA: Pseudo-F (2,29) = 7.981, p = 0.001; Fig. 2), but additional PERMANOVAs comparing site pairs revealed that these differences were not driven by the site where L. littorea was present. Rather, the southern site differed significantly from the northern site (Pseudo-F (1,20) = 7.977, p = 0.001) and the invaded site (Pseudo-F (1,20) = 12.336, p = 0.001). The site with L. littorea did not differ significantly from the boulder field to the north (Pseudo-F (1,18) = 1.742, p = 0.158). Overall, the southern boulder field had fewer mussels, more bare rock, and more native littorine snails than either the impacted or northern boulder fields (data not shown).
nMDS ordination (2D-stress = 0.14) of the community assemblage of the L. littorea introduction site and two neighbouring sites lacking L. littorea in the vicinity of Ferguson Point. As assessed by PERMANOVA, the southern boulder field was significantly different than the introduction site and the northern boulder field. Community assemblages were not significantly different between the introduction site and the northern boulder field
Morphological characteristics
Field-collected snails were all adults and ranged in shell length from 22.1 to 37.2 mm at Ferguson Point and from 19.6 to 30.2 mm at Acadia Beach. When considered together with the five market samples, there were significant differences in mean length among the seven samples (ANOVA: MS = 255, F 6,1538 = 44.7, p < 0.0001). Field-collected samples were as large as or larger than market samples (Fig. 3). Ferguson Point mean size was significantly larger than all other samples, and Acadia Beach snails were significantly larger than market snails in samples T and T1, T and T3, and RCSS1, and statistically similar to the other two market samples (Tukey–Kramer HSD post hoc comparisons, α = 0.05).
Shell size frequency distribution for two field samples (Acadia Beach and Ferguson Point) and five market samples: three from T&T Markets in Vancouver, BC (T&T 1 and 2) and Richmond, BC (T&T 3), and two from a Royal Canadian Superstore in Vancouver (RCSS 1 and 2). Mean size shown by dashed line, error bars represent 1 SD of the mean
Evidence of past attacks by crabs, in the form of existing and repaired shell chips, was observed in periwinkles collected at Ferguson Point (18.6 % of all shells) and Acadia Beach (27.6 % of all shells). Smaller snails were significantly more likely to have existing or recently repaired shell chips at both field sites (logistic regression: Ferguson Point χ2 = 5.68, p = 0.017; Acadia Beach χ2 = 17.3, p < 0.0001) (data not shown). However, it is unclear how much of this damage was incurred post-release on Vancouver beaches. The frequencies of shell damage in market samples spanned that observed in the field, ranging from 0 % of shells in the least damaged sample to 37.1 % of shells in the most damaged sample. We found very limited evidence (e.g., peeled or crushed shells) of successful crab predation in the field; of the 354 L. littorea collected from Vancouver beaches, none were crushed and only one was peeled sufficiently far up the body whorl to expose the soft tissues.
Hitchhikers
Algae were commonly found on market snails and primarily consisted of both non-calcifying encrusting red (most common) and crustose coralline red algae. An unidentified bleached and presumably dead crustose coralline alga was observed on 4 L. littorea collected from Acadia Beach—as native crusts do not occur intertidally at this site, the species was presumed to have been introduced with the periwinkles, although it is unclear whether the crusts were alive at the time of introduction. Preliminary checks for trematodes revealed abundant Cryptocotyle lingua in the guts of one L. littorea purchased from a market and one collected from Acadia Beach. Store-purchased L. littorea occasionally had barnacles (likely Semibalanus balanoides) living on their shells. Finally, we observed galleries consistent with the North Atlantic shell-boring polychaete Polydora ciliata in both market and field L. littorea shells; however, no live worms were found.
Feeding rates and diet
Algal mass loss in the herbivory experiment was significantly related to the interactive effects of herbivore treatment and algal species identity (herbivore × alga interaction p < 0.0001; Table 1). L. littorea and C. funebralis consumed similar quantities of Ulva, with significantly higher Ulva mass loss in the treatments with snails than in the snail-free controls (Fig. 4). Herbivore effects on Fucus were minimal, with neither herbivore reducing Fucus mass beyond levels measured in the no-herbivore control (Fig. 4). Mass loss for Ulva and Fucus in the no-herbivore controls was slight and did not differ between the two species of algae (Fig. 4).
Salinity tolerance
Reduced salinity had readily apparent effects on L. littorea behaviour in laboratory experiments. After 1 h, all snails held at 5 psu had detached from the substratum and withdrawn into their shells, and by 24 h the same was true of snails held at 10 psu. In contrast, all snails in the saltiest treatment (20 psu) remained attached to the substratum throughout the experiment. By the end of the 7 days exposure, there were significant differences among salinity treatments in the proportion of snails that were responsive to a tactile stimulus (ANOVA: MS = 6,460, F 2,6 = 11.6, p = 0.009); 8.3 ± 8.3 % (mean ± SE) were responsive at 5, 66.7 ± 22.0 % were responsive at 10, and 100 % were responsive at 20. However, after an additional 60 h of recovery, most snails were able to emerge from their shells and reattach to the substratum (5: 66.7 ± 22.0 %; 10: 83.3 ± 8.3 %; 20: 100 ± 0 %). Assuming the unattached snails were ecologically dead, mortality rates among treatments were slightly higher at lower salinities, but these differences were not statistically significant (ANOVA: MS = 833, F 2,6 = 1.50, p = 0.296).
Susceptibility to native predators
Both store-bought and field-collected L. littorea were tested for their avoidance response to chemical cue from three native predators: P. helianthoides, P. ochraceus, and M. magister. Crawl-out times differed with cue (p = 0.006; Fig. 5), but not with source (market vs. field; p = 0.466), and the cue × source interaction was not significant (p = 0.713, Table 2). For the main effect of cue, only the crab cue treatment was significantly different from the control (Tukey HSD, p < 0.05).
Response to predator cue, expressed as time to emerge from water containing predator effluent (mean ± SE). Non-native L. littorea did not differ in their behaviour based upon source (market or wild-caught). While having similar emergence times in control and sea–star (P. ochraceus or P. helianthoides) effluent water, L. littorea emerged significantly faster than controls from water containing dungeness crab (M. magister) cue
To further explore the potential vulnerability of L. littorea to Northeast Pacific sea stars, behavioural responses to tactile stimuli were examined for both non-native L. littorea and the similarly sized native herbivorous gastropod, C. funebralis. The responses of L. littorea to contact with predatory sea stars—as measured by post-contact crawling distance over a period of 60 s—differed from the responses of native C. funebralis as indicated by the significant snail species × stimulus type interaction (p = 0.008, Table 3). In the controls, crawling speeds were similar between the two gastropod species (Fig. 6). However, when contacted with predatory sea stars (P. helianthoides or P. ochraceus), native C. funebralis tended to increase their speed, whereas non-native L. littorea tended to decrease their speed, resulting in significant differences in velocity between the two gastropod species (Fig. 6). L. littorea touched with an inanimate probe also moved significantly slower than C. funebralis, although the magnitude of this difference was less than the interspecific difference in responses to sea stars (Fig. 6).
Response to predator contact, as expressed by net distance crawled in 60 s, for native C. funebralis and non-native L. littorea (mean ± SE). Differences in net movement patterns between the snail species only became pronounced in the treatments with a tactile stimulus present, particularly when the tactile stimulus was a live sea star. Letter codes treatment differences identified by Tukey’s HSD post hoc tests (α = 0.05)
When L. littorea and C. funebralis were held together in arenas with native predators, there were striking differences in susceptibility to predation (Fig. 7). In tanks with P. helianthoides, the majority (15/21) of the L. littorea were consumed within a week, during which time only 1/15 C. funebralis was consumed. In tanks with P. ochraceus, L. littorea was consumed even more rapidly (20/21 within a week), and C. funebralis were only eaten once the supply of L. littorea dropped to near zero. Interspecific differences in snail mortality were detected at day 4 and day 7 in P. ochraceus tanks (two-tailed paired t tests: t = −7.00, p = 0.0198, and t = −6.93, p = 0.0202, respectively). Interspecific differences in loss to predators were marginally significant in P. helianthoides tanks on day 4, and significant on day 7 (two-tailed paired t tests: t = −3.88, p = 0.0604, and t = −5.29, p = 0.0339, respectively).
Discussion
Introduced marine species have had dramatic impacts on their recipient communities by altering food web structure and causing substantial declines in abundance and local extirpations of native species (Byrnes et al. 2007; Galil 2007). In the case of L. littorea, which is a dominant ecological player elsewhere (Lubchenco 1978; Bertness 1984; Petraitis 1987; Buschbaum 2000; Lotze et al. 2002; Díaz et al. 2012), determining the likelihood of establishment and potential impacts in the Northeast Pacific are priorities for research. As with any potentially invasive species, understanding the risks associated with this introduction involves determining (1) propagule pressure, including pathways and probabilities of further introductions, (2) the ability of the introduced species to survive the abiotic conditions and biological interactions in the recipient habitat, and (3) the degree of ecological damage that the introduced species may inflict.
Potential mode of L. littorea introductions
The discovery of L. littorea at two sites in Vancouver in 2010 and 2011 represented the first confirmed records of this species in British Columbia. At both sites, L. littorea numbered well over 100 individuals, but these individuals were restricted to <100 m of shoreline and no juvenile periwinkles (<15 mm shell length) were found at either site. The distributional and size frequency patterns suggest that L. littorea were not recruiting from the plankton, but had been intentionally released as adults. Given the relative similarity in size frequency distributions in field and market samples, introduced periwinkles likely originated in the live seafood trade, as has been hypothesized for L. littorea introduced in California (Chang et al. 2011).
People may obtain live non-native animals from markets and release them into the wild for a number of reasons, including the desire to establish a harvestable local population and the desire to release captive animals for ethical or religious reasons. L. littorea is harvested in the Atlantic, and it is possible that someone intends to establish a viable population for recreational or commercial harvest in British Columbia. Alternatively, religious releases are becoming increasingly problematic in some areas (Agoramoorthy and Hsu 2005). Members of the Buddhist community in the greater Vancouver area are known to organize the release of live seafood including fish, bivalves, and crustaceans. In most cases, this cultural tradition is not well regulated, and it represents a possible pathway for recent and future introductions of L. littorea. Regardless of the motivation, the continued presence of live L. littorea in local seafood markets suggests that future introductions in British Columbia may be likely.
Surviving the environmental filter
Once introduced, the establishment of a non-native species depends on its ability to cope with local environmental conditions (Elton 1958). Thermal conditions in coastal British Columbia do not reach the extremes seen along the Northwest Atlantic coast (Steinhauser 1979), and therefore seem unlikely to exceed the thermal tolerance of L. littorea. However, temperature is not always the primary correlate to invasion success, and variation in other factors, notably salinity, may be more important in restricting the distribution of some non-native species (Dafforn et al. 2009). In the vicinity of Vancouver, the Fraser River spring freshet results in seasonally low salinity that may severely impact marine species (Held and Harley 2009). Thus, for species introduced near the mouth of the Fraser River (e.g., Vancouver), low salinity stress could potentially serve as a very strong filter.
With specific regard to L. littorea, however, the case for salinity limiting invasion success is weak. Although periwinkle activity levels were suppressed by realistic exposures to low salinity stress, the majority of snails were able to survive 7 days of continuous submergence even at a salinity of 5, and there were no significant differences in mortality among salinity treatments. It must be noted that our statistical power to detect differences in mortality was low, and longer exposures have been shown to result in complete L. littorea mortality even at salinities above those used in our experiments (Todd 1964). Nevertheless, field evidence indicates that at least some L. littorea can survive lengthy exposure to seasonal low surface water salinity in the Vancouver area; live snails were found throughout the low salinity spring/summer periods of 2010 and 2011 at Ferguson Point and Acadia Beach, respectively.
Surviving the biological filter
The potential for native species to “resist” the establishment and spread of introduced species has long been hypothesized (Elton 1958; Ruiz et al. 2000), and numerous examples of biotic resistance populate the invasion literature (e.g., Levine et al. 2004). Native predators, particularly generalists, have important but potentially overlooked impacts on non-native species, and their role in biotic resistance to invasions may be substantial (Sax et al. 2007; Carlsson et al. 2009). Indeed, we found that two native species of sea stars—both generalist predators—were highly efficient at capturing and consuming non-native L. littorea. This result may be due to inappropriate behavioural responses on the part of the periwinkle. Whereas native C. funebralis avoided sea star predators, or actively fled when directly contacted by a sea star, L. littorea tended to remain in the predator’s vicinity and, in many cases, ceased moving or even released their hold on the substratum and withdrew into their shells (CH, personal observation). While these behavioural responses may be an appropriate defence against crab predators, which could easily overtake a fleeing snail and attack exposed flesh, such strategies fail as a deterrent to sea star predation. This may reflect the evolutionary history of L. littorea in the Atlantic Ocean, where periwinkles fall prey to crabs far more often than they fall prey to sea stars (Perez et al. 2009).
Given the efficiency with which sea stars consume L. littorea in the laboratory, it is possible that sea stars could prevent the establishment or restrict the spread of periwinkles in the field. The strongest evidence for this form of biotic resistance, of course, would come from field-based observations or experiments involving sea star predation on periwinkles. To date, however, documented populations of L. littorea in California (Chang et al. 2011) and British Columbia (this study) are only found at sites where sea stars are rare or absent. Factors including low salinity and a paucity of rocky substrate preclude large sea star populations in the areas of San Francisco Bay where L. littorea have been found (A. Chang, pers. com.). Although sea star densities frequently exceed 10 per linear meter of shoreline in and around Vancouver (Harley, unpublished data), the two sites at which L. littorea were found were unusual in that they had naturally low sea star densities due to seascape-level features. Specifically, both sites consisted of a mid-intertidal boulder field bordering extensive low intertidal sand flats that act as a barrier to daily or seasonal patterns of Pisaster migration into the mid-intertidal zone (CH, personal observation; see Rilov and Schiel (2006a, b) for an analogous situation involving other rocky shore predators). It is possible that there is a bias towards finding L. littorea in sea star-free sites because the absence of such predators is a pre-requisite for longer-term persistence; such predator-driven patterns of non-native species distributions have been documented in other systems (e.g., Cheng and Hovel 2010). The degree to which predation influences observed L. littorea distributional patterns on the west coast of North America, however, cannot be determined without more information.
Native crabs may also form an important component of biotic resistance to L. littorea. Periwinkles exhibited escape responses in the presence of crab cue, and such modification of L. littorea behaviour may significantly blunt their impacts as grazers even in the absence of actual predation (Trussell et al. 2003). M. magister subadults do forage extensively in intertidal soft-sediment habitats in Washington, USA (Holsman et al. 2006), and Cancer productus influences the lower intertidal limits of native Littorina sitkana and L. scutulata via consumption and trait-mediated effects on protected rocky shores in British Columbia (Rochette and Dill 2000). Whether crab predation can limit the abundance of larger, mid-intertidal snails like L. littorea and C. funebralis, however, remains poorly explored in the Northeast Pacific.
Potential impacts on native species
There is no way of knowing exactly what the impacts of L. littorea would be unless they become established. We were not able to identify any ecological impacts of one British Columbian introduction, perhaps because local periwinkle density (well under 1 per m2) was orders of magnitude lower than densities on many Atlantic shores. Nevertheless, because L. littorea has had such a significant effect on Northwest Atlantic community structure (Lubchenco 1978; Petraitis 1987; Lotze et al. 2002), it is important to consider the potential impacts of this non-native grazer should it become established and reach similarly high densities in the Northeast Pacific. Our feeding assays indicated that non-native L. littorea preferred soft ephemeral algae (see also Lubchenco 1978), as did the native herbivorous snail. This finding is significant for two reasons: (1) should L. littorea be able to establish themselves in the same densities as they appear on the east coast, they will likely preferentially reduce the abundance of ephemeral algae, and (2) should L. littorea successfully establish and spread in British Columbia, they may directly compete with native herbivores such as C. funebralis, which were also found to prefer ephemeral Ulva sp. in our laboratory trials. This particular competitive scenario, however, would require L. littorea to spread beyond its current points of introduction and into areas occupied by C. funebralis.
Additional risks of L. littorea introductions are associated with the presence of hitchhikers, many of which could become invasive in British Columbia even if L. littorea does not. The occurrence of barnacles, trematodes, epizoic algae, and potentially of shell-boring polychaetes on/in market sample snails indicates a potential source of introduction for these epibionts and parasites. Indeed, the non-native trematode C. lingua was found in both market and field-collected periwinkles. This trematode infects multiple Littorina species on Atlantic shores (Blakeslee and Byers 2008), can affect community structure via alteration of host grazing rates (Wood et al. 2007), and could potentially have negative ecological impacts where it to become established in the Northeast Pacific. As with other types of live trade (e.g., Whittington and Chong 2007), more work is needed to characterize hitchhikers associated with L. littorea and similarly “introduceable” taxa in the live seafood trade in order to better assess the overall risks associated with such introductions.
Long-term prospects for L. littorea in British Columbia
To date, it is unclear whether L. littorea will successfully establish reproductive populations in British Columbia. As with the California populations (Chang et al. 2011), the Vancouver populations lacked juveniles and therefore did not appear to be increasing via reproduction, although we cannot rule out the possibility that offspring from the introduced populations have been dispersing elsewhere. Our eradication efforts seem to be making progress; densities at the two introduction sites have declined steadily to undetectably low numbers. However, given the current regulatory framework allowing the live import of this species and local cultural attitudes towards the release of live marine organisms, the probability of additional (current or future) introductions in the Vancouver area seems to be high. Adult periwinkles are able to survive for long periods on shorelines around Vancouver, and if the observation that Ferguson Point snails were larger than market specimens is meaningful, they are able to grow in these habitats as well.
Although the potential for L. littorea to become invasive is evident in the extensive invasion of the Northwest Atlantic, the success of L. littorea along the Pacific coast of North America has thus far been limited despite multiple introductions (Chang et al. 2011). On Northeast Pacific rocky shores in particular, local biotic resistance, particularly as enforced by native predatory sea stars (e.g., Paine 1966, 1969; Harley 2011), may be quite high. Although biotic resistance in the form of trophic interactions has been shown to be important in many systems (Levine et al. 2004; Carlsson et al. 2009), it has been hypothesized that such resistance will act to reduce non-native population size and distributional extent without necessarily preventing invasions (Levine et al. 2004). However, studies on biotic resistance mediated by predators typically involve invaders that are already well established (e.g., deRivera et al. 2005; Shinen et al. 2009; Cheng and Hovel 2010). In situations where the introduced species is not yet fully established, as appears to be the case with L. littorea in British Columbia, native predators could potentially prevent establishment by keeping introduced populations below thresholds of abundance or density required for successful reproduction, recruitment, and population growth. Indeed, local augmentation of native predator densities, coupled with public education and continued scientific monitoring, is one potential management strategy for invasions that are still in their early stages.
Conclusions
Considering the potential for biotic resistance by native predators in the light of L. littorea’s invasion history and the supply of live L. littorea available for future introductions, we suggest that L. littorea may eventually become established in British Columbia. However, we predict that predators, particularly sea stars, would substantially restrict the distribution and blunt the impacts of L. littorea in the Northeast Pacific. L. littorea could still become abundant in areas where sea stars either cannot forage (e.g., the high intertidal zone) or are locally rare (soft-sediment habitats as well as some rocky shores). In those areas, there is strong potential for negative ecological impacts on native algae via consumption and on native herbivores via competition. Furthermore, such local refuges may come to harbour source populations of hitchhiking species that arrive with L. littorea but are not obligately associated with them. For non-native species introductions in general and the Northeast Pacific case of L. littorea in particular, areas of low predator density should be prioritized for monitoring and management as they may represent important refuges in which non-native species can establish and subsequently spread.
References
Agoramoorthy G, Hsu MJ (2005) Religious freeing of wildlife promotes alien species invasion. Bioscience 55:5–6
Bertness MD (1984) Habitat and community modification by an introduced herbivorous snail. Ecology 65:370–381
Blakeslee AMH, Byers JE (2008) Using parasites to inform ecological history: comparisons among three congeneric marine snails. Ecology 89:1068–1078
Blakeslee AMH, Byers JE, Lesser MP (2008) Solving cryptogenic histories using host and parasite molecular genetics: the resolution of Littorina littorea’s North American origin. Mol Ecol 17:3684–3696
Buschbaum C (2000) Direct and indirect effects of Littorina littorea (L.) on barnacles growing on mussel beds in the Wadden Sea. Hydrobiologia 440:119–128
Byrnes JE, Reynolds PL, Stachowicz JJ (2007) Invasions and extinctions reshape coastal marine food webs. PLoS ONE 2:e295
Carlson RL, Shulman MJ, Ellis JC (2006) Factors contributing to spatial heterogeneity in the abundance of the common periwinkle Littorina littorea (L.). J Molluscan Stud 72:149–156
Carlsson NOL, Sarnelle O, Strayer DL (2009) Native predators and exotic prey—an acquired taste? Front Ecol Environ 7:525–532
Carlsson NOL, Bustamante H, Strayer DL, Pace ML (2011) Biotic resistance on the increase: native predators structure invasive zebra mussel populations. Freshw Biol 56:1630–1637
Carlton JT (1992) Introduced marine and estuarine mollusks of North America: an end-of-the-20th-century perspective. J Shellfish Res 11:489–505
Carlton JT (2007) Intertidal invertebrates from Central California to Oregon. University of California Press, Berkeley, CA
Chang AL, Blakeslee AMH, Miller AW, Ruiz GM (2011) Establishment failure in biological invasions: a case history of Littorina littorea in California, USA. PLoS ONE 6:e16035
Chapman MG (1999) Are there adequate data to assess how well theories of rarity apply to marine invertebrates? Biodivers Conserv 8:1295–1318
Chapman JW, Carlton JT, Bellinger MR, Blakeslee AMH (2007) Premature refutation of a human-mediated marine species introduction: the case history of the marine snail Littorina littorea in the northwestern Atlantic. Biol Invasions 9:737–750
Chapman JW, Blakeslee AMH, Carlton JT, Bellinger MR (2008) Parsimony dictates a human introduction: on the use of genetic and other data to distinguish between the natural and human-mediated invasion of the European snail Littorina littorea in North America. Biol Invasions 10:131–133
Cheng BS, Hovel KA (2010) Biotic resistance to invasion along an estuarine gradient. Oecologia 164:1049–1059
Choi FMP (2011) Assessing intertidal marine non-indigenous species in Canadian ports. Dissertation, University of British Columbia, Vancouver
Dafforn KA, Glasby TM, Johnston EL (2009) Links between estuarine condition and spatial distributions of marine invaders. Divers Distrib 15:807–821
Davenport J, Davenport JL (2005) Effects of shore height, wave exposure and geographical distance on thermal niche width of intertidal fauna. Mar Ecol Prog Ser 292:41–50
deRivera CE, Ruiz GM, Hines AH, Jivoff P (2005) Biotic resistance to invasion: native predator limits abundance and distribution of an introduced crab. Ecology 86:3364–3376
Dethier MN, Graham ES, Cohen S, Tear LM (1993) Visual versus random-point percent cover estimation: ‘objective’ is not always better. Mar Ecol Prog Ser 96:93–100
Díaz ER, Kraufvelin P, Erlandsson J (2012) Combining gut fluorescence technique and spatial analysis to determine Littorina littorea grazing dynamics in nutrient-enriched and nutrient-unenriched littoral mesocosms. Mar Biol 159:837–852
Elton C (1958) The ecology of invasions by animals and plants. Methuen, London
Galil BS (2007) Loss or gain? Invasive aliens and biodiversity in the Mediterranean Sea. Mar Pollut Bull 55:314–322
Hanna DG (1966) Introduced mollusks of western North America. Occa Pap Calif Acad Sci 48:1–108
Harley CDG (2011) Climate change, keystone predation, and biodiversity loss. Science 334:1124–1127
Held MBE, Harley CDG (2009) Responses to low salinity by the sea star Pisaster ochraceus from high- and low-salinity populations. Invertebr Biol 128:381–390
Holsman KK, McDonald PS, Armstrong DA (2006) Intertidal migration and habitat use by subadult Dungeness crab Cancer magister in a NE Pacific estuary. Mar Ecol Prog Ser 308:183–195
Jacobsen HP, Stabell OB (1999) Predator-induced alarm responses in the common periwinkle, Littorina littorea: dependence on season, light conditions, and chemical labelling of predators. Mar Biol 134:551–557
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989
Lewis JR (1964) The ecology of rocky shores. English Universities Press LTD, London
Lotze HK, Worm B (2002) Complex interactions of climatic and ecological controls on macroalgal recruitment. Limnol Oceanogr 47:1734–1741
Lotze HK, Worm B, Molis M, Wahl M (2002) Effects of UV radiation and consumers on recruitment and succession of a marine macrobenthic community. Mar Ecol Prog Ser 243:57–66
Lubchenco J (1978) Plant species diversity in a marine intertidal community: importance of herbivore preference and algal competitive abilities. Am Nat 112:23–39
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
McClintock JB (1985) Avoidance and escape responses of the sub-Antarctic limpet Nacella edgari (Powell) (Mollusca, Gastropoda) to the sea star Anasterias perrieri (Smith) (Echinodermata, Asteroidea). Polar Biol 4:95–98
Oliveira MD, Calheiros DF, Jacobi CM, Hamilton SK (2011) Abiotic factors controlling the establishment and abundance of the invasive golden mussel Limnoperna fortunei. Biol Invasions 13:717–729
Paine RT (1966) Food web complexity and species diversity. Am Nat 100:65–75
Paine RT (1969) The Pisaster-Tegula interaction: prey patches, predator food preference, and intertidal community structure. Ecology 50:950–961
Perez KO, Carlson RL, Shulman MJ, Ellis JC (2009) Why are intertidal snails rare in the subtidal? Predation, growth and the vertical distribution of Littorina littorea (L.) in the Gulf of Maine. J Exp Mar Biol Ecol 369:79–86
Petraitis PS (1987) Factors organizing rocky intertidal communities of New England: herbivory and predation in sheltered bays. J Exp Mar Biol Ecol 109:117–136
R Development Core Team (2004) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rilov G, Schiel DR (2006a) Seascape-dependent subtidal-intertidal trophic linkages. Ecology 87:731–744
Rilov G, Schiel DR (2006b) Trophic linkages across seascapes: subtidal predators limit effective mussel recruitment in rocky intertidal communities. Mar Ecol Prog Ser 327:83–93
Rochette R, Dill LM (2000) Mortality, behavior and the effects of predators on the intertidal distribution of littorinid gastropods. J Exp Mar Biol Ecol 253:165–191
Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH (2000) Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annu Rev Ecol Syst 31:481–531
Sax DF, Stachowicz JJ, Brown JH, Bruno JF, Dawson MN, Gaines SD, Grosberg RK, Hastings A, Holt RD, Mayfield MM, O’Connor MI, Rice WR (2007) Ecological and evolutionary insights from species invasions. Trends Ecol Evol 22:465–471
Shinen JS, Morgan SG, Chan AL (2009) Invasion resistance on rocky shores: direct and indirect effects of three native predators on an exotic and a native prey species. Mar Ecol Prog Ser 378:47–54
Shurin JB, Borer ET, Seabloom EW, Anderson K, Blanchette CA, Broitman B, Cooper SD, Halpern BS (2002) A cross-ecosystem comparison of the strength of trophic cascades. Ecol Lett 5:785–791
Sorte CJB, Jones SJ, Miller LP (2011) Geographic variation in temperature tolerance as an indicator of potential population responses to climate change. J Exp Mar Biol Ecol 400:209–217
Steinhauser F (1979) Climatic atlas of North and Central America. World Meteorological Organization, UNESCO Cartographia, Budapest
Thornber C (2007) Associational resistance mediates predator-prey interactions in a marine subtidal system. Mar Ecol Evol Persp 28:480–486
Todd ME (1964) Osmotic balance in Littorina littorea, L. littoralis, and L. saxatilis (Littorinidae). Physiol Zool 37:33–44
Trussell GC, Ewanchuk PJ, Bertness MD (2003) Trait-mediated effects in rocky intertidal food chains: predator risk cues alter prey feeding rates. Ecology 84:629–640
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Whittington RJ, Chong R (2007) Global trade in ornamental fish from an Australian perspective: the case for revised import risk analysis and management strategies. Prev Vet Med 81:92–116
Wood CL, Byers JE, Cottingham KL, Altman I, Donahue MJ, Blakeslee AMH (2007) Parasites alter community structure. Proc Natl Acad Sci USA 104(22):9335–9339
Acknowledgments
We thank D. Reid for assisting with the identification of the snails. R. Gooding brought the L. littorea population at Acadia Beach to our attention. The students in the University of British Columbia’s Experimental Biology of Invertebrates course assisted with data collection for the herbivory trials. M. Frey kindly surveyed sites at Clover Point (Victoria) and Sidney. Additional assistance in the field was provided by B. Arney, F. Choi, C. Murray, Z. Harley, C.G. Harley, M. Harley, B. Harley, J. Nelson, and M. Picard. R. Whippo assisted with the statistical analyses. Comments by P. Kraufvelin and four anonymous referees improved the manuscript. Funding for this work was provided by a National Science and Engineering Research Council (NSERC) Discovery Grant to CH and by the Canadian Aquatic Invasive Species Network (an NSERC Strategic Network Grant).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kraufvelin.
Rights and permissions
About this article
Cite this article
Harley, C.D.G., Anderson, K.M., Lebreton, C.AM. et al. The introduction of Littorina littorea to British Columbia, Canada: potential impacts and the importance of biotic resistance by native predators. Mar Biol 160, 1529–1541 (2013). https://doi.org/10.1007/s00227-013-2206-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-013-2206-8