Abstract
This paper offers the first study of diurnal variations in the use of an estuarine habitat by Indo-Pacific humpback dolphins. Passive acoustic data loggers were deployed in the Xin Huwei River Estuary, Western Taiwan, from July 2009 to December 2010, to collect biosonar clicks. Acoustic encounter rates of humpback dolphins on the riverside of the estuary changed significantly over the four tidal phases, instead of the two diurnal phases based on the recordings from 268 days. Among the tidal phases, the encounter rates were lowest during ebb tides. Additionally, circling movements associated with the hunt for epipelagic fish significantly changed in temporal and spatial presence over the four tidal phases, matching the overall pattern of encounter rate changes in the focal estuary. Our findings suggest that the occurrence pattern and habitat utilization of humpback dolphins are likely to be influenced by the tidal-driven activity of their epipelagic prey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Indo-Pacific humpback dolphin (Sousa chinensis) is a strictly coastal species found throughout the Indian and western Pacific oceans (Jefferson and Karczmarski 2001). Within their distribution range, the estuary has been reported as the core habitat (Parra 2006; Hung 2008). Although temporal variations in the dolphins’ use of the estuarine habitat have been studied at a few locations through visual observations (Parsons 1998; Parra 2006; Hung 2008), details regarding their periodic patterns of activity and movement remain unclear. Focused and continuous observation over a full day is necessary for further insight into their ecology.
To thrive in an estuary, the dolphins must be able to take advantage of the routine changes in aquatic environments of such a habitat. In particular, the estuarine waters are strongly influenced by tidal activities, as the water depth and current change periodically with the tidal cycle. The tidal current also induces hydrological turbulence by stratifying and mixing the freshwater and seawater (Largier 1993; McLusky and Elliott 2004). In response to these environmental changes, estuarine fish and decapods are known to migrate or behave differently between tidal phases (Krumme 2004, 2009). As the top predator, cetaceans are also believed to alter their behavioral patterns over different periods of time to increase the availability of their food sources (Karczmarski et al. 2000; Carlström 2005; Akamatsu et al. 2010; Soldevilla et al. 2010). Variations, for instance, in the temporal and spatial presence of estuarine bottlenose dolphins (Tursiops truncates) have been shown to correlate with the tidal cycle (Mendes et al. 2002; Fury and Harrison 2011).
The small and possibly isolated population of humpback dolphins off Western Taiwan has attracted considerable attention for its critically endangered status and conservation issues (Reeves et al. 2008; Wang et al. 2007, 2008; Ross et al. 2010). They commonly inhabit estuaries with semidiurnal tides and with tidal differences that reach to approximately four meters. As such, investigating the temporal variation in their estuarine habitat utilization is essential for understanding the ecology of humpback dolphins and is critical for the conservation of this endangered population.
In order to understand the temporal variation of humpback dolphin activities, continuous 24-h observations are necessary. Passive acoustic monitoring (PAM) has been widely employed to study the presence of cetaceans and is especially efficient for long-term monitoring (Moore et al. 2006; Mellinger et al. 2007; Todd et al. 2009; Akamatsu et al. 2010; Soldevilla et al. 2011). PAM also facilitates the study of the foraging behavior of odontocetes by analyzing their echolocations (Akamatsu et al. 2005b). Continuous acoustic recording enables the monitoring of cetaceans during nighttime and severe weather, thus collecting a wider range of temporal data unobtainable by visual observation.
The present study is the first to record the 24-h activities of Indo-Pacific humpback dolphins by PAM. The changes in acoustic activity of humpback dolphins at the monitored estuary were analyzed over the diurnal cycle and the tidal cycle during the one and a half year to understand their predominant occurrence pattern. The temporal and spatial presences of different movement types were investigated to understand the temporal variation of their estuarine habitat use. Our findings support the hypothesis that the habitat utilization pattern of humpback dolphins at the monitored estuary is correlated to the tidal cycle, instead of the diurnal cycle.
Methods
Study site
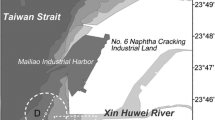
The acoustic monitoring site is located at the Xin Huwei River Estuary, off the west coast of central Taiwan (Fig. 1). Two reclaimed lands for industrial parks located on the northern and southern river banks restrict the width of the estuary to only 2 km. The humpback dolphins sighted at the nearby area are primarily distributed between 4 and 16 m depth ranges based on the onboard surveys conducted by the corresponding author. The area south of the Mailiao harbor, including the studied estuary, has been identified as a habitat with high sighting rates of humpback dolphins but not of other cetacean species (Chou et al. 2011).
Instrument deployment and data collection
Four long-life acoustic data loggers (A-tag, Marine Micro Technology Inc., Saitama, Japan) were deployed in the field from July 31, 2009 to December 21, 2010 to record the echolocation clicks produced by the humpback dolphins. The A-tags were attached to a fixed pile (N23°45.6′ E120°9.1′). The water depth at the pile ranged between 8 and 12 m depending on the tidal phase. The fixed pile insulated the high-frequency clicks coming from the opposite side so only clicks from one side were recorded by each A-tag. During each deployment, two A-tags were fixed at 4 m from the sea floor on opposite sides of the pile to separate the monitoring area into the riverside and the offshore side (Fig. 1). The riverside thus represents the area that is more influenced by river discharge, as the distance to the river mouth is shorter than the offshore side. We retrieved the A-tags every month, as the weather made it possible, to ensure data recovery and battery change.
The A-tag employed in this study is a self-contained event recorder powered by two D cells which can record continuously for about 30 days before the batteries are completely drained. Each A-tag has two hydrophones (MHP-140-70) placed 60 cm apart with an electronic band-pass filter between 55 and 235 kHz. One hydrophone of MHP-140-70 is most sensitive at 130 kHz (sensitivity: −200.0 dB re 1 V/μPa), and the other one is most sensitive at 70 kHz (sensitivity: −200.2 dB re 1 V/μPa, the frequency response curve is available at http://cse.fra.affrc.go.jp/akamatsu/A-tag/A-tagSpec.html). The peak frequency range (100–180 kHz) of humpback dolphin clicks (Goold and Jefferson 2004) is thus within the audible range of the A-tag. The dynamic range of A-tag is between 129 and 157 dB re 1 μPa peak to peak (Akamatsu et al. 2005a). The individual difference in sensitivity of the long-life A-tags was within ±1 dB according to the calibration using 100 kHz 5-cycle tone burst sound projected in an acoustic tank that was conducted by the second author. When ultrasonic pulses were detected, the time, pressure level, and the difference in arrival time of the sound between two hydrophones were stored in the flash memory of the A-tag. The sound arrival time difference was measured by the high-speed counter within the A-tag based on a resolution of 1.08 μs that enables the calculation of sound source bearing angle with a relatively short 60-cm baseline between the two hydrophones.
The detection ranges of A-tag have not been investigated for humpback dolphins. For the finless porpoises, empirical data show that the effective detection range reached to 300 m during the towed survey (Akamatsu et al. 2008). The simulation based on a spherical propagation model also showed that the estimated detection range reach to 1.25 km for the stationary monitoring when the received level is 140.4 dB p–p (re 1 μPa) (Kimura et al. 2010).
Acoustic data processing
The recorded ultrasonic pulse events were processed using Igor Pro 5.01 (Wave Metrics, Lake Oswego, OR, USA). Acoustic signals were filtered to remove background noise, reflections from the sea surface and bottom, and sounds other than those of humpback dolphins by using the following procedures modified from Akamatsu et al. (2010):
-
1.
Signals with sound pressure levels <133.3 dB p–p (re 1 μPa) were excluded in order to reduce most of the background noise.
-
2.
Successive clicks detected within 4 ms were eliminated to exclude potential surface or bottom reflections.
-
3.
Successive clicks with ICI (inter-click interval) variations between 50 and 200 % were extracted to avoid false detections from sounds other than those of humpback dolphins, for example, pulses with irregular ICIs from waves and other biological sound sources.
-
4.
Click trains <6 clicks or >500 clicks were discarded in the analysis to exclude isolated pulses and intense shipping noise. A click train was defined as a series of clicks separated by no more than 200 ms which was the maximum ICI recorded in this study.
-
5.
Click trains where the coefficient of variation of pressure level was <0.3 and the standard deviation (SD) of time difference between the two hydrophones <221 μs were used for the analysis. This was to ensure that the click trains were produced from a consistent bearing angle since a sound source (i.e., dolphin) should be located within a narrow bearing angle during a single click train.
Preliminary comparison between the A-tag data and wideband recording showed that the automatic filtering was effective. Some false alarms from boat sonar or wave noise were detected but were easily removed by visual examination of the extremely small variation of ICIs or the irregular change of time differences of noise train. In the present study, the click trains were visually confirmed by the first author after the automatic detection. For each filtered click train, the start time, end time, mean ICI, standard deviation of ICIs, and arrival time differences were saved for further analysis.
Temporal analysis of encounter rate
One dolphin could produce multiple click trains in a short time. To reduce the effect of double counting the clicks from the same individual, the encounter rate was used as a quantified index of humpback dolphin acoustic activities in this study. The encounter rate (e) was calculated as
where N and T represent the number of encounters and the recording duration, respectively. An encounter was defined as an independent sound source in a one minute time bin from each A-tag according to the method employed by Kimura et al. (2009). The independent sound source was discriminated by the change of sound source bearing angle among multiple click trains in the same time bin. Multiple encounters per one minute time bin could be recorded if two or more individuals were phonating at different bearing angles. In this study, encounters were individually recorded for the riverside and the offshore side to investigate the variation in humpback dolphin acoustic activity on different sides of the estuary.
The occurrence patterns were investigated based on the change in encounter rates between daytime and nighttime for the diurnal cycle and between high, ebb, low, and flood tides for each tidal cycle. Encounter rates for each diurnal and tidal phase were calculated for each week; the data sets from less than 7 days recording were excluded from the analysis. The daytime and nighttime were defined as the periods between the time of sunrise and sunset. The high and low tides were defined as the periods between 1.5 h before and after the time of high and low water, respectively. The ebb and flood tides were defined as the periods between high and low tides resulting in four periods for each tidal cycle. The time of sunrise, sunset, high, and low water were obtained from the Taiwan Central Weather Bureau. Since the distribution of encounter rate was not normally distributed, Mann–Whitney U test and Kruskal–Wallis ANOVA were applied to test the difference in encounter rates among the diurnal and the tidal phases.
Behavioral analysis of dolphin schools
In this study, the habitat-use pattern of humpback dolphins was described by the movement pattern of a dolphin school, rather than that of individual animal. A dolphin school was defined as a series of encounters with relatively close time cohesion. An encounter was considered to be within a school if it occurred within 10 min of any other encounter.
For each detected school, its movement pattern was categorized as circling, traveling, or unidentified movements based on the traces of the sound source bearing angle (Fig. 2). The movement was classified as circling when traces of the sound sources moved from one quadrant of the monitored area to the other quadrants and then circled back to the original quadrant. The four quadrants were defined by two axes, which are the boundary between the two monitoring sides and the zero time difference between two hydrophones of the A-tag. The circling movement, characterized by a back and forth moving pattern, has been linked to the foraging behaviors of bottlenose dolphins when dolphins were circling around or chasing a school of fish (Bel’kovich et al. 1991). A movement was classified as traveling when traces of the sound sources were shown to head in the same direction during the entire time. Movements not belonging to either the circling or traveling movements were categorized as unidentified. Schools with less than ten click trains were not categorized into any movements since the traces of the sound source bearing angles were not sufficient to discriminate their movement type.
Example data showing the recorded click train during circling and traveling movements of the humpback dolphin. Each dot represents a detected click. The time difference between two hydrophones on each A-tag could help identify the sound source bearing angle along the north–south direction. The circling movement describes a school of dolphins moving from north to south on the riverside, then moving to the offshore side, and finally moving back to the north on the riverside. The traveling movement describes at least two dolphins indicated by two independent sound sources at the same time, moving from south to north only on the offshore side
The behavior of each cataloged school was quantitatively compared based on the duration of sound reception and its echolocation use. The duration of sound reception, an index of the duration a school remained in the area, was calculated based on the time between the first and last detected clicks. The difference of duration in the three movement types was tested by Kruskal–Wallis ANOVA.
For the echolocation use, the maximum inspected distance (MID) of the dolphins (Thomas and Turl 1990) was calculated as
where v represents the underwater sound speed, and ICI represents the inter-click interval. The underwater sound speed ranged between 1,466 and 1,542 m/s based on the UNESCO equation when seawater temperature ranged between 15 and 30 °C and salinity ranged between 0 and 32 ppt. The temperature and salinity ranges were based on the data recorded by the DST CTD loggers (Star-Oddi, Gardabaer, Iceland) deployed in the study site. The maximum inspected distance does not represent the actual detection distance, as a time lag is included in the ICI in addition to the two-way transit time of a click (Au 1993). To compare the composition of maximum inspected distance of the three movement types, re-sampling analysis was employed for the reason that the mean ICIs of successive click trains are not totally independent. ICIs also vary within each click train since dolphins produce multiple clicks in each click train to continuously update their inspected distance. To prevent the pseudo-replication in sample size, only one click train was randomly selected from all click trains of each school at each re-sampling. Then, only one ICI was randomly extracted between the mean ± SD ICI from this selected click train. Based on the extracted ICI, the maximum inspected distance of each school was calculated and categorized as short (<15 m), medium (15–45 m), or long (>45 m) range, assuming that the underwater sound speed was 1,500 m/s on the average. The percentages of the three categories of maximum inspected distance among the three movements were compared by ANOVA and Tukey’s honestly significant difference (HSD) tests after re-sampling 10,000 times. The re-sampling-based analysis was performed in Matlab 7.1 (MathWorks Inc., Natick, MA, USA).
Temporal occurrence analysis of movement types
In order to identify occurrence probabilities of the three movement types in each tidal phase, the observed number (O) of each movement type was compared to the expected number (E) over the four tidal phases by chi-squared tests. The expected number of each movement (m) in each tidal phase (t) was calculated by multiplying the total observed number of each movement type (O m) by the occurrence probability of dolphin schools in each tidal phase (P t),
The P t was calculated as
where O t represents the number of observed schools in each tidal phase and O * represents the total number of observed schools. Observed schools with duration of sound reception that occurred during one tidal phase but extended into the next tidal phase were assigned to the phase that contained the greater portion of duration of sound reception.
Spatial distribution analysis of movement types
To investigate the spatial distribution in the monitoring area of the three movement types within the tidal cycle, the proportion of encounters at the riverside (R) of each school was calculated as
where the N r represents the number of encounters for a school detected on the riverside, and N * represents the total number of encounters for that school. If R is equal to one, the whole school was considered to be present only on the riverside; on the other hand, the whole school was considered to be present only on the offshore side if R equals zero. Kruskal–Wallis ANOVA and Mann–Whitney U tests were used to compare the proportion of encounters at the riverside encounters between tidal phases to understand the distribution change of each movement type.
Results
Due to the lack of sufficient A-tags in the first year and the limited days with fine weather in fall and winter to retrieve the A-tags, only 268 days (6,282 h) of effective recordings were obtained during the study period (Table 1). Indo-Pacific humpback dolphins were detected by the A-tags on 78 % of the monitoring days, with 0.29 encounters/h or 0.10 schools/h on the average.
Temporal variation in encounter rates
The difference between the daytime and nighttime encounter rates was not significant on both the riverside (Mann–Whitney U test, U = 487, N 1 = 32, N 2 = 32, P = 0.74) and the offshore side (Mann–Whitney U test, U = 432, N 1 = 32, N 2 = 32, P = 0.28) (Fig. 3). On the other hand, the variation in the encounter rates among the four tidal phases showed significant difference on the riverside (Kruskal–Wallis ANOVA, H 3,120 = 9.51, P = 0.02), but not on the offshore side (Kruskal–Wallis ANOVA, H 3,120 = 4.18, P = 0.24). On the riverside, the encounter rates during ebb tides were significantly lowest than those in the other tidal phases (Fig. 4). Accordingly, the succeeding analysis focused only on the tidal cycle rather than on the diurnal cycle.
Median encounter rates of humpback dolphins on the riverside and the offshore side during the four tidal phases. The shaded boxes represent the range of the first quartile to the third quartile, and the error bars represent the range of all data. The letters above the bars denote Mann–Whitney U test post hoc grouping
Behavioral analysis
The numbers of schools that were identified as displaying circling, traveling, or unidentified movements were 32, 44, and 37, respectively. These three movement types were associated with significantly different durations of sound reception (Kruskal–Wallis ANOVA, H 2,113 = 28.23, P < 0.001). Longest durations were observed during circling movements, while shortest duration was found during traveling (Table 2). The averaged ICI of all three movements showed bimodal distribution, with the first peak between 15 and 20 ms and the other peak between 30 and 50 ms (Fig. 5). The three movements had different compositions of maximum inspected distance, which is supported by the significant percentage differences for the three categories of maximum inspected distance between the three movement types in the re-sampling analysis (ANOVA, F (2,29997) = 953.04, 9130.37, and 14513.8 for short-, medium-, and long-range inspected distance, respectively, P < 0.001 for all cases). The circling movements used a higher percentage of medium-range inspected distance but a lower percentage of short- and long-range inspected distance than the traveling movements (Fig. 6). Maximum inspected distance longer than 15 m (medium- and long-range categories) suggested that the dolphins were inspecting a distance greater than the water depth of their primary habitat. This shows that the dolphins were not inspecting the seafloor vertically.
Mean percentages of the three categories of maximum inspected distance (MID) for the three movements. The short, medium, and long ranges represent <15, 15–45, and >45 m of MID, respectively. The error bars represent the SD of the 10,000 re-sampled data. The letters above the bars denote significant difference based on Tukey HSD test post hoc grouping
Temporal occurrence of movement types
The occurrence probabilities changed significantly over the tidal cycle for the circling movements (chi-square test, \( \chi_{4}^{2} \) = 11.14, P < 0.01), but not for either the traveling (chi-square test, traveling: \( \chi_{4}^{2} \) = 3.44, P < 0.33) or the unidentified movements (chi-square test, \( \chi_{4}^{2} \) = 0.47, P < 0.93). The circling movements occurred more frequently during flood tides and less frequently during ebb tide; however, other movement types did not exhibit a similar tidal presence pattern (Fig. 7).
Spatial distribution of movement types
The percentage of riverside encounters of circling movements changed significantly over the four tidal phases (Kruskal–Wallis ANOVA, H 3,32 = 9.62, P = 0.02). Compared to the relatively wider distribution range during flood tide, circling movements occurred mainly on the riverside in a narrower distribution range during high tide. Furthermore, the only circling school observed during ebb tide was found mainly on the offshore side (Fig. 8). In contrast, the percentage of riverside encounters of other movement types showed no significant changes over the four tidal phases (Kruskal–Wallis ANOVA, traveling: H 3,44 = 3.87, P = 0.28; unidentified: H 3,37 = 5.49, P = 0.14).
Median proportion of encounters at the riverside (R) of the three movement types in each tidal phase. When R equals to one, it means that all encounters were detected on the riverside; in contrast, all encounters were detected on the offshore side when R equals to zero. The shaded boxes represent the range of the first quartile to the third quartile, and the error bars represent the range of all data. The letters above the bars denote Mann–Whitney U test post hoc grouping
Discussion
The present study shows that the acoustic encounter rate of humpback dolphins varied significantly on the riverside as a function of the tidal cycle instead of the diurnal cycle. Furthermore, the three movement types, characterized by varied behavioral patterns, displayed different temporal and spatial presence patterns over the four tidal phases. The utilization pattern of humpback dolphins in their estuarine habitat is thus shown to be correlated with the tidal cycle.
To determine which periodic factor was related to the temporal variation in humpback dolphin habitat use, both the diurnal and tidal cycles were investigated. This level of comprehensive daily monitoring cannot be accomplished by visual observation and can only be achieved by PAM. Still, there are some limitations to PAM. Its detection range varies with the orientation of the echolocating animal, given that the beam pattern of clicks produced by odontocetes is highly directional (Au 1980; Au et al. 1987). The detection range is much longer if the dolphin head is directly oriented toward the hydrophone (on-axis). Any head orientation that is not on-axis would reduce the detection range. As a result, the presence of echolocating animals might have not been detected when they were not orientated toward our acoustic sensors or when they passed by the monitoring area quickly. However, the long duration and continuous monitoring in this study should be able to capture the humpback dolphins’ occurrence patterns, especially for frequently occurring behaviors.
Our finding that the acoustic encounter rates of the humpback dolphins on the riverside changed significantly over the tidal cycle is similar to the sighting rate variation of estuarine bottlenose dolphins (Mendes et al. 2002; Fury and Harrison 2011). Varied presence patterns in the tidal cycle between different behavioral states (e.g., foraging and traveling) were also reported for both the humpback dolphins and the costal bottlenose dolphins (Saayman and Tayler 1979; Hanson and Defran 1993). Still, the occurrence pattern of humpback dolphins can be site-specific. The tide-based presence pattern reported by the present study is different from those documented in Algoa Bay, South Africa, and in Hong Kong waters. The change of sighting rates was related to the diurnal cycle in Algoa Bay (Karczmarski et al. 2000), and the sighting rate was higher during ebb tides than flood tides in Hong Kong waters (Parsons 1998). These site-specific variations may be due to the influence exerted by river discharges in a given study area. At greater distances from the river mouth, the freshwater–seawater interaction induced by the tidal activity could be lessened to the point that the tidal cycle ceases to be a factor in determining the presence pattern of humpback dolphins. While a wider area that includes habitats less influenced by river discharges was covered, a different occurrence pattern of humpback dolphins could be found.
The tide-related presence pattern was not observed on the offshore side which shows that the humpback dolphins exhibit temporal and spatial variations in their habitat utilization. The habitat utilization of humpback dolphins was determined based on the traveling and circling schools, which represented two clearly different behavioral patterns. Despite absence of direct measurement on the inspected distance of humpback dolphins, the variations in the ICIs can be assumed to be directly proportional to the acoustic sensing range (Akamatsu et al. 2005b; Madsen et al. 2005). Short-range inspected distance occurs when the echolocating animal is inspecting its target from within only a few body lengths or scanning the seafloor. On the other hand, medium- and long-range inspected distances suggest that the echolocating dolphins are not vertically inspecting the seafloor. Our results show that the traveling movements mainly involve inspecting long-distance targets and passing through the monitored area without lingering. In contrast, circling movements, which showed not only more complex trajectories but also remained in an area for significantly longer periods of time, are thought to be related to foraging behaviors. Moreover, the lower percentage of short-range inspected distance and higher percentage of medium-range inspected distance in circling movements suggest that the behavior of vertically inspecting the seafloor occurs less often. Similar observations were recorded for Hawaiian spinner dolphins (Stenella longirostris) where short-range inspected distance was rarely recorded when they were cooperatively herding fish schools in circling formations (Benoit-Bird and Au 2009). In addition, the first author has observed humpback dolphins feeding on the epipelagic Perth herring (Nematalosa come) with back and forth movement which is similar to the circling movement observed through acoustic trajectories in this study. The circling movement is different from the widely dispersed group formation observed in the bottom feeding strategy (Karczmarski et al. 1997; Parra 2006). Therefore, the main prey targets of the circling dolphins in the present study are very likely to be epipelagic fish, rather than benthic fish.
Unlike the other movement types which had no significant periodic variation, circling movements occurred more frequently during flood tide and less frequently during ebb tide. Despite the small study area, the spatial distribution analysis revealed that dolphins displaying circling movements occurred on both the riverside and the offshore side during flood tide. They moved to the riverside during high tide and then moved back to the offshore side during ebb tide, which corresponded with the difference in encounter rates between the two monitoring sides during high and ebb tides. This spatial distribution pattern of circling humpback dolphins is similar to that of the bottlenose dolphins reported by Scott et al. (1990). They observed that the bottlenose dolphins follow the movement of striped mullet Mugil cephalus (Mugilidae) to shallow waters during flood tides and then return to deeper waters during ebb tides. This predator–prey interaction suggests that the distribution of the prey fish is a direct factor in determining the movement patterns of dolphins (Hastie et al. 2004). Marine animals, including decapods and fish, use selective tidal-stream transport to migrate between sub-tidal resting grounds and intertidal feeding grounds (Tankersley and Forward 2001; Gibson 2003). The prey species of humpback dolphins can include benthic fish (majorly family Sciaenidae) and epipelagic fish (family Engraulidae, Clupeidae, Trichiuridae, and Mugilidae) (Jefferson 2000; Barros et al. 2004; Parra and Jedensjö 2009). Although no clear tidal presence patterns of their benthic prey, such as croakers, have been reported, their epipelagic fish prey, including anchovies and mullets, have been observed to ride the flood tide into the intertidal zone and mill around during high tide (Krumme 2004, 2009). The tidal-driven movement of the epipelagic fish prey leads to the tidal occurrence of circling movement of humpback dolphins. Therefore, the occurrence pattern of humpback dolphins in the focal estuary follows the tidal cycle in order to maximize their foraging opportunities.
The tidal-driven activity of the estuarine dolphins and their prey can be influenced by the interaction between freshwater and seawater. Mendes et al. (2002) reported that the higher sighting rates of bottlenose dolphins during flood tides in the Moray Firth, Scotland, are correlated with the formation of the tidal intrusion front near the river mouth. The tidal front in an estuary can cause turbulence at the frontal region and result in an accumulation of higher plankton concentrations which can attract relevant predators (Franks 1992; Largier 1993). The activity of a tidal front should be investigated in future studies to understand the hydrological characteristics of the focal estuary. The monitoring area should be extended to the waters close to the river mouth to better understand the habitat-use pattern of humpback dolphins in an estuary and its relations with the tidal front. To determine whether epipelagic fish are the primary target of the humpback dolphins’ circling movement, an echo-sounder can be employed to investigate the presence of epipelagic fish. The proposed studies can help to understand the feeding habits of humpback dolphins, which are currently unknown for Western Taiwan population, and provide a solid base for the regulation of fishery activities in estuarine waters. The fishery activities can directly threaten the humpback dolphins through the entanglement or indirectly affect their habitat-use pattern by changing the availability of prey. The temporal variations, including the daily activity presented in this study and the seasonal variation need further investigation, should also assist the competent authorities of government to designate proper regulation on the fishery activities to decrease the impact on this vulnerable population of humpback dolphins.
References
Akamatsu T, Matsuda A, Suzuki S, Wang D, Wang K, Suzuki M, Muramoto H, Sugiyama N, Oota K (2005a) New stereo acoustic data logger for free-ranging dolphins and porpoises. Mar Technol Soc J 39:3–9
Akamatsu T, Wang D, Wang K, Naito Y (2005b) Biosonar behaviour of free-ranging porpoises. Proc R Soc Lond B 272:797–801
Akamatsu T, Wang D, Wang K, Li S, Dong S, Zhao X, Barlow J, Stewart BS, Richlen M (2008) Estimation of the detection probability for Yangtze finless porpoises (Neophocaena phocaenoides asiaeorientalis) with a passive acoustic method. J Acoust Soc Am 123:4403–4411
Akamatsu T, Nakamura K, Kawabe R, Furukawa S, Murata H, Kawakubo A, Komaba M (2010) Seasonal and diurnal presence of finless porpoises at a corridor to the ocean from their habitat. Mar Biol 157:1879–1887
Au WWL (1980) Echolocation signals of the Atlantic bottlenose dolphin (Tursiops truncatus) in open waters. In: Busnel RG, Fish JF (eds) Animal sonar systems. Plenum Press, New York, pp 251–282
Au WWL (1993) The sonar of dolphins. Springer, New York
Au WWL, Penner RH, Turl CW (1987) Propagation of beluga echolocation signals. The J Acoust Soc Am 83:807–813
Barros NB, Jefferson TA, Parsons ECM (2004) Feeding habits of Indo-Pacific humpback dolphins (Sousa chinensis) stranded in Hong Kong. Aquat Mamm 30:179–188
Bel’kovich VM, Ivanova EE, Yefremenkova OV, Kozarovitsky LR, Kharitonov SP (1991) Searching and hunting behavior in the bottlenose dolphin (Tursiops truncatus) in the Black Sea. In: Pryor K, Norris KS (eds) Dolphin societies: discoveries and puzzles. University of California Press, Berkeley and Los Angeles, pp 38–67
Benoit-Bird KJ, Au WWL (2009) Cooperative prey herding by the pelagic dolphin, Stenella longirostris. J Acoust Soc Am 125:125–137
Carlström J (2005) Diel variation in echolocation behavior of wild harbor porpoises. Mar Mamm Sci 21:1–12
Chou LS, Lee JD, Lee PF, Kao CC, Shao KT, Chuang CT, Chen MH, Chen CF, Wei RC, Yang WC, Tsai HC (2011) Population ecology, critical habitat and master planning for marine mammal protected area of Indo-Pacific humpback dolphins, Sousa chinensis. Final Report to Forest Bureau, Council of Agriculture, Republic of China. No. 99-FD-08.1-C-32. http://ethics.forest.gov.tw/public/Attachment/181616351971.pdf (in Chinese)
Franks PJS (1992) Sink or swim: accumulation of biomass at fronts. Mar Ecol Prog Ser 82:1–12
Fury CA, Harrison PL (2011) Seasonal variation and tidal influences on estuarine use by bottlenose dolphins (Tursiops aduncus). Estuar Coast Shelf Sci 93:389–395
Gibson RN (2003) Go with the flow: tidal migration in marine animals. Hydrobiologia 503:153–161
Goold JC, Jefferson TA (2004) A note on clicks recorded from free-ranging Indo-Pacific humpback dolphins, Sousa chinensis. Aquat Mamm 30:175–178
Hanson MT, Defran RH (1993) The behavior and feeding ecology of the Pacific coast bottlenose dolphin, Tursiops truncatus. Aquat Mamm 19:127–142
Hastie GD, Wilson B, Wilson LJ, Parsons KM, Thompson PM (2004) Functional mechanisms underlying cetacean distribution patterns: hotspots for bottlenose dolphins are linked to foraging. Mar Biol 144:397–403
Hung SK (2008) Habitat use of Indo-Pacific humpback dolphins (Sousa chinensis) in Hong Kong. Dissertation. The University of Hong Kong, Hong Kong
Jefferson TA (2000) Population biology of the Indo-Pacific hump-backed dolphin in Hong Kong waters. Wildl Monogr 144:1–65
Jefferson TA, Karczmarski L (2001) Sousa chinensis. Mamm Species 655:1–9
Karczmarski L, Thornton M, Cockcroft VG (1997) Description of selected behaviours of humpback dolphins, Sousa chinensis. Aquat Mamm 23:127–133
Karczmarski L, Cockcroft VG, McLachlan A (2000) Habitat use and preferences of Indo-Pacific humpback dolphins Sousa chinensis in Algoa Bay, South Africa. Mar Mamm Sci 16:65–79
Kimura S, Akamatsu T, Wang K, Wang D, Li S, Dong S, Arai N (2009) Comparison of stationary acoustic monitoring and visual observation of finless porpoises. J Acoust Soc Am 125:547–553
Kimura S, Akamatsu T, Li S, Dong S, Dong L, Wang K, Wang D, Arai N (2010) Density estimation of Yangtze finless porpoises using passive acoustic sensors and automated click train detector. J Acoust Soc Am 128:1435–1445
Krumme U (2004) Patterns in tidal migration of fish in a Brazilian mangrove channel as revealed by a split-beam echosounder. Fish Res 70:1–15
Krumme U (2009) Diel and tidal movements by fish and decapods linking tropical coastal ecosystems. In: Nagelkerken I (ed) Ecological connectivity among tropical coastal ecosystems. Springer Netherlands, Dordrecht, pp 271–324
Largier JL (1993) Estuarine fronts: how important are they? Estuaries 16:1–11
Madsen PT, Johnson M, de Soto NA, Zimmer WMX, Tyack P (2005) Biosonar performance of foraging beaked whales (Mesoplodon densirostris). J Exp Biol 208:181–194
McLusky DS, Elliott M (2004) The estuarine ecosystem: ecology, threats, and management. Oxford University Press, New York
Mellinger DK, Stafford KM, Moore SE, Dziak RP, Matsumoto H (2007) An overview of fixed passive acoustic observation methods for cetaceans. Oceanography 20:36–45
Mendes S, Turrell W, Lütkebohle T, Thompson P (2002) Influence of the tidal cycle and a tidal intrusion front on the spatio-temporal distribution of coastal bottlenose dolphins. Mar Ecol Prog Ser 239:221–229
Moore SE, Stafford KM, Mellinger DK, Hildebrand JA (2006) Listening for large whales in the offshore waters of Alaska. Bioscience 56:49–55
Parra GJ (2006) Resource partitioning in sympatric delphinids: space use and habitat preferences of Australian snubfin and Indo-Pacific humpback dolphins. J Anim Ecol 75:862–874
Parra GJ, Jedensjö M (2009) Feeding habits of Australian snubfin (Orcaella heinsohni) and Indo-Pacific humpback dolphins (Sousa chinensis). Project Report to the Great Barrier Reef Marine Park Authority, Townsville and Reef & Rainforest Research Centre Limitred, Cairns, Australia. http://www.rrrc.org.au/publications/downloads/141e-UQ-Parra-G-etal-2009-Feeding-Habits-of-Snubfin-and-Pacific-Humpback-Dolpins.pdf
Parsons ECM (1998) The behaviour of Hong Kong’s resident cetaceans: the Indo-Pacific hump-backed dolphin and the finless porpoise. Aquat Mamm 24:91–110
Reeves RR, Dalebout ML, Jefferson TA, Karczmarski L, Laidre K, O’Corry-Crowe G, Rojas-Bracho L, Secchi ER, Slooten E, Smith BD, Wang JY, Zhou K (2008) Sousa chinensis (eastern Taiwan Strait subpopulation). In: IUCN 2011. IUCN Red List of Threatened Species. Version 2011.1
Ross PS, Dungan SZ, Hung SK, Jefferson TA, Macfarquhar C, Perrin WF, Riehl KN, Slooten E, Tsai J, Wang JY, White BN, Würsig B, Yang SC, Reeves RR (2010) Averting the Baiji syndrome: conserving habitat for critically endangered dolphins in Eastern Taiwan Strait. Aquat Conserv 20:685–694
Saayman GS, Tayler CK (1979) The socioecology of humpback dolphins (Sousa sp.). In: Winn HE, Olla BL (eds) The behavior of marine animals, vol 3: Cetaceans. Plenum Press, New York, pp 165–226
Scott MD, Wells RS, Irvine AB (1990) A long-term study of bottlenose dolphins on the west coast of Florida. In: Leatherwood S, Reeves RR (eds) The bottlenose dolphin. Academic Press, San Diego, pp 235–244
Soldevilla MS, Wiggins SM, Hildebrand JA (2010) Spatial and temporal patterns of Risso’s dolphin echolocation in the Southern California Bight. J Acoust Soc Am 127:124–132
Soldevilla MS, Wiggins SM, Hildebrand JA, Oleson EM, Ferguson MC (2011) Risso’s and Pacific white-sided dolphin habitat modeling from passive acoustic monitoring. Mar Ecol Prog Ser 423:247–260
Tankersley RA, Forward RB (2001) Selective tidal-stream transport of marine animals. Oceanogr Mar Biol Annu Rev 39:305–353
Thomas JA, Turl CW (1990) Echolocation characteristics and range detection threshold of a false killer whale (Pseudorca crassidens). In: Thomas JA, Kastelein RA (eds) Sensory abilities of cetaceans: laboratory and field evidence. Plenum Press, New York, pp 321–334
Todd VLG, Pearse WD, Tregenza NC, Lepper PA, Todd IB (2009) Diel echolocation activity of harbour porpoises (Phocoena phocoena) around North Sea offshore gas installations. ICES J Mar Sci 66:734–745
Wang JY, Yang SC, Hung SK, Jefferson TA (2007) Distribution, abundance and conservation status of the eastern Taiwan Strait population of Indo-Pacific humpback dolphins, Sousa chinensis. Mammalia 71:157–165
Wang JY, Hung SK, Yang SC, Jefferson TA, Secchi ER (2008) Population differences in the pigmentation of Indo-Pacific humpback dolphins, Sousa chinensis, in Chinese waters. Mammalia 72:302–308
Acknowledgments
This study was supported by the Formosa Plastics Group and Research and Development Program for New Bio-industry Initiatives of Japan. We thank the Tainan Hydraulics Laboratory, National Cheng Kung University, and Industrial Development Bureau, Ministry of Economic Affairs, Taiwan, for allowing us access the fixed pile in the Xin Huwei River Estuary. We appreciate Li-Cing Mei, Long-Jhen Lin, Siou-Syong Wu, and the members of the Cetacean Laboratory, National Taiwan University for their dedicated support to the field surveys on the Indo-Pacific humpback dolphins of the west coast of Taiwan. We thank Wu-Jung Lee, Shiang-Lin Huang, Shane Guan, and Florence Evacitas for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Siebert.
Rights and permissions
About this article
Cite this article
Lin, TH., Akamatsu, T. & Chou, LS. Tidal influences on the habitat use of Indo-Pacific humpback dolphins in an estuary. Mar Biol 160, 1353–1363 (2013). https://doi.org/10.1007/s00227-013-2187-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-013-2187-7