Abstract
This study examined the response of a coral holobiont to thermal stress when the bacterial community was treated with antibiotics. Colonies of Pocillopora damicornis were exposed to broad and narrow-spectrum antibiotics targeting coral-associated α and γ-Proteobacteria. Corals were gradually heated from the control temperature of 26 to 31 °C, and measurements were made of host, zooxanthellar and microbial condition. Antibiotics artificially reduced the abundance and activity of bacteria, but had minimal effect on zooxanthellae photosynthetic efficiency or host tissue protein content. Heated corals without antibiotics showed significant declines in F V /F M , typical of thermal stress. However, heated corals treated with antibiotics showed severe tissue loss in addition to a decline in F V /F M . This study demonstrated that a disruption to the microbial consortium diminished the resilience of the holobiont. Corals exposed to antibiotics under control temperature did not bleach, suggesting that temperature may be an important factor influencing the activity, diversity and ecological function of the holobiont bacterial community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reef-building corals exist as a unique multi-partner symbiosis, generally recognised to comprise the cnidarian host and a dinoflagellate algal partner, known as zooxanthellae (genus Symbiodinium). The term ‘holobiont’ has been used to describe this host–algal partnership functioning as a whole (Rowan 1998; Baird et al. 2009). However, there is increasing evidence to suggest that a third essential partner has been largely overlooked until recently in the holobiont symbioses: the coral microbial consortium (Rohwer et al. 2002). As a relatively recent field of study, and requiring different sampling approaches compared to the other holobiont components, our understanding of the microbial consortium of corals and its function within the holobiont remains comparatively limited (Rosenberg et al. 2007a; Ainsworth et al. 2010).
The coral microbial consortium contains a highly diverse community comprising Bacteria, viruses and Archaea (Rosenberg et al. 2007b). Bacteria in particular have been found to demonstrate specific associations with certain coral species (Rohwer et al. 2002) as well as within different habitats of the coral structure (Rosenberg et al. 2007b). These habitats include the surface mucus layer (SML), within the cnidarian and zooxanthellae tissue and within the host skeleton (Bourne and Munn 2005; Koren and Rosenberg 2006; Sweet et al. 2011a). While less is known about the influence of viruses (Thurber et al. 2008; van Oppen et al. 2009) and Archaea found on corals (Rosenberg et al. 2007a), Bacteria are known to perform several important roles including pathogen defence, particularly associated with the SML (Ritchie and Smith 2004; Ritchie 2006; Rypien et al. 2010), nitrogen fixation (Shashar et al. 1994; Lesser et al. 2004, 2007), cycling of sulphur compounds (Raina et al. 2009), chitin decomposition (Ducklow and Mitchell 1979) and photosynthesis (Rosenberg et al. 2007a). Thus, bacteria contribute significant beneficial ecological services to the collective function of the holobiont (Rosenberg et al. 2007a; Ainsworth et al. 2010).
This expanding knowledge of the functional roles of bacteria has provided new perspectives on coral health and disease to challenge the existing paradigm concerning the phenomenon of coral bleaching (Toren et al. 1998; Kushmaro et al. 2001; Ben-Haim and Rosenberg 2004; Rosenberg 2004; Reshef et al. 2006; Rosenberg et al. 2007a). The current coral bleaching paradigm suggests that stress caused by high light and elevated temperature (as small as 1–2 °C above the summer average; Hoegh-Guldberg 1999) causes expulsion of the zooxanthellae or degradation of algal pigments (Glynn 1996). This leads to a paled or ‘bleached’ appearance due to the visibility of the coral skeleton (Jones 1997). These stressors are well understood to cause zooxanthellae dysfunction in the early stages of bleaching, suggesting that the algal symbiont is the initial site of the holobiont collapse (Jones et al. 1998; Warner et al. 1999; Hill et al. 2004, 2011). Bacterial infection also leads to coral mortality, though the mechanism of infection caused by each bacterial species differs (Ben-Haim and Rosenberg 2002; Rosenberg and Ben-Haim 2002). These studies have demonstrated that bacterial bleaching may occur as a result of both external pathogen infection (Fig. 1b; Kushmaro et al. 1998) and from a naturally occurring bacterium whose pathogenicity increases under elevated temperature (Fig. 1c; Ben-Haim et al. 2003; Ben-Haim and Rosenberg 2004). The latter process is known as the ‘Bacterial Bleaching Hypothesis’ (BBH) (Rosenberg 2004) and is particularly relevant to thermal stress as demonstrated in laboratory studies where bacterial species Vibrio coralliilyticus and V. shioli have been separately identified as causative agents for coral bleaching in at least two coral species (Rosenberg et al. 2007a). However, it is still a matter of debate as to whether bacterial infection is the primary cause of coral bleaching and not merely a secondary invasion following bleaching via environmental stress (Rosenberg 2004; Ainsworth et al. 2008) (Fig. 1a).
Schematic diagram of potential pathways of coral bleaching (a–d) and this study’s experimental approach (e). a Environmental bleaching: environmental stress causes a breakdown in holobiont function causing bleaching, followed by external or opportunistic pathogen colonisation resulting in disease as a secondary response; b Disease: introduced pathogen successfully colonises healthy coral and causes a bleaching response; c Bacterial bleaching: temperature stress causes a shift in the natural microbial assemblage (increases virulence of endemic pathogens) which leads to bleaching as a primary response; (d) Environmental stress: environmental stress causes a shift in the natural microbial assemblage which either leads to coral resilience or causes disease. (e) Experimental manipulation: coral bacterial community altered by antibiotic exposure followed by thermal stress, predicted to result in holobiont deprivation of essential bacterial services leading to mortality, or alternatively, continued provision of bacterial services to the holobiont, including thermal stress resilience
In contrast to their potential negative impacts associated with bleaching and disease, there has been much less focus on the positive (i.e. nutritional or prophylactic) role bacteria provide as a necessary component for maintaining coral health and function or increasing their resilience to future environmental stress (Fig. 1d; Mouchka et al. 2010). Previous studies have involved isolating and identifying bacterial strains to uncover specific functions they provide to the holobiont (Koh 1997; Lesser et al. 2004; Ritchie and Smith 2004; Ritchie 2006; Rypien et al. 2010). However, detailing bacterial interactions between holobiont partners may be of great importance for corals in the future, in determining their adaptability and resilience to stress in the face of climate change. To address this knowledge gap, we assessed the role of the coral microbial consortium in holobiont resilience to thermal stress.
In this study, corals were treated with antibiotics to artificially manipulate their microbial consortium and were then exposed to thermal stress conditions (Fig. 1e). Our hypothesis was that antibiotic exposure would decrease the abundance and activity of bacteria associated with the mucus and coral tissue, and reduce any positive effect on the condition of the cnidarian and zooxanthellae partners, such that the holobiont would deteriorate more quickly under heat stress.
Materials and methods
Coral specimens
The study was conducted using the thermally sensitive, reef-building coral, Pocillopora damicornis (Ulstrup et al. 2006). Colonies were collected from Heron Island reef flat (151°55′E, 23°27′S) in July, 2009. Colonies were broken up into experimental units (nubbins) 2–3 cm in length and suspended from nylon strings, following the methods of Davies (1984). Nubbins were acclimated over a minimum of 3 weeks in recirculating artificial seawater (carbonate [140 ppm] and synthetic sea salt [Aquasonic ‘Ocean Nature’] adjusted to salinity of 33 in reverse osmosis water) at 26 ± 0.1 °C and a 12:12 h light:dark cycle (on at 0900 h and off at 2100 h) at a light intensity of 150 μmol photons m−2 s−1. For experimentation, nubbins were transferred into four separate 12 L surface-sterilised treatment tanks. Each tank was filled to 5 L capacity with 0.2 μm artificial filtered seawater (FSW). Transparent plastic film was applied over each tank to limit the potential for external airborne bacteria entering the aquaria. Tanks were aerated using autoclaved air-stones and tubing with a 0.2 μm inlet filter to further limit external bacterial introduction.
Experimental design
The experimental design involved the comparison of four treatments on P. damicornis to assess the outcome of an intact versus disrupted microbial community at control (26 °C) versus heat-stressed (up to 31 °C) conditions. Antibiotics were used to reduce bacterial activity and/or abundance on nubbins over 5 days. In the elevated temperature treatment, temperature was gradually ramped to reach 31 °C by 2100 h on day 3. The experiment involved the removal of 4 nubbins (n = 4) at T (time) 0 h for analyses, detailed below. An additional three nubbins were also removed at T 0 h for protein measurements and again on the final experimental day, T 120 h (n = 3). For regular analyses (including measurements of: F V /F M , chlorophyll a and c 2 content, zooxanthellae abundance and microbial consortium activity), four nubbins (n = 4) were taken at T 24, 72 and 120 h, while three nubbins (n = 3) were taken at T 96 h due to limitations in sample availability. In all cases of n = 3, the replication was sufficient to detect significant variation.
Nubbins were numbered using labels attached to nylon strings and were randomly selected for measurement on each day. Once removed from their treatment, nubbins were photographed to record their physical condition. All corals were transferred to tanks filled with fresh filtered seawater (FSW) (0.2 μm pore size) following overnight antibiotic exposure regardless of treatment. This allowed for any variation in tank conditions to reset in all treatments each day.
Coral bacterial consortia were manipulated through exposing relevant nubbins to an initial 12 h overnight dose of antibiotics, while those not receiving antibiotics were maintained in 0.2 μm FSW at their allocated treatment temperature. Antibiotic exposure was repeated each night of the experiment (2100 to 0900 h). A combination of Ampicillin (1 mg mL−1), Streptomycin (1 mg mL−1), Ciprofloxacin (0.25 mg mL−1) and Naladixic Acid (0.1 mg mL−1) was applied each night, followed by 12 h in fresh FSW. These antibiotics were selected to target bacteria types found to be associated with P. damicornis, including tissue and skeletal dwelling γ-Proteobacteria species such as Vibrio spp. and α-Proteobacteria species particularly found to inhabit the SML (Bourne and Munn 2005). All treatments were transferred to FSW at temperatures relevant to their treatments at the beginning of the 12 h light period at 0900 h each day to maintain sample handling between treatments. The temperature, salinity and pH of each treatment were monitored daily for the duration of the 5 day experiment.
Coral nubbins were removed from treatments for destructive analysis of host, zooxanthellae and microbial parameters at the beginning of the experiment (T 0 h), prior to temperature ramping of heated treatments (T 24 h), post-temperature ramping (T 72 h) and each day at 31 °C (T 96 and 120 h).
Destructive sampling involved removal of coral tissue and mucus using the airbrushing technique (Levy et al. 2006) by placing nubbins in individual zip-lock bags with 5 mL of 0.2 μm artificial FSW. The resultant slurry was transferred to a sterile 50 mL falcon tube and the zip-lock bag rinsed twice with 5 mL of 0.2 μm artificial FSW to collect any residual sample and added to the falcon tube. The slurry was used for measuring zooxanthellae abundance, chlorophyll a and c 2 concentration and bacterial activity. The additional nubbins were used for protein analysis at T 0 and T 120 h and were airbrushed using 0.2 μm filtered Milli-Q water to prevent salinity interference with spectrophotometry. All skeletons were kept for subsequent surface area determination.
Zooxanthellae abundance
The abundance of algal symbionts was determined by direct counts using a haemocytometer. Airbrushed samples were centrifuged at 1,000×g for 5 min and the supernatant removed. The concentrated zooxanthellae pellet was re-suspended in 10 mL of 0.2 μm of FSW. Samples were inverted twice before sub-sampling to keep zooxanthellae in suspension. Each haemocytometer grid was observed under a light microscope at 400× magnification. A total of eight replicate counts per nubbin sample were conducted, and cell density was determined per cm2 following surface area calculations.
Chlorophyll a and c 2 content
The re-suspended algal cells from zooxanthellae enumeration were centrifuged at 1,000×g for 5 min to re-isolate the remaining zooxanthellae pellet. The pellet was re-suspended in 4 mL of 90 % acetone and vortexed for 1 min. Samples were kept in darkness at 4 °C for 20 h to extract pigments. Vortexing was repeated before a second centrifugation to obtain particle-free supernatant. Spectrophotometric analysis at 630 and 664 nm was conducted and chlorophyll a and c 2 concentrations were determined following the methods of Jeffrey and Humphrey (1975). Data were normalised to nubbin surface area to achieve final units of μg pigments cm−2.
Microbial consortium activity
Ninety-six-well Biolog EcoPlates (Biolog Inc., Hayward, CA, USA) were used to monitor microbial activity and were measured every 24 h over the 5 day experimental period. Such plates have been used to assess heterotrophic bacteria activity in marine environments (Schultz and Ducklow 2000; Sala et al. 2005) and were used to assess both the viability of bacteria and their metabolic diversity. The airbrushed coral tissue slurry was vortexed in a falcon tube until homogenised. Samples were then centrifuged at 1,000×g for 5 min, isolating the zooxanthellae. The remaining supernatant was filtered through a 40 μm mesh to remove any remaining host tissue material. While some bacteria may have been retained in the mesh, this step was undertaken to limit the introduction of additional carbon sources derived from the cnidarian fraction of the slurry. The homogenate was poured into sterile containers and 150 μL dispensed into each 96-well plate under sterile laminar flow conditions. The container was gently mixed before drawing up each aliquot to keep the sample homogenous. A stratified random approach was used when allocating individual nubbin samples between Biolog EcoPlates to accommodate potential variability between plates. Plates were then incubated under low light (10 μmol photons m−2 s−1) for 5 days and monitored for colour development each day, following the protocol of Choi and Dobbs (1999).
Biolog EcoPlates contain triplicate wells of 31 different substrates comprising sugars, phosphates, carbohydrates, polymers, amino acids, carboxylic acids and amines plus one blank control. Utilisation of the substrate causes reduction in the tetrazolium dye and leads to colour development within wells (Stefanowicz 2006). Each day, a plate reader (FluroStar Optima) was used to conduct duplicate readings of absorbance at both 590 nm (A590) and 750 nm (A750). Microbial substrate utilisation was determined by the formula A590–A750, using 750 nm measurements to correct for any turbidity (i.e. bacterial growth) in each well (Wang et al. 2008). Final absorbance values were determined following the subtraction of the control well signal from each substrate well.
Protein content
Holobiont protein (measured at T 0 and T 120 h) was determined using a 0.1 % solution of sodium dodecyl sulphate (SDS) added to the coral slurry as described by Berner et al. (1993). Following centrifugation at 3,500×g for 10 min, 4 mL of supernatant was added into quartz cuvettes for spectrophotometric absorbance readings at 235 and 280 nm following methods of Whitaker and Granum (1980): Protein = (A235) − (A280). The results were standardised against bovine serum albumin (BSA) standard with an R 2 value = 1.000 and reported per cm2 of coral surface.
Coral surface area
Host, symbiont and bacterial parameters were all standardised to coral surface area to account for variation in nubbin sizes. Surface area of each nubbin was determined using a modification of the wax weight method of Stimson and Kinzie (1991), where coral skeletons were dipped in melted Paraffin wax for 2 s at 65 °C. Skeletal waxing was repeated three times, or until variation in wax weights were reduced to <5 %.
Maximum quantum yield (F V /F M ) of photosystem II
Non-destructive Pulse Amplitude Modulated (PAM) fluorometry was conducted daily on pigmented tissue of all nubbins using a Mini-PAM fluorometer (Walz, Germany). A 6-mm fibre optic was used to assess photosystem II photosynthetic efficiency of zooxanthellae. Corals were dark-adapted for 10 min prior to the application of a 0.8 s saturating pulse (>4,000 μmol photons m−2 s−1) to measure maximum quantum yield of PSII (F V /F M ) (Schreiber 2004) (measuring light intensity <0.15 μmol photons m−2 s−1, gain = 2).
Statistical analyses
Multivariate analyses were conducted on Biolog EcoPlate data to assess similarities between microbial consortia using PRIMER software (version 5.2.4, Plymouth Marine Laboratories, UK). Specifically, the non-metric multi-dimensional scaling (MDS) ordination program was chosen to generate a similarity matrix using square-root transformed absorbance values and the Bray-Curtis similarity measure. Analysis of similarities (ANOSIM) and similarity percentage (SIMPER) analyses were then applied to assess the substrates that contributed most to the differences within and between treatments.
All other parameters were assessed using one-way or repeated measures ANOVA (rmANOVA; SPSS version 14.1) to identify any significant differences between treatments, or over time within the same treatment, respectively. Prior to running the analysis, statistical tests for homogeneity of variances (Levene’s test) and normality (Kolmogrov–Smirnov test) were carried out to ensure data met the assumptions of ANOVA. In cases where the assumptions of ANOVA were not met, data were transformed using arcsine transformation. Tukey’s post hoc comparisons were used to identify where significant differences were present between treatments.
Results
Microbial consortium activity
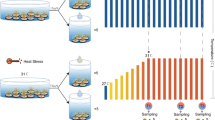
Biolog EcoPlate analysis revealed modification of the coral microbial consortia when exposed to antibiotics (Fig. 2). Heat did not cause a change in the pattern of substrate utilization by bacteria but median absorbance values after 96 h of incubation were lower in the control treatment (ranging from 0.094 to 0.155) compared to the heated treatment without antibiotics (median absorbance values between 0.214 and 0.261). Absorbance values were greater than 0.25 (indicating above background utilisation of polymers and carbohydrates) in 37 and 50 % of substrates for control and heat-stressed samples, respectively. Corals treated with antibiotics (irrespective of temperature treatment) showed reduced median absorbances of between 0.011 and 0.071. Only 2 % of wells from the antibiotic control treatment showed substrate utilization (principally carbohydrates and carboxylic acids) and there was no detectable colour development in any of the wells for the heat + antibiotic treatment. This indicates a reduction in bacterial activity and abundance as a result of antibiotic exposure. The multivariate analysis supported this finding with the MDS ordination showing more similarity in bacteria community metabolism with exposure to antibiotics compared with exposure to elevated temperature (Fig. 2).
Coral condition
Host protein was found to be significantly lower in the heat + antibiotics treatment by T 120 h compared to the antibiotic exposed control treatment (P = 0.048; Fig. 3). Visual comparison at T 120 h showed distinct differences between heated and control nubbins (Online resource 1). Corals maintained at 26 °C, regardless of antibiotic treatment, showed no visual differences (Online resource 1a, b). In contrast, corals subjected to thermal stress began paling by T 96 h with those exposed to antibiotics suffering prominent tissue loss (necrosis), by T 120 h (Online resource 1d). Corals under thermal stress without antibiotics were paler by T 120 h; however, retained coral tissue (Online resource 1c).
Host protein content of corals at T 0 h (white bar) and T 120 h (grey bars). Treatments are initial control (T 0), final day: control (C), antibiotic treatment (A), Heat-stress treatment (H) and heat-stress with antibiotics (H + A). Significant differences between T 0 h and heat-stressed antibiotic treatment indicated by asterisk. Mean ± SE (n = 3) are shown
Symbiont condition
Chlorophyll (chl) a and c 2 concentrations (μg cm−2) were stable across all treatments except in the heated treatment with antibiotics where Chl a declined from T 0 to T 120 h (P = 0.003) (Fig. 4a). The ratio of chl a to chl c 2 also remained relatively stable, with no significant differences between treatments.
a Chlorophyll a and b c 2 pigment concentrations normalised to coral surface area, c maximum quantum yield (F V /F M ) of PSII, and d zooxanthellae abundance in corals normalised to coral surface area. Data from T 0 to 120 h are shown for the control (black bars), antibiotic (dark grey bars), heat stressed (light grey bars) and heat stressed + antibiotics (white bars) treatments. Mean ± SE are shown (n = 4 at T 24, 72 and T 120 h, and n = 3 at T 96 h). Asterisk indicates significant declines from T 0 to T 120 h. Hash indicates significant differences between treatments
The maximum quantum yield (F V /F M ) of both heated treatments (±antibiotics) declined significantly from T 0 to T 120 h (P = 0.017 and 0.021) in comparison with the two unheated treatments that showed no significant change over time (P = 0.233 and 0.306). Only the heated treatment without antibiotics was found to be significantly lower than the unheated treatments at T 120 h (P = 0.001 and <0.001) (Fig. 4c), while the heat + antibiotics treatment was not significantly different from any of the other three treatments (Tukey’s post hoc comparisons).
Zooxanthellae abundance was variable in both control and antibiotic treatments maintained at 26 °C but heated corals exposed to antibiotics had lower zooxanthellae abundance than the other treatments at T 120 h (P = 0.001), showing a similar relationship with host tissue loss (Fig. 4d). Given the relatively constant chl a concentration and zooxanthellae abundance, the chl a content per zooxanthellae was not significantly different between treatments at T 120 h.
Discussion
This study revealed that an intact microbial consortium provides resilience against thermal stress. Exposure of the holobiont to antibiotics at control temperature resulted in no apparent change in host or zooxanthellae health compared to unexposed corals, despite a decline in the activity of bacteria. However, when heat was applied, antibiotic-treated corals experienced a more rapid and severe stress response with complete loss of host tissue, compared to heat-stressed corals with intact bacterial consortia that only experienced loss of zooxanthellae. This suggests bacteria are indirectly involved in thermal tolerance of the holobiont and could affect the likelihood and potential rate of recovery from bleaching events.
While Biolog plates are less sensitive than molecular techniques for assessing microbial activity, the EcoPlates were successful in assessing the effectiveness of the antibiotic treatments in reducing coral-associated bacterial activity. Treatments not receiving antibiotics had strong colour development (indicating substrate utilisation) in ≥37 % of substrates within 48 h of plate incubation. In contrast, there was very little colour development in any of the 32 wells containing antibiotic-treated samples during the 5 day plate incubation. While we cannot exclude that antibiotics caused changes in viability as well as mortality, we assume the changes revealed by Biolog plates reflect differences in the microbial community as a whole. Furthermore, antibiotic-treated nubbins were incubated in fresh seawater for 2 h before extracting material for Biolog analysis, so it is unlikely this result is due to carry-over of antibiotics into the well plates, preventing growth during the metabolism assays.
Contrary to recent studies demonstrating bacterial community shifts under increased temperature conditions (Bourne et al. 2008; Thurber et al. 2009; Rypien et al. 2010), this study found no measurable difference between community metabolism of culturable bacteria between heated and control treatments (−antibiotics). This likely reflects the limited sensitivity of the Eco Biolog plates in accommodating growth of only a small proportion (<1 %) of existing bacteria (Amann et al. 1995; Stefanowicz 2006). However, despite their potentially limited sensitivity, Eco Biolog plates showed a high degree of similarity in community substrate utilisation when P. damicornis extracts were incubated in a separate pilot study. Colour development within the wells was similar in eight of nine control samples, consistent with evidence of species-specific relationships between corals and bacteria (Bourne and Munn 2005; Ritchie 2006; Rosenberg et al. 2007a).
The visual appearance of corals provided clear evidence of tissue damage in the heated treatments, consistent with well-documented thermal bleaching of P. damicornis (D’Croz and Maté 2004; Jokiel 2004; Barron et al. 2010). Concentrations and ratios of chl a and c 2 pigments at optimal temperatures were consistent with previous studies on P. damicornis (Jones 1997; Hueerkamp et al. 2001). Significant declines in chl a concentrations were only detected between T 0 and T 120 h for the heated treatment receiving antibiotics (Fig. 4a) and can be attributed to the loss of host tissue by T 120 h, rather than a decline in chl a per zooxanthella. Zooxanthellae abundance was found to be relatively consistent in unheated treatments, as well as the heat stressed (antibiotic treatment) despite visual paling of corals at T 120 h (Fig. 4d). In contrast, corals receiving antibiotics under thermal stress displayed a significant decrease in zooxanthellae abundance from T 72 to T 120 h, correlating with tissue necrosis and chl a pigment declines at T 120 h.
The exposure to antibiotics reduced coral resilience to thermal stress and resulted in tissue detachment from the coral skeleton, not merely pigment/zooxanthellae loss. Release of whole-host cells, with symbionts still inside, from heat- and cold-stressed bleaching corals and the anemone Aiptasia pulchella has previously been reported (Gates et al. 1992; Brown et al. 1995). Tissue loss began at T 96 h and probably accounted for the significant decline in host protein content for the heat-stressed + antibiotic treatment in the final days of the experiment (Fig. 3). In comparison, host protein content for all other treatments remained consistent over time, indicating stable host condition.
The reason behind this enhanced tissue damage in heated treatments exposed to antibiotics is not yet clear. Our observations indicate that different mechanisms of zooxanthellae loss occur due to differing types and/or degrees of stress perceived by the holobiont. While application of antibiotics did not appear to decrease host and symbiont condition (although measurement of additional parameters such as host respiration rates may have increased our capacity to detect stress), the two stressors interacted to change the resilience of the holobiont. Changes to the coral-associated bacterial consortium (as caused by antibiotic treatment) could either enhance beneficial members of the consortium or increase virulence of pathogenic species (Ben-Haim et al. 2003), ultimately impacting the resilience of the holobiont. In combination, these stressors could have indirectly affected the holobiont through modifying the coral-associated bacteria community including the defence against pathogens. Sweet et al. (2011b), for example, examined the development of a modified bacterial community following antibiotic disturbance on the coral Acropora muricata. There was an initial dominance by antibiotic-resistant species from the natural microbiota that survived the treatment which were found to succeed in the absence of their usual bacterial competitors. This dominance was gradually reversed over 96 h as the original community began recovering (Sweet et al. 2011b). While Sweet et al. (2011b) allowed recruitment of bacteria from the surrounding water onto recovering corals, our experimental design limited the introduction of external bacteria into experimental tanks through use of plastic film to cover tanks and sterilized aquarium stones and tubing. Thus, bacteria dynamics are unlikely to have involved additions to the bacteria community, but rather involved dominance of antibiotic-resistant and/or thermally tolerant species that played different functional roles for the holobiont.
The antibiotic mixture applied in this study targeted γ-Proteobacteria including Vibrio species, yet corals in these treatments showed a more severe stress response than other treatments not exposed to antibiotics. Given antibiotics caused a decline in bacterial activity (Fig. 2), this suggests that the active remaining bacteria conferred thermal resilience to corals and was not the primary cause of bleaching under thermal stress in this species. An alternative explanation of these findings could be a temperature-modification to the activity of coral-associated viruses, which were not measured in this study. There is very limited understanding surrounding the influence of viruses to the coral holobiont (Thurber et al. 2008; van Oppen et al. 2009). Previous investigations have demonstrated that virus-like partices (VLP) appear to increase on corals and zooxanthellae in response to elevated temperature environments (Wilson et al. 2001, 2005) and may have the potential to contribute to coral bleaching (Wilson et al. 2005).
To our knowledge, this is the first study to investigate the role of coral-associated bacteria in heat-stressed colonies using a direct application of antibiotics to artificially manipulate the resident bacterial community. Our experiment shows that an undisturbed bacterial consortium ameliorates the thermal stress response, resulting in loss of zooxanthellae, not complete host cell (including zooxanthellae) detachment under heat stress. Intact microbial consortia therefore offer the holobiont resilience to thermal stress and increase the likelihood and potential rate of recovery after bleaching events.
References
Ainsworth TD, Fines M, Roff G, Hoegh-Gildberg O (2008) Bacteria are not the primary cause of bleaching in the Mediterranean coral Oculina patigonica. ISME J 2:67–73
Ainsworth TD, Thurber RV, Gates RD (2010) The future of coral reefs: a microbial perspective. Trends Ecol Evol 25:233–240
Amann RI, Ludwig W, Schleifer K-H (1995) Phylogenic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59:143–169
Baird AH, Bhagooli R, Ralph PJ, Takahashi S (2009) Coral bleaching: the role of the host. Trends Ecol Evol 24:16–20
Barron MG, McGill CJ, Courtney LA, Marcovich DT (2010) Experimental bleaching of a reef-building coral using a simplified recirculating laboratory exposure system. J Mar Biol Article ID 415167:8
Ben-Haim Y, Rosenberg E (2002) A novel Vibrio sp. pathogen of the coral Pocillopora damicornis. Mar Biol 141:47–55
Ben-Haim Y, Rosenberg E (2004) Temperature-regulated bleaching and tissue lysis of Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, New York, pp 301–324
Ben-Haim Y, Zicherman-Keren M, Rosenberg E (2003) Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl Envir Microbiol 69:4236–4242
Berner T, Baghdasarian G, Muscatine L (1993) Repopulation of a sea anemone with symbiotic dinoflagellates: analysis by in vivo fluorescence. J Exp Mar Biol Ecol 170:145–158
Bourne DG, Munn CB (2005) Diversity of bacteria associated with the coral Pocillopora damicornis from the Great Barrier Reef. Environ Microbiol 7:1162–1174
Bourne D, Iida Y, Uthicke S, Smith-Keune C (2008) Changes in coral-associated microbial communities during a bleaching event. ISME J 2:350–363
Brown BE, Le Tissier MDA, Bythell JC (1995) Mechanisms of bleaching deduced from histological studies of reef corals during a natural bleaching event. Mar Biol 122:655–663
Choi KH, Dobbs FC (1999) Comparison of two kinds of Biolog microplates (GN and ECO) in their ability to distinguish among aquatic microbial communities. J Microbiol Methods 36:203–213
D’Croz L, Maté JL (2004) Experimental responses to elevated water temperature in genotypes of the reef coral Pocillopora damicornis from upwelling and non-upwelling environments in Panama. Coral Reefs 23:473–483
Davies PS (1984) The role of zooxanthellae in the nutritional energy requirements of Pocillopora eydouxi. Coral Reefs 2:181–186
Ducklow HW, Mitchell R (1979) Bacterial populations and adaptations in the mucus layers on living corals. Limnol Oceanogr 24:715–725
Gates RD, Baghdasarian G, Muscatine L (1992) Temperature stress causes host cell detachment in symbiotic cnidarians: implications for coral bleaching. Biol Bull 182:324–332
Glynn PW (1996) Coral reef bleaching: facts, hypotheses and implications. Glob Change Biol 2:495–509
Hill R, Larkum A, Frankart C, Kühl M, Ralph PJ (2004) Loss of functional Photosystem II reaction centres in zooxanthellae of corals exposed to bleaching conditions: using fluorescence rise kinetics. Photosynth Res 82:59–72
Hill R, Brown CM, DeZeeuw K, Campbell DA, Ralph PJ (2011) Increased rate of D1 repair in coral symbionts during bleaching is insufficient to counter accelerated photo-inactivation. Limnol Oceanogr 56:139–146
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839–866
Hueerkamp C, Glynn PW, D’Croz L, Maté JL, Colley SB (2001) Bleaching and recovery of five eastern Pacific corals in an El Niño-related temperature experiment. Bull Mar Sci 69:215–236
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c 1 and c 2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanzen 167:191–194
Jokiel PL (2004) Temperature stress and coral bleaching. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, New York, pp 401–425
Jones RJ (1997) Zooxanthellae loss as a bioassay for assessing stress in corals. Mar Ecol Prog Ser 149:163–171
Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U (1998) Temperature induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant, Cell Environ 21:1219–1230
Koh EG (1997) Do scleractinian corals engage in chemical warfare against microbes? J Chem Ecol 23:379–398
Koren O, Rosenberg E (2006) Bacteria associated with mucus and tissues of the coral Oculina patagonica in summer and winter. Appl Environ Microbiol 72:5254–5259
Kushmaro A, Rosenberg E, Fine M, Ben-Haim Y, Loya Y (1998) Effect of temperature onbleaching of the coral Oculina patagonica by Vibrio shiloi AK-1. Mar Ecol Prog Ser 171:131–137
Kushmaro A, Banin E, Loya Y, Stackebrandt E, Rosenberg E (2001) Vibrio shiloi sp. nov., the causative agent of bleaching of the coral Oculina patagonica. Int J Syst Evol Microbiol 51:1383–1388
Lesser MP, Mazel CH, Gorbunov MY, Falkowski PG (2004) Discovery of symbiotic nitrogen-fixing cyanobacteria in corals. Science 305:997–1000
Lesser MP, Falcón LI, Rodríguez-Román A, Enríquez S, Hoegh-Guldberg O, Iglesias-Prieto R (2007) Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Mar Ecol Prog Ser 346:143–152
Levy O, Dubinsky Z, Achituv Y, Erez J (2006) Diurnal polyp expansion behavior in stony corals may enhance carbon availability for symbionts photosynthesis. J Exp Mar Biol Ecol 333:1–11
Mouchka ME, Hewson I, Harvell CD (2010) Coral-associated bacterial assemblages: current knowledge and the potential for climate-driven impacts. Integr Comp Biol 50:662–674
Raina J-B, Tapiolas D, Willis BL, Bourne DG (2009) Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Appl Environ Microbiol 75:3492–3501
Reshef L, Koren O, Loya Y, Zilber-Rosenberg I, Rosenberg E (2006) The coral probiotic hypothesis. Environ Microbiol 8:2068–2073
Ritchie KB (2006) Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar Ecol Prog Ser 322:1–14
Ritchie KB, Smith GW (2004) Microbial communities of coral surface mucopolysaccharide layers. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, New York, pp 259–264
Rohwer F, Seguritan V, Azam F, Knowlton N (2002) Diversity and distribution of coral-associated bacteria. Mar Ecol Prog Ser 243:1–10
Rosenberg E (2004) The bacterial disease hypothesis of coral bleaching. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, New York, pp 445–461
Rosenberg E, Ben-Haim Y (2002) Microbial diseases of corals and global warming. Environ Microbiol 4:318–326
Rosenberg E, Kellog CA, Rowher F (2007a) Coral microbiology. Oceanography 20:146–154
Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I (2007b) The role of microorganisms in coral health, disease and evolution. Nat Rev Microbiol 5:355–362
Rowan R (1998) Diversity and ecology of zooxanthellae on coral reefs. J Phycol 34:407–417
Rypien KL, Ward JR, Azam F (2010) Antagonistic interactions among coral-associated bacteria. Environ Microbiol 12:28–39
Sala MM, Arin L, Balagué V, Felipe J, Guadayol Ò, Vaqué D (2005) Functional diversity of bacterioplankton assemblages in western Antarctic seawaters during late spring. Mar Ecol Prog Ser 292:13–21
Schreiber U (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. In: Papageorgiou GC, Govindjee S (eds) Chlorophyll fluorescence: a signature of photosynthesis. Kluwer, Dordrecht, pp 279–319
Schultz GE, Ducklow H (2000) Changes in bacterioplankton metabolic capabilities along a salinity gradient in the York River estuary, Virginia, USA. Aquat Microb Ecol 22:163–174
Shashar N, Cohen Y, Loya Y, Sar N (1994) Nitrogen fixation (acetylene reduction) in stony corals: evidence for coral–bacteria interactions. Mar Ecol Prog Ser 111:259–264
Stefanowicz A (2006) The Biolog Plates technique as a tool in ecological studies of microbial communities. Polish J Environ Stud 15:669–676
Stimson J, Kinzie RA III (1991) The temporal pattern and rate of release of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus) under nitrogen-enrichment and control conditions. J Exp Mar Biol Ecol 153:63–74
Sweet MJ, Croquer A, Bythell JC (2011a) Bacterial assemblages differ between compartments within the coral holobiont. Coral Reefs 30:39–52
Sweet MJ, Croquer A, Bythell JC (2011b) Dynamics of bacterial community development in the reef coral Acropora muricata following experimental antibiotic treatment. Coral Reefs 30:1121–1133
Thurber RLV, Barott KL, Hall D, Liu H, Rodriguez-Mueller B, Desnues C, Edwards RA, Haynes M, Angly FE, Wegley L, Rohwer FL (2008) Metagenomic analysis indicates that stressors induce production of herpes-like viruses in the coral Porites compressa. Proc Natl Acad Sci USA 105:18413–18418
Thurber RV, Willner-Hall D, Rodriguez-Mueller B, Desnues C, Edwards RA, Angly F, Dinsdale E, Kelly L, Rohwer F (2009) Metagenomic analysis of stressed coral holobionts. Environ Microbiol 11:2148–2163
Toren A, Landau L, Kushmaro A, Loya Y, Rosenberg E (1998) Effect of temperature on adhesion of Vibrio strain AK-1 to Oculina patagonica and on coral bleaching. Appl Environ Microbiol 64:1379–1384
Ulstrup KE, Berkelmans R, Ralph PJ, van Oppen MJH (2006) Variation in bleaching sensitivity of two coral species across a latitudinal gradient on the Great Barrier Reef: the role of zooxanthellae. Mar Ecol Prog Ser 314:135–148
van Oppen MJH, Leong J, Gates RD (2009) Coral-virus interactions: a double-edged sword? Symbiosis 47:1–8
Wang G-H, Liu J–J, Qi X-N, Jin J, Wang Y, Liu X-B (2008) Effects of fertilization on bacterial community structure and function in a black soil of Dehui region estimated by Biolog and PCR-DGGE methods. Acta Ecologica Sinica 28:220–226
Warner ME, Fitt WK, Schmidt GW (1999) Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc Natl Acad Sci USA 96:8007–8012
Whitaker JR, Granum PE (1980) An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem 109:156–157
Wilson WH, Francis I, Ryan K, Davy SK (2001) Temperature induction of viruses in symbiotic dinoflagellates. Aquat Microb Ecol 25:99–102
Wilson WH, Dale AL, Davy JE, Davy SK (2005) An enemy within? Observations of virus-like particles in reef corals. Coral Reefs 24:145–148
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kühl.
Electronic supplementary material
Below is the link to the electronic supplementary material.
227_2012_1967_MOESM1_ESM.eps
Photographs showing coral condition representative of each treatment after 120 h. a Control, b + Antibiotics, c Heat-stressed d Heat-stress + Antibiotics (EPS 3.47 mb)
Rights and permissions
About this article
Cite this article
Gilbert, J.A., Hill, R., Doblin, M.A. et al. Microbial consortia increase thermal tolerance of corals. Mar Biol 159, 1763–1771 (2012). https://doi.org/10.1007/s00227-012-1967-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-1967-9