Abstract
Shifts in the timing and magnitude of the spring plankton bloom in response to climate change have been observed across a wide range of aquatic systems. We used meta-analysis to investigate phenological responses of marine and freshwater plankton communities in mesocosms subjected to experimental manipulations of temperature and light intensity. Systems differed with respect to the dominant mesozooplankton (copepods in seawater and daphnids in freshwater). Higher water temperatures advanced the bloom timing of most functional plankton groups in both marine and freshwater systems. In contrast to timing, responses of bloom magnitudes were more variable among taxa and systems and were influenced by light intensity and trophic interactions. Increased light levels increased the magnitude of the spring peaks of most phytoplankton taxa and of total phytoplankton biomass. Intensified size-selective grazing of copepods in warming scenarios affected phytoplankton size structure and lowered intermediate (20–200 μm)-sized phytoplankton in marine systems. In contrast, plankton peak magnitudes in freshwater systems were unaffected by temperature, but decreased at lower light intensities, suggesting that filter feeding daphnids are sensitive to changes in algal carrying capacity as mediated by light supply. Our analysis confirms the general shift toward earlier blooms at increased temperature in both marine and freshwater systems and supports predictions that effects of climate change on plankton production will vary among sites, depending on resource limitation and species composition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A large number of studies have reported that the timing and magnitude of seasonal plankton blooms are shifting in response to climate change (Adrian et al. 2006; Edwards and Richardson 2004; Meis et al. 2009; Shimoda et al. 2011). Plankton blooms are important features in seasonal aquatic environments where they drive many ecosystem and community processes and are a major source of energy input for higher trophic levels (Hjermann et al. 2007; Smayda 1997; Winder and Cloern 2010). Seasonal plankton succession is a community phenomenon that is controlled by processes that regulate population dynamics of various primary producers and consumers (Sommer et al. 1986). Blooms are triggered by individual species’ life history and physiological responses to changing abiotic conditions. Timing and magnitude of blooms are controlled by population feedbacks and mediated through resource dynamics and predator–prey interactions (Carpenter et al. 2001; Jäger et al. 2008; Sommer et al. 1986). In aquatic systems, seasonally changing abiotic conditions involve both changes in the supply of basal resources, most notably light, and in physical conditions, most notably temperature. The latter differentially affects the rates of various metabolic processes involved in resource acquisition and respiration of both producers and consumers (Englund et al. 2011; Eppley 1972; Vasseur and McCann 2005). It can therefore be expected that influences of climatic change on plankton succession act both directly by affecting the availability of resources and indirectly by altering the balance of metabolic processes in interacting populations.
The onset of plankton spring blooms is usually initiated by changes in water temperature and light supply. In deep systems, spring phytoplankton blooms are coupled to the onset of thermal stratification, which increases the mean light exposure of phytoplankton cells in the mixed surface layer. Under these conditions, spring blooms are triggered by correlated increases in temperature and seasonal light availability (Edwards and Richardson 2004; Peeters et al. 2007; Winder and Schindler 2004). In shallow, well-mixed systems, phytoplankton blooms are strongly coupled to the external light regime that is influenced by ice cover, cloud cover or day length, and can occur independently of temperature change (Adrian et al. 1999; Sommer and Lengfellner 2008). In both deep and shallow systems, increasing temperature and food availability trigger population growth of zooplankton that in turn can feed back on phytoplankton bloom dynamics through tight trophic coupling (Sommer and Lewandowska 2011). From a physiological perspective, rates of photosynthesis are less sensitive to temperature than is the metabolism of heterotrophic organisms (Allen et al. 2005; Lopez-Urrutia et al. 2006). Warming should therefore increase consumption by herbivores more strongly than primary production. This can strengthen top–down control over primary production by increasing grazing rates under warmer conditions, as has been shown in marine and freshwater experimental studies (Kratina et al. 2012; O’Connor et al. 2009; Sommer and Lewandowska 2011).
In contrast to temperature, light supply affects not only the growth rate but also the carrying capacity of primary producers (Jäger et al. 2008, 2010). Increased primary production at high light and sufficient nutrient supply can enhance zooplankton production, which may then feed back on phytoplankton. Under this scenario, earlier occurrence of the peak of a phytoplankton spring bloom is not necessarily just a direct phytoplankton growth response to increased temperature and light supply, but may result from faster zooplankton growth and higher grazing rates that terminate the phytoplankton bloom earlier (Berger et al. 2010; Jäger et al. 2008). Grazing impacts depend, however, on the taxonomic identity of the dominant primary consumers, which typically differ between marine and freshwater systems. Copepods dominate marine mesozooplankton communities, whereas cladocerans (mainly Daphnia) often dominate in lentic freshwater systems. Copepods have complex life histories with relatively long development times and graze selectively on larger-sized algae (>20 μm) and ciliates, which releases small-sized cells from grazing by microzooplankton (Sommer et al. 2001; Stibor et al. 2004). In contrast, Daphnia feed non-selectively on a broad size range of algae and protists, and parthenogenetic reproduction enables fast population growth (Sommer et al. 2001). It might therefore be expected that warming results in faster grazer responses and stronger top–down control of overall phytoplankton bloom development in cladoceran dominated systems and in more subtle shifts in phytoplankton species composition in copepod-dominated systems (Lewandowska and Sommer 2010).
As a result of the complex interactions of physical drivers with food web processes, phenological responses to climate change are not trivial to predict. Mesocosm experiments have been identified as a tool for studying the underlying mechanisms (Berger et al. 2007; McKee and Atkinson 2000; Sommer et al. 2007). Such experiments in marine and freshwater systems have yielded diverse patterns of phytoplankton and zooplankton bloom development in response to altered temperature and light supply (Aberle et al. 2007; Berger et al. 2010; Lewandowska and Sommer 2010; Sommer and Lengfellner 2008). Yet, responses across systems and trophic levels have never been compared in a systematic way. Here, we present a meta-analysis testing direct and interacting effects of temperature and light manipulations on marine and freshwater plankton communities from mesocosm experiments and disentangling the interplay of physical drivers with density-dependent processes. We hypothesize that warming advances the bloom timing of most functional plankton groups in both marine and freshwater systems. Responses of the magnitude of bloom peaks are, however, expected to be much more variable among taxa and systems and influenced by light intensity and trophic interactions.
Methods
Experimental mesocosm design
We used data from five marine and three freshwater mesocosm experiments (labeled by year) that were designed to investigate the effects of water temperature and light intensity on the spring succession of plankton communities (Table 1). This resulted in a total of 66 individual mesocosm from which we extracted phenological responses to temperature and of 24 mesocosm for light treatments. Detailed descriptions of the respective experiments are available elsewhere (Berger et al. 2007, 2010; Sommer et al. 2007; Sommer and Lewandowska 2011) and are summarized briefly below.
The marine experiments were conducted in temperature-controlled rooms at GEOMAR in spring using four (2005 and 2006) or twelve (2008 and 2009) gently stirred mesocosm (volume: 1,400 L; depth: 1 m), each with a separate light unit. Mesocosms were filled with seawater from Kiel Fjord, Western Baltic Sea in January or February, containing the over-wintering populations of phytoplankton, bacteria, and microzooplankton. Mesozooplankton, mainly consisting of copepods, was added from net catches. Plankton communities were acclimated to the mesocosms for some days under identical conditions before they were exposed to their respective temperature and light treatments (Table 1). The natural seasonal temperature increase was simulated during the experiments based on the decadal (1993–2002) mean sea surface temperature in Kiel Fjord and a 6 °C warming scenario (Table 1), the latter corresponding to the most drastic prediction of temperature increase by the end of this century (IPCC 2007). Light intensities were set to daily light curves and seasonal light patterns calculated from astronomic equations (Brock 1981) and February 4 (2005 and 2006) or 15 (2008 and 2009) were set as the simulated starting date for the temperature and light regimes. Irradiance differed between experiments to account for clouds and underwater light attenuation; 3 light levels were used in the 2008 experiment and 1 light level in all others (Table 1). Starting nutrient concentrations ranged 0.8–0.6 μmol L−1 for phosphorus (PO4), 8.7–21.5 μmol L−1 for NO3, 1.3–5.6 μmol L−1 for NH4, and 18.9–30.5 μmol L−1 for Si. A study by Sommer et al. (2012) suggests that variation in nutrient levels over these ranges does not affect phytoplankton peak magnitude.
The three freshwater experiments were conducted in Lake Brunnsee (47°56′N, 12°26′E), Germany, during the springs of 2005, 2006, and 2007. Lake Brunnsee is a small (area 5.8 ha) hardwater lake with a maximum depth of 19 m. The lake is poor in total phosphorus (<10 μg L−1) but rich in silica and nitrate (>2 mg L−1) and usually freezes over in November or December. Ice-off, followed by the onset of stratification, occurs between February and April. The experiments were initiated as early as possible after ice breakup (March or April, Table 1). For each experiment, twelve (six in 2006) mesocosms with a diameter of 0.95 m and a total length of 10 m were filled with lake water filtered through 30 μm gauze. This excluded most crustacean zooplankton but preserved microzooplankton and the natural phytoplankton community of Lake Brunnsee, which typically does not include large single-celled taxa or colonial growth forms. Instead, the spring community was dominated by small taxa with initial amounts of 30–70 % centric diatoms (Cyclotella), 10–30 % small chlorophytes (Oocystis), and 3–21 % small crysophytes (Rhodomonas). Prior to the establishment of the mixing regimes, all mesocosms were fertilized down to the bottom to an initial total phosphorus content of ~25 μg L−1 to mimic the nutrient pulse associated with spring overturn. Each mesocosm was kept well mixed to a pre-defined stratification depth. Small inocula of Daphnia hyalina, which is the naturally occurring species in Lake Brunnsee, were added to all mesocosms once to twice per week over the first 2–4 weeks of the experiments to simulate spring recruitment from an egg bank. All Daphnia were descendants of three to four clones that had been isolated from Lake Brunnsee and had been pre-cultured separately at 20 °C (see also Sebastian et al. 2012). Prior to stocking, the Daphnia were gradually acclimated to appropriate lower temperatures in a climate chamber.
Two temperature treatments (ambient and cold) were cross-classified with one to three light levels in the mixed surface layer. High, medium, and low light levels were accomplished with different stratification depths (2, 4, 8 m in 2005, 3 m in 2006, and 1.5, 3.5, 6.5 m in 2007, Table 1). Expressed as percent of incident light, this corresponded to depth-averaged mean light intensities in the mixed surface layers of 30–51 % (high), 20–30 % (intermediate), and 15–17 % (low) in different years (Table 1). Mesocosms experiencing ambient temperatures were placed freely in the lake, while cold mesocosms were placed inside a large limnocorral. The latter was permanently mixed down to 12 m depth, thus providing a cold water bath. Both temperature treatments experienced seasonal increases in water temperature, which were stronger in ambient treatments. Averaged over the entire experimental duration, the mean temperature difference between ambient and cold mesocosms ranged from 2 to 4 °C (Table 1).
Mesocosm sampling
Samples in the marine experiments were taken three times per week for phytoplankton and once per week for zooplankton (Aberle et al. 2007; Lewandowska and Sommer 2010; Sommer et al. 2007). Samples from the mixed surface layer of freshwater mesocosms were taken once or twice a week from close to the surface (phytoplankton and ciliates) and by vertical net hauls (Daphnia). In 2006, phytoplankton biomass was only measured as chlorophyll-a. In all other experiments, phytoplankton and ciliates were identified to species or genus level, counted under an inverted microscope and approximated to geometric standards for biovolume calculations; the latter were converted to carbon equivalents (for details, see Aberle et al. 2007; Berger et al. 2010; Sommer and Lewandowska 2011). Mesozooplankton was counted and identified to species or genus level under a dissecting microscope, and life (copepodid) stages were determined for copepods (except in the MA 2005 experiment).
Data analysis
Blooms of various taxa are cardinal phenological events in early seasonal plankton succession. For all functional and taxonomic plankton groups, we therefore focused our analyses on the peaks of their respective spring blooms, which can be characterized by two straightforward descriptors: (1) peak timing, which was defined as the day of the maximum in density or biomass during an experiment, with day zero being the starting date on which the treatments were first applied; (2) peak magnitude, which was defined as the difference between the largest and smallest density or biomass values observed during an experiment. We subsequently investigated how peak timing and peak magnitudes of different plankton groups were affected by temperature and light treatments across all experiments.
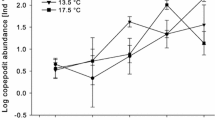
Phytoplankton was categorized into taxonomic groups including chlorophytes, chrysophytes, cryptophytes, diatoms, autotrophic dinoflagellates, and marine heterotrophic dinoflagellates. We also distinguished three phytoplankton size classes according to their largest linear dimension as nano- (<20 μm), micro- (20–200 μm), and mesophytoplankton (>200 μm) following Sieburth et al. (1978). For marine copepods, data were included from the 2006-2 and 2008 experiments; for the 2006-2 experiment copepodid and adult stages and for the 2008 experiment the C1 stage of calanoids were used as a proxy for copepod development, because other copepodid and adult stages did not develop clear peaks over the duration of the experiment. For nauplii, copepod C1 stages, and chlorophytes in the 2008 cold marine treatments peak timing were set to the last day of the experiment because no clear peak developed during the experiment (Fig. 1).
Succession of total phytoplankton and representative zooplankton taxa in marine (MA) and freshwater (FW) mesocosm experiments. Data represent averages across temperature (Tw low, Tc high) and light intensity (Ll low, and Lm intermediate, and Lh high) treatments of the respective year of the experiment. Phytoplankton and ciliate biomass calculations follow Sommer and Lewandowska (2011) and Aberle et al. (2007), respectively
The log-response ratio (LR) was used as an index of effect size, which is one of the most commonly used effect metrics in ecological meta-analysis (Hedges et al. 1999). The LR for temperature was calculated as ln(X w/X c) from each pair of mean values (X) of the warm (w) over the cold (c) treatment at a given light level for each experiment (Table 1). The LR for light was correspondingly calculated as ln(X h/X l), where h and l are the high and low light treatments at a given temperature level, respectively. Thus, LR measures the bloom peak responses in the warm relative to the cold treatments and in the high light relative to the low light treatments. Light effect size was calculated from the marine 2008 and freshwater 2005 and 2007 experiments (excluding intermediate light treatments) because other experiments had a single light treatment (Table 1). A factorial meta-analysis approach was therefore not possible, and the effect sizes of temperature and light responses were calculated separately.
For each experiment, the size and variance of temperature and light effects, weighted by the number of replicates, was calculated. Weighted LRs and variance from these independent studies were used to calculate overall effect sizes and 95 % confidence intervals (CI) for the different taxonomic and functional groups separately for the marine and freshwater systems using random effects meta-regression with restricted maximum-likelihood estimator (Viechtbauer 2010). LR is a dimensionless ratio. A value of 0.69 corresponds to a 100 % increase and a value of −0.69 to a 50 % decrease, respectively, in performance of the response variable.
Random-effect models were used to examine for significant influences of light intensity on the average sizes of temperature effects on timing and magnitude of phytoplankton peaks by including light as a moderator (Viechtbauer 2010). To assess heterogeneity due to the influence of light manipulation, the Q statistics based on a Chi-squared test was used to describe the difference between categories. Therefore, P-values associated with Q between (Q b) were examined, which describe the variation in effect sizes that can be ascribed to differences between light categories. Analysis was carried out using the “wq” (Jassby and Cloern 2011) and “metafor” (Viechtbauer 2010) libraries in the R software environment 2.14.1 (R Development Core Team 2009).
Results
Temporal trajectories and dominant taxa
Total phytoplankton biomass and the dominant taxonomic groups of phytoplankton and zooplankton developed pronounced peaks over time in the vast majority of treatments in both marine and freshwater experiments (Fig. 1). Blooms of marine primary producers were dominated by diatoms and to a lesser extent by cryptophytes; these taxonomic groups plus chlorophytes also dominated the freshwater phytoplankton (Table 2). Ciliates, heterotrophic dinoflagellates, and copepods (nauplii and copepodid stages) were the dominant zooplankton taxa in the marine experiments (Table 2). In the freshwater experiments, zooplankton was dominated by ciliates (not available for 2006) and Daphnia (Table 2).
Peak timing
Increased temperature significantly advanced peak timing of (1) total phytoplankton biomass and (2) all phytoplankton size fractions and taxonomic groups, with cryptophytes showing the strongest response in both marine and freshwater systems (Fig. 2a) and the exception that increased temperature had no statistically significant effect on chrysophytes in the freshwater system (Fig. 2a). In addition, increased temperature significantly advanced peak timing of ciliates, copepods, nauplii, and heterotrophic dinoflagellates in marine experiments and of Daphnia in freshwater experiments (Fig. 2b). Shifts in peak timing of phytoplankton taxa in response to increased temperature were comparable in marine and freshwater experiments, whereas responses of zooplankton were stronger in marine experiments.
Log ratio effect size of temperature increase (Temp) and light intensity (Light) on peak timing of a phytoplankton functional groups and b zooplankton taxonomic groups for marine and freshwater mesocosm experiments. Bars denote ±95 % confidence intervals. A mean effect is significantly different from zero when its 95 % confidence interval does not bracket zero. Negative mean effect sizes indicate that peak timing is earlier in warm or high light relative to cold or low light intensity treatments, respectively. Dashed lines are zero effects; values within the figure indicate upper confidence interval
Effects of light on peak timing were generally weaker and less consistent than effects of temperature. Diatoms were the only group that responded consistently to light in both marine and freshwater experiments. In addition, marine chrysophytes and freshwater mesophytoplankton reached their peaks earlier at higher light levels, while peak timing of the remaining categories of phytoplankton was not significantly affected by light (Fig. 2). Because diatoms were the by far dominating marine phytoplankton taxon, peak timing of total phytoplankton in the marine experiments was also accelerated by higher light levels. The timing of both marine and freshwater zooplankton peaks was not significantly affected by light intensity (Fig. 2).
Analysis of heterogeneity did not reveal any significant influence of light on the effects of temperature on the timing of phytoplankton peaks (p = 0.5), with the exception that marine dinoflagellates showed a reduced response to temperature increase at medium light level (Q b = 6.9, P = 0.03; data not shown).
Peak magnitude
In the marine experiments, increased temperature significantly reduced the magnitude of the biomass peaks of (1) diatoms, (2) total phytoplankton, and (3) all phytoplankton size fractions except nanophytoplankton, while peak magnitude of all other phytoplankton categories and of ciliates was unaffected by temperature (Fig. 3). Peak magnitudes of marine chlorophytes (Tetraselmis sp.) and chrysophytes were also affected by temperature, but these groups made only negligible contributions to total marine phytoplankton biomass (Table 2). In contrast, peak magnitudes of all freshwater phytoplankton and zooplankton categories were unaffected by temperature (Fig. 3). Peak magnitudes of marine calanoids increased significantly at higher temperature, which was strongly pronounced in the 2006-2 experiment and less in 2008 (Figs. 3b, 1).
Log ratio effect size of temperature increase (Temp) and light intensity (Light) on peak magnitude of a phytoplankton functional groups and b zooplankton taxonomic groups for marine and freshwater mesocosm experiments. Negative mean effect sizes indicate that peak magnitude is higher in cold or low light relative to warm or high light intensity treatments, respectively. See Fig. 2 for a more detailed description
Higher light levels significantly increased the magnitude of the biomass peaks of (1) diatoms, cryptophytes, and dinoflagellates, (2) total phytoplankton, and (3) all phytoplankton size fractions except mesophytoplankton in both systems (Fig. 3a). The qualitative light response of these different taxa and of total phytoplankton was consistent across marine and freshwater experiments, but more pronounced in freshwater experiments. Freshwater cryptophytes showed a particularly strong light-dependent increase in peak magnitude. Freshwater ciliates and Daphnia showed also higher peak densities at high compared to low light (Fig. 3b). In contrast, light did not affect the magnitude of marine copepod peaks and biomass of marine heterotrophic dinoflagellates decreased at high light intensity.
Analysis of heterogeneity did not reveal any significant influence of light on the effects of temperature on the magnitude of phytoplankton peaks, with the exception that freshwater chrysophyte peak biomass was significantly higher at high light and warm temperature compared to low light conditions (Q b = 14.1, P < 0.001; data not shown).
Discussion
Our meta-analysis of mesocosm experiments identified several general patterns that seem to hold across both marine and freshwater systems. First, increased temperature shifted the timing of spring peaks in essentially all taxonomic groups toward earlier appearance. Second, increased light levels increased the magnitude of the spring peaks of most phytoplankton taxa and of total phytoplankton biomass. Third, most observed effects of temperature and light appeared to be independent of each other. There were also responses that differed between marine and freshwater systems. Observed differences cannot always be attributed to biological characteristics of the community (e.g., copepod vs. daphnid dominance), but were likely influenced by design differences between marine and freshwater experiments. Owing to the specific research questions and to practical constraints, light treatments within a single experiment differed more strongly in the freshwater experiments, while absolute temperatures were lower and temperature differences between treatments were higher in the marine experiments (Table 1). We therefore focus the discussion primarily on general patterns observed across both marine and freshwater systems, and emphasize differences between the systems only when they can be attributed to biological characteristics such as community composition.
Increased temperature advanced spring peaks consistently across systems and taxonomic groups, which agrees with predictions from dynamical models of pelagic producer–grazer systems (De Senerpont Domis et al. 2007; Schalau et al. 2008) and with long-term observations in lakes and marine systems (Edwards and Richardson 2004; Shimoda et al. 2011; Weyhenmeyer et al. 1999; Winder and Schindler 2004). The degree of advance in primary producers varied considerably among taxonomic groups. Cryptophytes and diatoms, the most abundant taxa in all experiments (Berger et al. 2010; Lewandowska and Sommer 2010), showed the strongest response to warming. This is in agreement with physiological characteristics of these taxa (Gervais 1997; Litchman et al. 2007). Both have high maximum growth and nutrient uptake rates and are able to outcompete other groups under nutrient replete conditions, which were the starting condition in all experiments. Similarly, chlorophytes have relatively high growth rates under sufficient nutrient supply (Litchman et al. 2007) and warming accelerated growth of this group in both freshwater and marine systems. Chrysophytes, which contributed only a small proportion to overall biomass in both systems, were the least responsive to warming.
Similar to primary producers, peak timing of micro- and mesozooplankton species advanced at increased temperature, which is consistent with widespread observations in freshwater and marine systems (Blenckner et al. 2007; Straile 2002; Thackeray et al. 2010). Overall, the successional pattern suggests that zooplankton responded more strongly to warming than phytoplankton, as indicated by the faster acceleration of initial population growth rates in high temperature treatments (Fig. 1). This suggests that the earlier occurrence of phytoplankton peaks at higher temperatures is most likely driven by negative feedback from faster growing grazers, that is, increased grazing pressure at higher temperatures terminated the phytoplankton spring bloom earlier. This explanation is consistent with the observed negative (marine) or neutral (freshwater) response of phytoplankton peak magnitude to warming, in spite of higher phytoplankton growth rates at higher temperatures.
Evidence for tight phytoplankton–grazer interactions is particularly suggestive in the marine experiments. Here, the magnitudes of phytoplankton and zooplankton peaks showed opposite responses to warming. Nauplii and copepodid developed faster and reached higher densities at higher temperatures over the experimental duration. Intensified copepod grazing at higher temperature is suggested by the significantly lower peak magnitude of intermediate phytoplankton cells (20–200 μm) and of diatoms and chrysophytes that largely contributed to this size class. Diatoms, dominated by Skeletonema costatum and Thalassiosira spp., and the chrysophyte Dinobryon balticum are within the preferred prey size range of copepod species (Granéli and Turner 2002; Nejstgaard et al. 2001). These results agree with previous observations from marine plankton experiments (Aberle et al. 2007; O’Connor et al. 2009; Sommer and Lewandowska 2011) and confirm that intensified copepod grazing at increased temperature can affect phytoplankton size structure (Lewandowska and Sommer 2010). A caveat of our analysis is that, due to the long generation time of copepods at the prevailing low temperatures (~3 °C), copepods did not reach peak densities in the cold treatments, except in 2006-2. The strong temperature effect on peak magnitude of copepods is therefore likely an artifact of delayed development in the cold treatments and limited duration of the experiment. Despite this methodological shortcoming, enhanced top–down control can be expected at increased temperature given sufficient food supply as shown in long-term freshwater experiments (Kratina et al. 2012).
Model investigations of phytoplankton-Daphnia dynamics predict that the magnitude of algal and Daphnia spring peaks is fairly insensitive to changes in temperature (De Senerpont Domis et al. 2007; Schalau et al. 2008) but rather sensitive to changes in algal carrying capacity as mediated by light supply (Jäger et al. 2008; Schalau et al. 2008). These expectations were confirmed in the freshwater experiments, that is, the magnitudes of phytoplankton and Daphnia peaks were unaffected by temperature, but increased with increasing light supply. While field observations have shown higher abundances of Daphnia in warm (high NAO) years (Straile 2002), this trend can be attributed to shifts in timing of spring maxima rather than to overall increases in abundance (Blenckner et al. 2007), which is consistent with our results. Overall, the results indicate that light limitation had pronounced effects on plankton succession in lentic experiments and suggests that tight predator–prey coupling can suppress a response of phytoplankton and grazer peaks to increased temperature.
Comparable phytoplankton responses to increased light intensity can, in principle, be expected between marine and freshwater environments. The somewhat weaker response in the 2008 marine experiment is most likely a consequence of the rather modest light treatment range in the 2008 marine (factor 1.5) compared to the freshwater (factor 2–3) experiments (Table 1). Comparably strong, positive responses of phytoplankton peak height to increased light supply became, however, apparent when earlier marine experiments (2005 and 2006) covering lower light levels were included in the analysis (Fig. 4). These earlier experiments could not be included in the meta-analysis of light effects because each experiment used a single light treatment (Sommer et al. 2007). Light dose, however, differed between experiments and the response of peak magnitude across all marine experiments confirmed that high light intensity increased phytoplankton bloom magnitude (r = 0.62, p < 0.001, Fig. 4), similar to observations in freshwater systems (Berger et al. 2010). Effects of light supply on the timing of phytoplankton peaks were largely consistent between freshwater and marine systems. Specifically, light affected bloom timing in diatoms and chrysophytes independently of temperature. This supports observations that light is an important trigger for population growth of fast-growing diatoms, which often dominate spring blooms (Sommer et al. 1986). In natural systems, diatom blooms are linked to increasing radiation such as after ice breakup in shallow systems (Adrian et al. 1999) or after the onset of stratification in deep systems (Winder and Schindler 2004).
Phytoplankton peak magnitude as a function of light dose across the five marine experiments (see Table 1) in the low (Tc) and high (Tw) temperature (Temp) treatments. Experiments that were included in the meta-analysis of light effect size are indicated by filled circles. Points represent peak phytoplankton magnitude of individual mesocosm; dashed line represents a linear regression
While climate-related phenological shifts toward earlier spring events are widespread across marine and freshwater habitats (Thackeray et al. 2010), there is less consensus on bloom magnitude (Adrian et al. 1999; Boyce et al. 2010; Straile 2002; Taucher and Oschlies 2011). Our analysis confirms the general shift toward earlier blooms at increased temperature and supports predictions that the effects of climate change on plankton production will vary among sites, depending on resource limitation and species composition. In the short term, the great sensitivity of heterotrophs to temperature change can strengthen top–down control over primary production by increasing grazing rates. However, resource availability and food web feedbacks can constrain trophic control at increased temperature. The response of phytoplankton to warming will depend on light intensity and likely also on nutrient availability, which was not addressed in our experiments. Our study indicates that plankton bloom dynamics are influenced by a complex interplay of physical and physiological effects on predator–prey interactions and that climate impacts on bloom dynamics can therefore be strongly modulated by trophic interactions.
References
Aberle N, Lengfellner K, Sommer U (2007) Spring bloom succession, grazing impact and herbivore selectivity of ciliate communities in response to winter warming. Oecologia 150:668–681
Adrian R, Walz N, Hintze T, Hoeg S, Rusche R (1999) Effects of ice duration on plankton succession during spring in a shallow polymictic lake. Freshw Biol 41:621–632
Adrian R, Wilhelm S, Gerten D (2006) Life-history traits of lake plankton species may govern their phenological response to climate warming. Glob Change Biol 12:652–661
Allen AP, Gillooly JF, Brown JH (2005) Linking the global carbon cycle to individual metabolism. Funct Ecol 19:202–213
Berger SA, Diehl S, Stibor H, Trommer G, Ruhenstroth M, Jäger C, Striebel M (2007) Water temperature and mixing depth affect timing and intensity of events during spring succession of the plankton. Oecologia 150:643–654
Berger SA, Diehl S, Stibor H, Trommer G, Ruhenstroth M (2010) Water temperature and stratification depth independently shift cardinal events during plankton spring succession. Glob Change Biol 7:1954–1965
Blenckner T, Adrian R, Livingstone DM et al (2007) Large-scale climatic signatures in lakes across Europe: a meta-analysis. Glob Change Biol 13:1314–1326
Boyce DG, Lewis MR, Worm B (2010) Global phytoplankton decline over the past century. Nature 446:591–596
Brock TD (1981) Calculating solar radiation for ecological studies. Ecol Model 14:1–19
Carpenter SR, Cole JJ, Hodgson JR et al (2001) Trophic cascades, nutrients, and lake productivity: whole-lake experiments. Ecol Monogr 71:163–186
De Senerpont Domis LN, Mooij WM, Hulsmann S, van Nes EH, Scheffer M (2007) Can overwintering versus diapausing strategy in Daphnia determine match-mismatch events in zooplankton-algae interactions? Oecologia 150:682–698
Edwards M, Richardson AJ (2004) Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430:881–884
Englund G, Ohlund G, Hein CL, Diehl S (2011) Temperature dependence of the functional response. Ecol Lett 14:914–921
Eppley RW (1972) Temperature and phytoplankton growth in sea. Fish Bull 70:1063–1085
Gervais F (1997) Light-dependent growth, dark survival, and glucose uptake by Cryptophytes isolated from a freshwater chemocline. J Phycol 33:18–25
Granéli E, Turner JT (2002) Top-down regulation in ctenophore-copepod-ciliate-diatom-phytoflagellate communities in coastal waters: a mesocosm study. Mar Ecol Prog Ser 239:57–68
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156
Hjermann DO, Bogstad B, Eikeset AM, Ottersen G, Gjosaeter H, Stenseth NC (2007) Food web dynamics affect Northeast Arctic cod recruitment. Proc R Soc Lond B 274:661–669
IPCC (2007) Climate change 2007: the physical science basis. In: Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Jäger CG, Diehl S, Matauschek C, Klausmeier CA, Stibor H (2008) Transient dynamics of pelagic producer-grazer systems in a gradient of nutrients and mixing depths. Ecology 89:1272–1286
Jäger CG, Diehl S, Emans M (2010) Physical determinants of phytoplankton production, algal stoichiometry, and vertical nutrient fluxes. Am Nat 175:E91–E104
Jassby AD, Cloern JE (2011) wq: exploring water quality monitoring data. Version R package, version 0.3–3. Available from:http://cran.r-project.org/web/packages/wq/index.html
Kratina P, Greiga HS, Thompson PL, Carvalho-Pereiraa TSA, Shurin JB (2012) Warming modifies trophic cascades and eutrophication in freshwater ecosystems. Ecology. http://dx.doi.org/10.1890/11-1595.1
Lewandowska A, Sommer U (2010) Climate change and the spring bloom: a mesocosm study on the influence of light and temperature on phytoplankton and mesozooplankton. Mar Ecol Prog Ser 405:101–111
Litchman E, Klausmeier CA, Schofield OM, Falkowski PG (2007) The role of functional traits and trade-offs in structuring phytoplankton communities: scaling from cellular to ecosystem level. Ecol Lett 10:1170–1181
Lopez-Urrutia A, San Martin E, Harris RP, Irigoien X (2006) Scaling the metabolic balance of the oceans. Proc Natl Acad Sci USA 103:8739–8744
McKee D, Atkinson D (2000) The influence of climate change scenarios on populations of the mayfly Cloeon dipterum. Hydrobiologia 441:55–62
Meis S, Thackeray SJ, Jones ID (2009) Effects of recent climate change on phytoplankton phenology in a temperate lake. Freshw Biol 54:1888–1898
Nejstgaard JC, Hygum BH, Naustvoll LJ, Bamstedt U (2001) Zooplankton growth, diet and reproductive success compared in simultaneous diatom- and flagellate-microzooplankton-dominated plankton blooms. Mar Ecol Prog Ser 221:77–91
O’Connor MI, Piehler MF, Leech DM, Anton A, Bruno JF (2009) Warming and resource availability shift food web structure and metabolism. PLoS Biol 7:e1000178
Peeters F, Straile D, Lorke A, Livingstone DM (2007) Earlier onset of the spring phytoplankton bloom in lakes of the temperate zone in a warmer climate. Glob Change Biol 13:1898–1909
Schalau K, Rinke K, Straile D, Peeters F (2008) Temperature is the key factor explaining interannual variability of Daphnia development in spring—a modelling study. Oecologia 157:531–543
Sebastian P, Stibor H, Berger S, Diehl S (2012) Effects of water temperature and mixed layer depth on zooplankton body size. Mar Biol (in this issue). doi:10.1007/s00227-012-1931-8
Shimoda Y, Azim ME, Perhar G, Ramin M, Kenney MA, Sadraddini S, Gudimov A, Arhonditsis GB (2011) Our current understanding of lake ecosystem response to climate change: what have we really learned from the north temperate deep lakes? J Great Lakes Res 37:173–193
Sieburth J, Smetacek V, Lenz J (1978) Pelagic ecosystem structure—heterotrophic compartments of plankton and their relationship to plankton size fractions—comment. Limnol Oceanogr 23:1256–1263
Smayda TJ (1997) What is a bloom? A commentary. Limnol Oceanogr 42:1132–1136
Sommer U, Lengfellner K (2008) Climate change and the timing, magnitude, and composition of the phytoplankton spring bloom. Glob Change Biol 14:1199–1208
Sommer U, Lewandowska A (2011) Climate change and the phytoplankton spring bloom: warming and overwintering zooplankton have similar effects on phytoplankton. Glob Change Biol 17:154–162
Sommer U, Gliwicz ZM, Lampert W, Duncan A (1986) The PEG-model of seasonal succession of planktonic events in fresh waters. Arch Hydrobiol 106:433–471
Sommer U, Sommer F, Santer B, Jamieson C, Boersma M, Becker C, Hansen T (2001) Complementary impact of copepods and cladocerans on phytoplankton. Ecol Lett 4:545–550
Sommer U, Aberle N, Engel A, Hansen T, Lengfellner K, Sandow M, Wohlers J, Zollner E, Riebesell U (2007) An indoor mesocosm system to study the effect of climate change on the late winter and spring succession of Baltic Sea phyto- and zooplankton. Oecologia 150:655–667
Sommer U, Aberle N, Lengfellner K, Lewandowska A (2012) The Baltic Sea spring phytoplankton bloom in a changing climate: an experimental approach. Mar Biol (in this issue). doi:10.1007/s00227-012-1897-6
Stibor H, Vadstein O, Diehl S, Gelzleichter A, Hansen T, Hantzsche F, Katechakis A, Lippert B, Løseth K, Peters C, Roederer W, Sandow M, Sundt-Hansen L, Olsen Y (2004) Copepods act as a switch between alternative trophic cascades in marine pelagic food webs. Ecol Lett 7:321–328
Straile D (2002) North Atlantic Oscillation synchronizes food-web interactions in central European lakes. Proc R Soc Lond B 269:391–395
Taucher J, Oschlies A (2011) Can we predict the direction of marine primary production change under global warming? Geophys Res Lett 38:L02603
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. http://www.R-project.org
Thackeray SJ, Sparks TH, Frederiksen M et al (2010) Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob Change Biol 16:3304–3313
Vasseur DA, McCann KS (2005) A mechanistic approach for modeling temperature-dependent consumer-resource dynamics. Am Nat 166:184–198
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48
Weyhenmeyer GA, Blenckner T, Pettersson K (1999) Changes of the plankton spring outburst related to the North Atlantic Oscillation. Limnol Oceanogr 77:1788–1792
Winder M, Cloern JE (2010) The annual cycles of phytoplankton biomass. Philos Trans R Soc B Biol Sci 365:3215–3226
Winder M, Schindler DE (2004) Climatic effects on the phenology of lake processes. Glob Change Biol 10:1844–1856
Acknowledgments
Technical assistance by T. Hansen and C. Meyer for the marine and by A. Wild, A. Weigert, and M. Feissel for the freshwater experiments is gratefully acknowledged. This work was supported by the DFG (Deutsche Forschungsgemeinschaft) within the priority program 1162 “AQUASHIFT” (the impact of climate variability on aquatic ecosystems).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Adrian.
Rights and permissions
About this article
Cite this article
Winder, M., Berger, S.A., Lewandowska, A. et al. Spring phenological responses of marine and freshwater plankton to changing temperature and light conditions. Mar Biol 159, 2491–2501 (2012). https://doi.org/10.1007/s00227-012-1964-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-1964-z