Abstract
Many species of marine invertebrate larvae settle and metamorphose in response to chemicals produced by organisms associated with the adult habitat, and histamine is a cue for larvae of the sea urchin Holopneustes purpurascens. This study investigated the effect of histamine on larval metamorphosis of six sea urchin species. Histamine induced metamorphosis in larvae of three lecithotrophic species (H. purpurascens, Holopneustes inflatus and Heliocidaris erythrogramma) and in one planktotrophic species (Centrostephanus rodgersii). Direct comparisons of metamorphic rates of lecithotrophic and planktotrophic larvae in assays cannot be made due to different proportions of larvae being competent. Histamine (10 μM) induced metamorphosis in 95% of larvae of H. purpurascens and H. inflatus after 1 h, while the coralline alga Amphiroa anceps induced metamorphosis in 40–50% of these larvae. Histamine (10 μM) and A. anceps induced 40 and 80% metamorphosis, respectively, in the larvae of H. erythrogramma after 24 h. Histamine (10 μM) and the coralline alga Corallina sp. induced 30 and 70% metamorphosis, respectively, in the larvae of C. rodgersii after 24 h. No metamorphosis of any larval species occurred in seawater controls. Larvae of two planktotrophic species (Tripneustes gratilla and Heliocidaris tuberculata) did not metamorphose in response to histamine. Seagrasses, the host plants of H. inflatus, induced rapid metamorphosis in larvae of the two Holopneustes species, and several algae induced metamorphosis in C. rodgersii larvae. Histamine leaching from algae and seagrasses may act as a habitat marker and metamorphic cue for larvae of several ecologically important sea urchin species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine invertebrate larvae may spend days, weeks or months in the plankton, depending on their mode of development. Larval settlement, metamorphosis and recruitment into adult populations are fundamental ecological processes that help determine the structure of benthic communities (Underwood and Keough 2000). A number of studies have shown that marine larvae are not passive participants during the critical life-history transition marked by settlement out of the plankton and metamorphosis (Butman 1987; Finelli and Wethey 2003). The larvae of echinoderms, molluscs and polychaetes respond to environmental factors that influence the timing and/or site of settlement and metamorphosis (Crisp 1974; Pawlik 1992). Most larvae become competent to settle and metamorphose after a period of development in the plankton (Strathmann 1985). The settlement process often begins with the descent of competent larvae to the benthos, which is followed by benthic exploration and sometimes attachment. Metamorphosis is defined by the loss of larval features and the irreversible morphological transformation into the juvenile (Hadfield 2000). Even though settlement and metamorphosis are separate phenomena, they generally occur in close succession in response to an inducer referred to as a metamorphic cue (reviewed by Hadfield and Paul 2001; Hadfield 2010). Metamorphic cues may act as signposts for marine invertebrate larvae indicating favourable habitats for future success (Larsson and Jonsson 2006). A number of strategies are utilised by competent larvae for settlement and metamorphosis (Pechenik 1990; Bishop et al. 2006). Some larvae have stringent requirements for metamorphosis, particularly larvae of habitat/feeding specialists such as the corallivorous nudibranch Phestilla sibogae (reviewed by Hadfield 1998) and the opisthobranch Alderia modesta (Krug and Manzi 1999). In other species, spontaneous metamorphosis of larvae occurs in the absence of cues at the onset of competency or in older larvae (Fenaux and Pedrotti 1988; Gibson and Chia 1995; Krug 2001).
Metamorphic cues can be physical factors such as light (Baird et al. 2003) and surface texture (Berntsson et al. 2004), or more complex biological and chemical cues (Keough and Raimondi 1995; Clare and Matsumura 2000). The most commonly reported metamorphic cues are chemicals produced by organisms associated with the juvenile/adult habitat such as prey (Hadfield and Scheuer 1985), hosts (Williamson et al. 2000), conspecifics (Burke 1984) or microbial biofilms (Weiczorek and Todd 1998). Chemical cues for metamorphosis range from large, insoluble, surface-bound glycoproteins and carbohydrates to small peptides dissolved in seawater (Zimmer-Faust and Tamburri 1994; Krug and Manzi 1999; Clare and Matsumura 2000). Although the notion that chemicals dissolved in seawater could affect the behaviour of settling larvae was initially dismissed (Crisp 1974), many species are now known to respond to waterborne cues (Hadfield and Scheuer 1985; Turner et al. 1994; Krug and Manzi 1999; Finelli and Wethey 2003). Numbers of naturally occurring, surface-bound and dissolved cues inducing metamorphosis in invertebrate larvae have been partially described (e.g., proteinaceous), but only a few have been completely characterised (Yvin and Chevolet 1985; Tsukamoto et al. 1999; Swanson et al. 2004).
Histamine is a naturally occurring chemical dissolved in seawater that induces metamorphosis in the lecithotrophic larvae of the Australian sea urchin Holopneustes purpurascens (Swanson et al. 2004). Holopneustes purpurascens is an unusual urchin because it lives in the canopy of its host plants, Delisea pulchra and Ecklonia radiata, consuming the algae that it also uses as habitat (Steinberg 1995). Larvae of H. purpurascens metamorphose rapidly in the presence of the red alga D. pulchra, in response to a metamorphic cue derived from this alga that is dissolved in seawater (Williamson et al. 2000). This cue was subsequently identified as histamine (Swanson et al. 2004). Histamine at 10 μM triggers rapid metamorphosis in larvae of H. purpurascens in static bioassays (Swanson et al. 2004, 2007). Histamine is produced in high quantities by D. pulchra (~100–300 μg g−1 dw [dry weight]) and in lower quantities by the kelp E. radiata and brown algae Sargassum linearifolium and Homeostrichus olsenii (0.1–3.0 μg g−1 dw; Swanson et al. 2006). This chemical is also present in trace amounts concentrated on the surface of coralline algae (0.01–0.1 μg g−1 dw, Tebben 2008), which are known to provide metamorphic cues for the larvae of corals, molluscs and echinoderms (reviewed by Hadfield and Paul 2001). Red (including corallines) and brown algae induced metamorphosis in larvae of H. purpurascens in the laboratory with varying efficacy (Williamson et al. 2000; Swanson et al. 2006; Tebben 2008). Very low concentrations of histamine (~5–15 nM) were measured in seawater collected adjacent to D. pulchra (Swanson et al. 2006; Tebben 2008), and some of these samples induced metamorphosis of larvae of H. purpurascens (Swanson et al. 2006). These findings are the only instance where a characterised metamorphic cue has been quantified in the habitat and shown to relate to the recruitment of the organism (Swanson et al. 2006).
The discovery that histamine acts as a metamorphic cue for larvae of H. purpurascens is intriguing as this is a well-known neural signalling molecule in invertebrates and vertebrates (Reite 1972; Stuart 1999). This study addressed the hypothesis that histamine is a common metamorphic cue for sea urchin larvae. Sea urchins exhibit a diverse array of developmental modes ranging from simple non-feeding (lecithotrophic) larvae that develop in several days, to feeding (planktotrophic) larvae that develop over weeks or months (Strathmann 1985). Here, we investigated whether histamine acts as a metamorphic cue for the larvae of six sea urchins, three with lecithotrophic development (H. purpurascens, Holopneustes inflatus and Heliocidaris erythrogramma) and three with planktotrophic development (Heliocidaris tuberculata, Tripneustes gratilla and Centrostephanus rodgersii), species for which development and metamorphosis are well characterised (Morris 1995; Byrne et al. 2001; Huggett et al. 2006; Dworjanyn and Pirozzi 2008; Soars et al. 2009). These species are also common and ecologically important in intertidal and sublittoral habitats. Four of these species co-occur, and so their larvae might use similar cues for settlement and metamorphosis.
Methods
Gamete collection and larval culture
Six echinoid species representing three orders (Table 1) were collected from the shallow sublittoral at several sites along the coast of New South Wales, Australia, between September 2003 and July 2011. The following urchins were collected from Botany Bay (Sydney) during their reproductive periods: Holopneustes spp. (spring 2004, Williamson and Steinberg 2002), Heliocidaris erythrogramma and Heliocidaris tuberculata (spring 2003 and winter 2004, respectively, Laegdsgaard et al. 1991). The Coffs Harbour populations of Tripneustes gratilla and Centrostephanus rodgersii are reproductive in summer and winter, respectively; however, broodstock held at the National Marine Science Centre (NMSC—Coffs Harbour) are reproductive year round (Byrne et al. 1998; Mos et al. 2011). Broodstock of T. gratilla and C. rodgersii held at the NMSC were spawned in February 2005 and July 2011, respectively. Urchins (except Holopneustes spp.) were spawned by intra-coelomic injection of 2–5 mL of 0.5–2.0 M KCl. For each of these species, embryos were pooled from two separate fertilisations of at least 3 females and 3 males. For H. purpurascens and H. inflatus, 20 urchins of each species were held in separate 20-L buckets with air until they spawned pooling the embryos from each bucket (usually within ~48 h of collection).
Seawater was either autoclaved and antibiotics added (22 mg L−1 of penicillin G and 37 mg L−1 of streptomycin sulphate–sterile seawater, SSW) or was filtered (1-μm) and UV-sterilised (FSW). Larvae were cultured at the ambient temperature at which adults were collected. The lecithotrophic larvae (H. purpurascens, H. inflatus and H. erythrogramma) were cultured at 19°C in SSW (salinity, 36) in 2-L beakers with gentle aeration. Lecithotrophic larvae were stocked at a maximum density of 4-larvae mL−1. Seawater (SSW) was changed daily until competency (3–5 days). The echinoplutei of H. tuberculata were cultured at 19°C in FSW (salinity, 36) in 2-L beakers and stirred gently with a paddle system. Larvae were stocked at an initial density of 10 larvae mL−1, which decreased to 2 larvae mL−1 after 1 week and 1 larva mL−1 by 7 week. These larvae were fed Chaetoceras muelleri at 2 × 104 cells mL−1 every other day from 3rd day onwards, and FSW was changed every 3–4 days until competency (~7 week). Tripneustes gratilla and C. rodgersii were cultured at 25 and 21°C, respectively, in FSW (salinity, 37) in 125-L rearing tanks. Larvae were initially stocked at 5 larvae mL−1, which was reduced to 1 larva mL−1 towards the end of the rearing period. These larvae were fed Proteomonas sulcata at 5 × 103 cells mL−1 daily from 3rd day onwards and gradually increased to 4 × 104 cells mL−1 at the 8-arm pluteus stage (~5 and 11 weeks, respectively). Seawater in which larvae of T. gratilla and C. rodgersii were cultured was cleaned daily by exchanging two tank volumes of FSW, and larval cultures were transferred into clean tanks weekly.
Histamine assays
All assays followed the same basic protocol; specific details for each assay are shown in Table 2. The response of larvae of H. purpurascens to histamine is well established (Swanson et al. 2004, 2006, 2007). The effects of histamine on larvae of H. purpurascens and H. inflatus were tested to directly compare the response of two closely related species. All tests were done in static conditions under a 12-h light : 12-h dark photoperiod at the temperature at which larvae were cultured (19–25°C). Replicates were randomly assigned among treatments. Diluted stock solutions of histamine in seawater were prepared on the day of the assay from a concentrated stock solution of histamine (10 mg mL−1 in Milli-Q). Aliquots of diluted stock solutions of histamine were added to sterile Petri dishes (36 mm) followed by 4–5 mL of SSW or FSW. Histamine concentrations tested ranged from 0.01 to 200 μM with a subset of concentrations tested on each species depending on the availability of larvae. The higher concentrations of histamine (>10 μM) were not tested against larval Holopneustes spp. as a maximal response was observed with 10-μM histamine.
The rate of development of larvae to competency can vary within a single cohort, particularly for planktotrophic species that develop over a number of weeks (Hadfield and Strathmann 1996). Thus, a proportion of larvae used in each assay may not have been competent to metamorphose. Coralline algae known to induce metamorphosis of larvae of Holopneustes spp. (Swanson et al. 2006), H. erythrogramma (Huggett et al. 2006), H. tuberculata (Byrne et al. 2011), T. gratilla (Dworjanyn and Pirozzi 2008) and C. rodgersii (herein) were used in each assay to indicate the proportion of larvae that were competent to metamorphose (Table 2). Lecithotrophic larvae used in assays were 6 days old for Holopneustes species and 4 days old for H. erythrogramma, that is, one day older than the typical age that these larvae attain competence in our hands (Table 1). Larvae of H. tuberculata were deemed to be competent at 5 weeks old when, in a preliminary assay, >90% of a subset of larvae metamorphosed in the presence of the coralline alga Corallina officinalis. Larvae of T. gratilla were used in assays at 5 weeks old when at least 50% had large rudiments and were deemed competent to metamorphose (Dworjanyn and Pirozzi 2008). Larvae of C. rodgersii were 11 weeks old at the time of the assay and had multiple pedicellaria and/or tube-feet, structures indicating competency (B. Mos and S. Dworjanyn, in prep.). Sterile seawater or FSW treatments were used as controls for spontaneous metamorphosis. Larvae were added once all dishes were prepared. Planktotrophic larvae were not fed during the histamine assay. The number of larvae used for each species depended on their availability. Percent metamorphosis was recorded over time (h) in repeated observations (Table 2). Attachment, an early stage of the settlement process, was scored in tests with H. tuberculata as a significant proportion of larvae showed this behaviour, but did not metamorphose, in response to histamine. A larva was deemed to have attached firmly to the dish if it could not be dislodged by a gentle stream of water from a pipette.
Algal and seagrass assays
Investigations into the effects of algae on metamorphosis of larval H. purpurascens, H. erythrogramma and larval T. gratilla have been reported elsewhere (Huggett et al. 2006; Swanson et al. 2006; Dworjanyn and Pirozzi 2008). Following the induction of metamorphosis of larval H. inflatus and C. rodgersii by histamine, seagrasses and algae from the habitat were tested in assays with larvae to see whether they induced metamorphosis. There were not enough H. tuberculata larvae available to do an algal assay.
Host plant assay with Holopneustes species
Seagrasses, the host plants for H. inflatus, were assessed to see whether they would induce metamorphosis in larvae of H. inflatus. As the response of larval H. inflatus and H. purpurascens to histamine was similar, the effects of common host plants of both species were investigated in this assay with both larval species. Three species of seagrass (Posidonia australis, Halophila ovalis and Zostera capricornia), the algae Delisea pulchra and Amphiroa anceps, and the kelp Ecklonia radiata were collected on the day of the assay. Six-day-old larvae of H. inflatus (N = 10 [3 per dish]) and H. purpurascens (N = 10 [5 per dish]) were added to dishes containing 4 mL of SSW and 10–30 mg of seagrass or alga, or histamine (10 μM). Percent metamorphosis was scored at 1 and 24 h.
Algal assay with Centrostephanus rodgersii
Algae common in the habitat of Centrostephanus rodgersii were assessed for metamorphic activity. The following algae were collected on the day of the assay: the red algae A. anceps, Corallina officinalis and Laurencia sp.; the brown algae Sargassum linearifolium, Dictyota dichotoma, Dilophus marginata and Ecklonia radiata; and the green alga Ulva sp. Eleven-week-old larvae of C. rodgersii (N = 10 [20–30 per dish]) were added to dishes containing 4 mL of FSW and 15 mm2 of algae. Percent metamorphosis was scored at 24 and 48 h.
Statistical analysis
Proportional data were transformed (arcsine) prior to analysis. Percent metamorphosis of larvae of H. purpurascens and H. inflatus in response to histamine were analysed by repeated measures 2-factor ANOVA (with species and histamine concentration as random factors). Percent metamorphosis of larvae H. purpurascens and H. inflatus to seagrass and algae was analysed by a repeated measures 2-factor ANOVA (with species and plant as fixed factors). All other analyses (percent metamorphosis of larvae of H. erythrogramma in response to histamine at 24 h, percent attachment of larvae of H. tuberculata in response to histamine at 48 h and percent metamorphosis of C. rodgersii in response to histamine and algae at 24 h) were conducted by permutational multivariate analysis of variance (PERMANOVA) using Primer 6 (Primer-E, Plymouth) with PERMANOVA+ extension (v.6.1.7) software. PERMANOVA was used here as proportional data did not meet the assumptions of normality and/or constant variance required for ANOVA (Anderson 2001; McArdle and Anderson 2001; Anderson 2005). Pair-wise comparisons of untransformed data were generated using Euclidean distance, utilising approximately 9,999 permutations of the raw data. Monte Carlo P-values (P-MC) were used when the number of unique permutations was low (Anderson 2005). Pair-wise post hoc tests were performed if PERMANOVA results indicated that there were significant differences between treatments.
Results
Histamine assays
Species with lecithotrophic larvae
Histamine (10 μM) induced rapid metamorphosis in >90% of larvae of Holopneustes purpurascens and H. inflatus within 1 h of exposure (Fig. 1). Histamine (1 μM) induced metamorphosis in 35–40% of larvae of both species of Holopneustes after 1 h, which increased to 90–95% metamorphosis by 24 h (Fig. 1). The geniculate coralline alga Amphiroa anceps induced metamorphosis in 45–50% of larval H. purpurascens and H. inflatus after 1 h, which increased to 95% of larvae by 24 h (Fig. 1). Lower concentrations of histamine (0.01 and 0.1 μM) and SSW induced minimal metamorphosis (<5%) in both species after 24 h. Thus, histamine induced metamorphosis in larvae of H. inflatus at the same level as for larvae of H. purpurascens, at the range of concentrations tested (Fig. 1, Table 3; species effect: F (1,90) = 0.662, P = 0.418).
There was no effect of histamine or A. anceps on larvae of Heliocidaris erythrogramma after 1 h. By 24 h, however, histamine (10 μM) induced metamorphosis in 40% of H. erythrogramma larvae while A. anceps induced metamorphosis in 80% of larvae (Fig. 2a). Concentrations ≥10 μM of histamine (10, 50, 100 and 200 μM) were equally effective at inducing metamorphosis at 24 h (PERMANOVA—Pseudo F (6,63) = 22.04, P-MC = 0.0001, pair-wise comparisons P-MC > 0.05). Histamine at 1 μM and FSW did not elicit a response from the larvae (Fig. 2a).
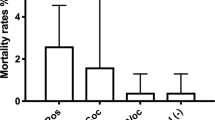
Percent metamorphosis (mean ± SE) of larvae of a Heliocidaris erythrogramma (N = 10) and b Centrostephanus rodgersii (N = 10) after 24 h in response to algae (Amphiroa anceps) (Amphiroa) or Corallina officinalis (Corallina), histamine at 1 and 10 μM and filtered UV-sterilised seawater (SW). Note that response of H. erythrogramma larvae was also recorded after 1 h, and no larvae had metamorphosed in any treatment at this time
Species with planktotrophic larvae
Histamine did not induce metamorphosis in larvae of Heliocidaris tuberculata at any concentration tested (1, 10, 50, 100 and 200 μM). The geniculate coralline alga Corallina officinalis induced metamorphosis in 95% of larvae of H. tuberculata by 24 h. FSW did not induce any larval metamorphosis during the assay. While metamorphosis was not observed in response to histamine at 24–48 h, a significant proportion of larval H. tuberculata became attached to dishes containing 10–200 μM histamine. Between 12 and 20% of larval H. tuberculata were firmly attached to dishes at 24 h, which increased to 24–44% of larvae attached in dishes containing 100–200 μM of histamine at 48 h (PERMANOVA—Psuedo F (5, 24) = 6.729, P-MC = 0.0003; pair-wise comparison of 100/200 μM vs. FSW, P-MC < 0.05). Histamine at 10–50 μM induced attachment of <10% of larvae by 48 h. Histamine at 1 μM and FSW did not induce attachment of larvae.
Histamine did not induce metamorphosis in larvae of Tripneustes gratilla at any concentration tested (0.1, 1, 10 and 100 μM). Approximately 30% of larval T. gratilla metamorphosed in response to C. officinalis by 48 h, indicating that a significant proportion of these larvae were not competent to metamorphose because this alga is a reliable cue. FSW did not induce any larval metamorphosis after 48 h.
Histamine at 10 μM induced metamorphosis in 30% of larvae of Centrostephanus rodgersii, and 100-μM histamine induced >95% of larvae to metamorphose by 24 h. Approximately 70% of larvae of C. rodgersii metamorphosed in response to C. officinalis by 24 h (Fig. 2b; PERMANOVA—Psuedo F (4, 45) = 111.2, P-perm = 0.0001; pair-wise comparison of 1/10 μM vs. FSW, P-MC < 0.001)). Histamine at 1 μM and FSW induced minimal metamorphosis (<5%) of larvae by 24 h. A further 20% of larval C. rodgersii had metamorphosed in response to histamine (1 and 10 μM) and C. officinalis by 48 h.
Host plant assay with Holopneustes species
Larvae of H. inflatus and H. purpurascens were induced to metamorphose by all species of seagrass and algae assayed with varying efficacy (Fig. 3). Interestingly, rates of larval metamorphosis after 1 h favoured the host plants of each species. That is, more larvae of H. purpurascens (than H. inflatus) had metamorphosed in response to D. pulchra and A. anceps at 1 h, whereas more larvae of H. inflatus (than H. purpurascens) had metamorphosed in response to the three seagrasses at 1 h (Fig. 3a). The different responses of larvae to host plants that were apparent at 1 h (Fig. 3a) had diminished by 24 h (Fig. 3b) as indicated by the time x species x plant interaction (P = 0.034, Table 4). Although there was no effect of (larval) species in the analysis, there was a significant species x (host) plant interaction (Table 4) suggesting that larvae of H. inflatus and H. purpurascens were responding differently to host plants.
Percent metamorphosis (mean ± SE) of larvae (N = 10) of Holopneustes purpurascens (black bars) and H. inflatus (grey bars) in response to histamine (10 μM), different host plants and sterile seawater (SW) after (a) 1 h and (b) 24 h. Delisea = D. pulchra, Amphiroa = A. anceps, Ecklonia = E. radiata, Posidonia = P. australis, Halophila = H. ovalis, Zostera = Z. capricornia
Algal assay with Centrostephanus rodgersi
All algal species assayed induced metamorphosis in larvae of C. rodgersii with the red algae inducing 35–68% metamorphosis by 24 h (Fig. 4; PERMANOVA—Psuedo F (8, 81) = 15.368, P-perm = 0.0001; pair-wise comparison of alga vs. FSW, each P-MC < 0.01). Brown algae induced 9–31% metamorphosis in larvae of C. rodgersii by 24 h, while FSW had no effect on larvae.
Percent metamorphosis (mean ± SE) of larvae of Centrostephanus rodgersii (N = 10) in response to common algae from its habitat and filtered UV-sterilised seawater (SW) after 24 h. Corallina = C. officinalis, Laurencia = Laurencia sp., Amphiroa = A. anceps, Sargassum = S. linearifolium, Dictyota = D. dichotoma, Dilophus = D. marginata, Ulva = Ulva sp, Ecklonia = E. radiata
Discussion
As documented previously for Holopneustes purpurascens (Swanson et al. 2004), histamine is also an effective metamorphic cue for the lecithotrophic larvae of Holopneustes inflatus (Temnopluridae) and Heliocidaris erythrogramma (Echinometridae), and the planktotrophic larvae of Centrostephanus rodgersii (Diadematidae). In contrast, histamine did not induce metamorphosis in the echinoplutei of Heliocidaris tuberculata (Echinometridae) and Tripnesutes gratilla (Toxopneustidae) although some larvae of H. tuberculata attached in response to histamine. Thus, the species that responded to histamine were a disparate assemblage of species with regards to developmental mode and phylogeny (Table 1). It is difficult to make direct comparisons of the larval response to histamine as different proportions of each larval species were competent to metamorphose during the assay. Competency is indicated by the number of larvae that metamorphosed in response to the coralline alga included in the assay (Table 2). The larval responses to selected algae suggest that 80–95% of larvae of the lecithotrophic species, and approximately two-third of the planktotrophic species C. rodgersii, were competent to metamorphose in the assays at 24 h. Histamine was most effective at inducing metamorphosis in larvae of the two Holopneustes species as 1-μM histamine induced metamorphosis in >90% of these larvae at 24 h, compared with ~2% of larvae of H. erythrogramma and C. rodgersii.
Habitat similarities among the species that responded to histamine may have influenced the pattern of response of larvae in laboratory assays. Three of the species that metamorphosed in response to histamine, that is, H. purpurascens, H. erythrogramma and C. rodgersii, co-occur in subtidal rocky reefs. We propose that dissolved histamine that leaches from algae and kelp, as shown for Delisea pulchra and Ecklonia radiata (Swanson et al. 2006; Tebben 2008), may act as a signpost of algal-dominated habitats to competent sea urchin larvae, providing a cue for an appropriate site in which to settle and metamorphose. Peptides and amino acids leak from algae (Agrawal and Sharma 1996) and sea urchin larvae have been shown to use amino acids and sugars as signals for morphological change (Shilling 1995). Histamine may also leak from algae and serve as an exogenous cue for metamorphosis for sea urchin larvae. A quantitative survey of the histamine content of common algae from a typical habitat of H. purpurascens, H. erythrogramma, H. tuberculata and C. rodgersii confirmed the production of this naturally occurring metamorphic cue in all seasons (Swanson et al. 2006). Quantitative analysis of the histamine content of several species of red and brown algae showed that they contained 0.01–300 μg g−1 (dry weight) histamine (Swanson et al. 2006; Tebben 2008). Given that histamine comes from the decarboxylation of the amino acid histidine, it is probable that other species of red and brown algae also contain at least some level of histamine. Epiphytic growth of filamentous algae (fouling) on the surface of macroalgae generally increases during the warmer months (Hellio et al. 2004), and fouling taken from the laminae of E. radiata contained 100-fold higher concentrations of histamine than unfouled laminae of E. radiata (Swanson et al. 2006). Thus dense beds of E. radiata and Sargassum spp., as well as beds of D. pulchra, might provide a source of histamine in the habitat, particularly during the warmer months.
Heliocidaris tuberculata co-occurs with H. purpurascens, H. erythrogramma and C. rodgersii in algal-dominated habitats, but the larvae of this species only attached in response to histamine and did not metamorphose. The slow attachment of larvae in response to relatively high concentrations of histamine (10–200 μM) indicates a weak effect of histamine on larvae of H. tuberculata. Dissolved histamine may, however, influence settlement behaviour bringing the competent larvae of this species closer to metamorphic cues in the habitat. Serotonin and its precursor 5-hydroxytryptophan, invoke a negative phototaxis in larvae of the bryozoan Bugula neritina implying a neurochemical regulatory role for these biogenic amines in larval settlement (Pires and Woollacott 1997). Heliocidaris tuberculata may use separate cues for settlement and metamorphosis, as shown for a nudibranch Onchidoris bilamellata and a barnacle Balanus amphitrite (Chia and Koss 1988; Clare and Matsumura 2000). Low concentrations of dissolved histamine may be effective in nature, where synergistic effects of water flow and chemical cues affect the behaviour of settling larvae (Wright and Boxshall 1999; Alteri 2003).
Holopneustes inflatus inhabits seagrass beds, rather than the algal-dominated rocky reefs of its congener. Histamine induced rapid metamorphosis in 80% of larval H. inflatus and the seagrasses (Posidonia australis, Halophila ovalis and Zostera capricornia) induced >90% metamorphosis by 24 h (Fig. 3). It is not known whether H. inflatus recruits to seagrass beds in Botany Bay, but it seems likely, given that seagrasses produce a metamorphic cue for larval H. inflatus, and juvenile H. inflatus were found there in this study. Sea urchins are found in temperate, subtropical and tropical seagrass beds (Lawrence 1975) where they are among the most common macrograzers (Vergés et al. 2007; Eklöf et al. 2008). Larvae of both species of Holopneustes were induced to metamorphose by the favoured host plants of their congener albeit at slower rates initially. Seagrasses contain similar levels of histamine as brown algae (~0.5 μg g−1 dw) based on semi-quantitative analyses (Swanson et al. 2006; Swanson 2007). The similar response of larval H. inflatus and H. purpurascens to histamine, seagrass and algae suggests that they respond to the same metamorphic cue that is likely to be histamine. Dense beds of seagrass may leach histamine into surrounding seawater, a potential signpost for seagrass beds for competent H. inflatus larvae. In this instance, the calmer conditions in which seagrass beds typically occur may lead to longer retention of dissolved histamine of seagrass origin (Krug and Zimmer 2000).
The different habitats occupied by adult H. purpurascens, H. inflatus, H. erythrogramma and C. rodgersii may influence their different larval sensitivities to histamine. Holopneustes purpurascens and H. inflatus are atypical echinoids in that they are specialists in habitat use and diet. That is, H. purpurascens and H. inflatus live in the canopy of their host plants, algae/kelp and seagrass, respectively, using it as habitat as well as food (Steinberg 1995; Williamson et al. 2004). Conversely, the life style of H. erythrogramma and C. rodgersii as generalists foraging on the sea floor (Hill et al. 2003; Ling et al. 2010) is more typical of urchins. Larvae of habitat specialists such as H. purpurascens and H. inflatus, which live on a discrete range of algae/plants, may have evolved a more sensitive and rapid response to cues from their host plants to enhance post-larval success. Generalist marine herbivores such as H. erythrogramma and C. rodgersii, on the other hand, are less restricted in their habitat use and diet and thus might be expected to have a lower specificity in their metamorphic cues (Huggett et al. 2006). Centrostephanus rodgersii demonstrated low specificity in its cues for metamorphosis by responding to a wide range of red, brown and green algae. Only ~2% of larvae of H. erythrogramma and C. rodgersii metamorphosed in response to 1-μM histamine whereas most larvae of the Holopneustes species had metamorphosed by 24 h. This difference in sensitivity to histamine as metamorphic inducer may reflect the ability of H. erythrogramma and C. rodgersii larvae to respond to cues from a wide range of algae present in the habitat.
Histamine had no effect on metamorphosis in larvae of another generalist urchin T. gratilla. Even though only a third of larval T. gratilla were competent to metamorphose in the assay, no effect of histamine (0.1, 1.0, 10 and 100 μM) was observed over 48 h, with similar results seen in preliminary assays. Tripneustes gratilla is a widely distributed tropical Indo-West Pacific species often found in habitats dominated by Sargassum spp. and seagrass (Juinio-Meňez and Bangi 2010). As a generalist, T. gratilla also appears to have low specificity in its cues for settlement and metamorphosis as these processes are triggered by a variety of algae, conspecifics and biofilms enriched with diatoms (Dworjanyn and Pirozzi 2008). When Sargassum linearifolium was cleaned to reduce the abundance of surface bacteria, the alga no longer induced metamorphosis of T. gratilla (Dworjanyn and Pirozzi 2008). These observations suggest a biofilm-derived metamorphic cue for T. gratilla and support the hypothesis of Steinberg et al. (2001) that larvae of generalist herbivores are likely to metamorphose in response to biofilms.
The mechanism by which histamine induces metamorphosis in sea urchin larvae may be twofold and may differ among species. The rapid metamorphic response of larvae of Holopneustes spp. to histamine suggests induction via an external histamine receptor, which is the expectation for a true metamorphic cue in nature. A sea urchin homologue of the metabotropic histamine H1 receptor of vertebrates (suH1R) has been identified on the surface of Strongylocentrotus purpuratus eggs, and this receptor is an integral part of the Ca2+ release pathway leading to fertilisation (Leguia and Wessel 2006). It is possible that suH1R is expressed in other sea urchins and may be involved in histamine-induced metamorphosis of sea urchin larvae, but this hypothesis requires further investigation. Rather than only acting via an external receptor, histamine may also be an endogenous signalling molecule involved in the control of metamorphosis in sea urchin larvae. Dissolved histamine in the water may lead to an increase in endogenous levels of histamine over 24 h, triggering metamorphosis. Endogenous levels of the other biogenic amines (dopamine, serotonin) and hormones (thyroxine) appear to control or modulate competency and metamorphosis in hydrozoan, molluscan and echinoderm larvae (Pires et al. 2000; Leise et al. 2001; Pechenik et al. 2002; Heyland and Hodin 2004). Biogenic amines, all products of the decarboxylation of amino acids, are known to play critical roles in initiating and controlling behaviour and in the physiology of invertebrates, by acting as classical neurotransmitters, neuromodulators and neurohormones (Katz 1995). Thus, histamine may have a regulatory role in the settlement and metamorphosis of sea urchin larvae.
Under laboratory conditions, 10-μM histamine induced metamorphosis of larvae of H. purpurascens, H. inflatus, H. erthyrogramma and C. rodgersii. In nature, however, measured concentrations of histamine in seawater samples (10 mL) collected adjacent to D. pulchra were much lower, between 5 and 15 nM, while seawater collected at the sea surface contained 0.5-nM histamine (Swanson et al. 2006). Contrary to past assumptions, dissolved chemicals emanating from substrata do not form smooth concentration gradients (Crisp 1974). Rather, chemical cues are dispersed in fine filaments of high concentration swirling in clean water, where wave-driven flow generates wider filaments of higher concentration compared to unidirectional flow (Koehl 2006). Thus inductive concentrations of histamine (μM) may accumulate in such fine-scale filaments, at spatial scales (nm, μm) appropriate for larval detection (Hadfield and Koehl 2004) but may have been diluted in the 10-mL seawater samples that were analysed for histamine concentration (Swanson et al. 2006). This phenomenon of fine-scale spatial distribution of dissolved cues was shown to occur in the turbulent oscillatory flow above coral reefs in which larvae of Phestilla sibogae are transported (Hadfield and Koehl 2004). These larvae sank when encountering filaments of odour (containing a metamorphic cue) emanating from a coral reef exposed to wave action, and then resumed swimming in odour (cue)-free seawater (Hadfield and Koehl 2004). Larval behaviour of sinking in response to dissolved cues in the water column can increase the rate of larval transport to the substratum in turbulent wave-driven flow, thereby enhancing the rate of metamorphosis (Hadfield and Koehl 2004). Oyster larvae Crassostrea virginica in a flume boundary layer exhibited rapid downward acceleration, or “dive-bombing,” in response to dissolved cues, a behaviour bringing them into contact with the substratum (Finelli and Wethey 2003). Larval behaviours in response to dissolved cues are increasingly recognised as important contributors to the recruitment patterns of marine invertebrates.
Increasing evidence suggests that histamine is a ubiquitous signalling molecule in the nervous systems of a diverse range of invertebrates and vertebrates (Reite 1972; Claiborne and Selverston 1984; Bayer et al. 1989; Stuart 1999; Zimmer and Zimmer 2008). Many chemical signals involved in neurotransmission or neuromodulation at the cellular level (e.g. amino acids, peptides and hormones) also function as external chemical signals in the aquatic environment and so act as both exogenous and endogenous signalling molecules (Haldane 1954; Carr 1988; Heyland et al. 2005; Heyland and Moroz 2005). Histamine may prove to be such a compound. Additional measurements of dissolved histamine in seawater surrounding algae/kelp, and in seagrass beds, are needed to address the hypothesis that histamine may be a naturally occurring metamorphic cue for larvae of sea urchins; H. purpurascens, H. inflatus, H. erthrogramma and C. rodgersii. The potential role of endogenous levels of histamine in regulating metamorphosis in sea urchin larvae is a worthy avenue for further research.
References
Agrawal SC, Sharma UK (1996) Chemical and biological properties of culture filtrates of Westiellopsis prolific and Chaetophora attenuate. Israel J Plant Sci 44:43–48
Alteri AH (2003) Settlement cues in the locally dispersing temperate cup coral Balanophyllia elegans. Biol Bull 204:241–245
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Anderson MJ (2005) PERMANOVA: a FORTRAN computer program for permutational multivariate analysis of variance. Department of Statistics, University of Auckland, NZ
Baird AH, Babcock RC, Mundy CP (2003) Habitat selection by larvae influences the depth distribution of six common coral species. Mar Ecol Prog Ser 252:289–293
Bayer TA, McClintock TS, Grunert U, Ache BW (1989) Histamine-induced modulation of olfactory receptor neurones in two species of lobster, Panulirus argus and Homarus americanus. J Exp Biol 145:133–146
Berntsson KM, Jonsson PR, Larsson AI, Holdt S (2004) Rejection of unsuitable substrata as a potential driver of aggregated settlement in the barnacle Balanus improvisus. Mar Ecol Prog Ser 275:199–210
Bishop CD, Huggett MJ, Heyland A, Hodin J, Brandhorst BP (2006) Interspecific variation in metamorphic competence in marine invertebrates: the significance for comparative investigations into the timing of metamorphosis. Integr Comp Biol 46:662–682
Burke RD (1984) Pheromonal control of metamorphosis in the Pacific sand dollar, Dendraster excentricus. Science 225:440–441
Butman CA (1987) Larval settlement of soft-sediment invertebrates: the spatial scales of pattern explained by active habitat selection and the emerging role of hydrodynamical processes. Oceanogr Mar Bio Ann Rev 25:113–165
Byrne M, Andrew NL, Worthington DG, Brett PA (1998) The influence of latitude and habitat on reproduction in the sea urchin Centrostephanus rodgersii in New South Wales, Australia. Mar Biol 132:305–318
Byrne M, Emlet RB, Cerra A (2001) Ciliated band structure in planktotrophic and lecithotrophic larvae of Heliocidaris species (Echinodermata: Echinoidea): a demonstration of conservation and change. Acta Zool 82:189–199
Byrne M, Selvakumaraswamy P, Ho MA, Nguyen HD (2011) Sea urchin development in a global change hot spot, potential for southerly migration of warm adapted propagules. Deep-Sea Res 58:712–719
Carr WES (1988) The molecular nature of chemical stimuli in the aquatic environment. In: Atema J, Fay RR, Popper AN, Tavolga WN (eds) Sensory biology of aquatic animals. Springer, New York, pp 3–27
Chia FS, Koss R (1988) Induction of settlement and metamorphosis of the veliger larvae of the nudibranch Onchidoris bilamellata. Int J Invert Repro Dev 14:53–70
Claiborne BJ, Selverston AI (1984) Histamine as a neurotransmitter in the stomatogastric nervous system of the spiny lobster. J Neurosci 4:708–721
Clare AS, Matsumura K (2000) Nature and perception of barnacle settlement pheromones. Biofouling 15:57–71
Crisp DJ (1974) Factors influencing the settlement of marine invertebrate larvae. In: Grant PT, Mackie AM (eds) Chemoreception in marine organisms. Academic Press, London, pp 177–265
Dworjanyn SA, Pirozzi I (2008) Induction of settlement in the sea urchin Tripneustes gratilla by macroalgae, biofims and conspecifics: a role for bacteria? Aquaculture 274:624–633
Eklöf JS, de la Torre-Castro M, Gullström M, Uku J, Muthiga N, Lyimo T, Bandeira SO (2008) Sea urchin overgrazing of seagrasses: a review of current knowledge on causes, consequences and management. Estuar Coast Shelf Sci 79:569–580
Fenaux L, Pedrotti ML (1988) Metamorphosis of echinoid larvae in midwater. Mar Ecol 9:93–107
Finelli CM, Wethey DS (2003) Behavior of oyster (Crassostrea virginica) larvae in flume boundary layer flows. Mar Biol 143:703–711
Gibson GD, Chia FS (1995) Developmental variability in the poecilogonous opisthobranch Haminaea callidegenita: life-history traits and effects of environmental parameters. Mar Ecol Prog Ser 121:139–155
Hadfield MG (1998) The D. P. Wilson Lecture: research on settlement and metamorphosis of marine invertebrate larvae: past, present and future. Biofouling 12:9–29
Hadfield MG (2000) Why and how marine-invertebrate larvae metamorphose so fast. Semin Cell Dev Biol 11:437–443
Hadfield MG (2010) Biofilms and marine invertebrate larvae: what bacteria produce that larvae use to choose settlement sites. Ann Rev Mar Sci 3:453–470
Hadfield MG, Koehl MAR (2004) Rapid behavioral responses of an invertebrate larva to dissolved settlement cue. Biol Bull 207:28–43
Hadfield MG, Paul VJ (2001) Natural chemical cues for settlement and metamorphosis of marine invertebrate larvae. In: McClintock JB, Baker BJ (eds) Marine chemical ecology. CRC Press, Florida, pp 431–461
Hadfield MG, Scheuer D (1985) Evidence for a soluble metamorphic inducer in Phestilla: ecological, chemical and biological data. Bull Mar Sci 37:556–566
Hadfield MG, Strathmann MF (1996) Variability, flexibility and plasticity in life histories of marine invertebrates. Oceanol Acta 19:323–334
Haldane JBS (1954) La signalisation animale. Année Biologique 58:89
Hellio C, Marechal JP, Veron B, Bremer G, Clare AS, Le Gal Y (2004) Seasonal variation of antifouling activities of marine algae from the Brittany Coast (France). Mar Biotech 6:67–82
Heyland A, Hodin J (2004) Heterochronic developmental shift caused by thyroid hormone in larval sand dollars and its implications for phenotypic plasticity and the evolution of non-feeding development. Evolution 58:524–538
Heyland A, Moroz LL (2005) Cross-kingdom hormonal signalling: an insight from thyroid hormone functions in marine larvae. J Exp Bio 208:4355–4361
Heyland A, Hodin J, Reitzel AM (2005) Hormone signalling in evolution and development: a non- model system approach. BioEssays 27:64–75
Hill NA, Blount C, Poore AGB, Worthington D, Steinberg PD (2003) Grazing effects of the sea urchin Centrostephanus rodgersii in two contrasting rocky reef habitats: effects of urchin density and its implications for the fishery. Mar Freshw Res 54:691–700
Huggett MJ, Williamson JE, de Nys R, Kjelleberg S, Steinberg PD (2006) Larval settlement of the common Australian sea urchin Heliocidaris erythrogramma in response to bacteria from the surface of coralline algae. Oecologia 149:604–619
Juinio-Meňez MA, Bangi HG (2010) Extrinsic and intrinsic factors affecting the metamorphic rate of Tripneustes gratilla. Mar Ecol Prog Ser 402:137–145
Katz PS (1995) Intrinsic and extrinsic modulation of motor circuits. Curr Opin Neurobiol 5:799–808
Keough MJ, Raimondi PT (1995) Responses of settling invertebrate larvae to bioorganic films: effects of different types of films. J Exp Mar Biol Ecol 185:235–253
Koehl MAR (2006) The fluid mechanics of arthropod sniffing in turbulent odor plumes. Chem Senses 31:93–105
Krug PJ (2001) Bet-hedging dispersal strategy of a specialist marine herbivore: a settlement dimorphism among sibling larvae of Alderia modesta. Mar Ecol Prog Ser 213:177–192
Krug PJ, Manzi AE (1999) Waterborne and surface-associated carbohydrates as settlement cues for larvae of the specialist marine herbivore Alderia modesta. Biol Bull 197:94–103
Krug PJ, Zimmer RK (2000) Larval settlement: chemical markers for tracing production, transport and distribution of a waterborne cue. Mar Ecol Prog Ser 207:283–296
Laegdsgaard P, Byrne M, Anderson DT (1991) Reproduction of sympatric populations of Heliocidaris erythrogramma and H. tuberculata (Echinoidea) in New South Wales. Mar Biol 110:359–374
Larsson AI, Jonsson PR (2006) Barnacle larvae actively select flow environments supporting post-settlement growth and survival. Ecology 87:1960–1966
Lawrence JM (1975) On the relationship between marine plants and urchins. Oceanogr Mar Biol Ann Rev 13:213–286
Leguia M, Wessel GM (2006) The histamine H1 receptor activates the nitric oxide pathway at fertilization. Mol Repro Dev 73:1550–1563
Leise EM, Thavaradhava K (2001) Serotonin and nitric oxide regulate metamorphosis in the marine snail Ilyanassa obsoleta. Am Zool 41:258–267
Ling SD, Ibbott S, Sanderson JC (2010) Recovery of canopy-forming macroalgae following removal of the enigmatic sea urchin Heliocidaris erythrogramma. J Exp Mar Biol Ecol 395:135–146
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82:290–297
Morris VB (1995) Apluteal devlopment of the sea urchin Holopneustes purpurescens Agassiz (Echinodermata: Euechinoidea). Zool J Linnean Soc 114:349–364
Mos B, Cowden KL, Nielsen SJ, Dworjanyn SA (2011) Do cues matter? Highly inductive settlement cues don’t ensure high post-settlement survival in sea urchin aquaculture. PLoS One 6(12):e28054
Pawlik JR (1992) Chemical ecology of the settlement of benthic marine invertebrates. Oceanog Mar Biol Ann Rev 30:273–335
Pechenik JA (1990) Delayed metamorphosis by larvae of benthic marine invertebrates. Does it occur? Is there a price to pay? Ophelia 32:63–94
Pechenik JA, Li W, Cochrane DE (2002) Timing is everything: the effects of putative dopamine antagonists on metamorphosis vary with larval age and experimental duration in the prosobranch gastropod Crepidula fornicata. Biol Bull 202:137–147
Pires AR, Woollacott RM (1997) Serotonin and dopamine have opposite effects on phototaxis in larvae of the bryozoan Bugula neritina. Biol Bull 192:399–409
Pires AR, Croll RP, Hadfield MG (2000) Catecholamines modulate metamorphosis in the opistobranch gastropod Phestilla sibage. Biol Bull 198:319–331
Reite OB (1972) Comparative histology of histamine. Physiol Rev 52:778–819
Shilling FM (1995) Morphological and physiological responses of echinoderm larvae to nutritive signals. Am Zool 35:399–414
Soars NA, Prowse TAA, Byrne M (2009) Overview of phenotypic plasticity in echinoid larvae, ‘Echinopluteus transversus’ type vs. typical echinoplutei. Mar Ecol Prog Ser 383:113–125
Steinberg PD (1995) Interaction between the canopy dwelling echinoid Holopneustes purpurescens and its host kelp Ecklonia radiata. Mar Ecol Prog Ser 127:169–181
Steinberg PD, de Nys R, Kjellberg S (2001) Chemical mediation of surface colonization. In: McClintock JB, Baker BJ (eds) Marine chemical ecology. CRC Press, Florida, pp 355–387
Strathmann RR (1985) Feeding and non-feeding larval development and life-history evolution in marine invertebrates. Ann Rev Ecol Sys 16:339–361
Stuart AE (1999) From fruit flies to barnacles, histamine is the neurotransmitter of arthropod photoreceptors. Neuron 22:431–433
Swanson RL (2007) Histamine—a naturally occurring settlement cue for larvae of the Australian sea urchin Holopneustes purpurascens. Dissertation, University of New South Wales, Sydney, Australia
Swanson RL, Williamson JE, de Nys R, Kumar N, Bucknall MP, Steinberg PD (2004) Induction of settlement of larvae of the sea urchin Holopneustes purpurascens by histamine from a host alga. Biol Bull 206:161–172
Swanson RL, de Nys R, Huggett MJ, Green JK, Steinberg P (2006) In situ quantification of a natural settlement cue and recruitment of the Australian sea urchin Holopneustes purpurascens. Mar Ecol Prog Ser 314:1–14
Swanson RL, Marshall DJ, Steinberg PD (2007) Larval desperation and histamine: how simple responses can lead to complex changes in larval behaviour. J Exp Biol 210:3228–3235
Tebben J (2008) Chemical separation and identification of water-soluble algal settlement cues for Australian sea urchins. Diploma thesis, Institute for Chemistry and Biology of the Marine Environment, University of Oldenburg, Germany
Tsukamoto S, Kato H, Hirota H, Fusetani N (1999) Lumichrome: a larval metamorphosis inducing substance in the ascidian Halocynthia roretzi. Eur J Biochem 264:785–789
Turner EJ, Zimmer-Faust RK, Palmer MA, Luckenbach M, Pentcheff ND (1994) Settlement of oyster (Crassostrea virginica) larvae: effects of water flow and water-soluble chemical cue. Limnol Oceanog 39:1579–1593
Underwood AJ, Keough MJ (2000) Supply-side ecology: the nature and consequences of variations in recruitment of intertidal organisms. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology. Sinauer Associates, Sunderland, MA, pp 183–200
Vergés A, Becerro MA, Alcoverro T, Romero J (2007) Experimental evidence of chemical deterrence against multiple herbivores in the seagrass Posidonia oceanica. Mar Ecol Prog Ser 343:107–114
Weiczorek SK, Todd CD (1998) Inhibition and facilitation of settlement of epifaunal marine invertebrate larvae by microbial biofilm cues. Biofouling 12:81–118
Williamson JE, Steinberg PD (2002) Reproductive cycle of the sea urchin Holopneustes purpurascens (Temnopluridae: Echinodermata). Mar Biol 140:519–532
Williamson JE, de Nys R, Kumar N, Carson DG, Steinberg PD (2000) Induction of metamorphosis in the sea urchin Holopneustes purpurascens by a metabolite complex from the algal host Delisea pulchra. Biol Bull 198:332–345
Williamson JE, Carson DG, de Nys R, Steinberg PD (2004) Demographic consequences of an ontogenetic shift by a sea urchin in response to host plant chemistry. Ecology 85:1355–1371
Wright JR, Boxshall AJ (1999) The influence of small-scale flow and chemical cues on the settlement of two congeneric barnacle species. Mar Ecol Prog Ser 183:179–187
Yvin JC, Chevolet L (1985) First isolation of jacarone from an alga, Delesseria sanguinea: a metamorphosis inducer of Pecten larvae. J Nat Prod 48:814–816
Zimmer RK, Zimmer CA (2008) Dynamic scaling in chemical ecology. J Chem Ecol 34:822–836
Zimmer-Faust RK, Tamburri MN (1994) Chemical identity and ecological implications of a waterborne, larval settlement cue. Limnol Oceanogr 39:1075–1087
Acknowledgments
This research was supported by an Australian Postgraduate Award to RLS and the Centre for Marine Bio-Innovation, UNSW and an ARC grant (MB). BM was supported by an Australian Postgraduate Scholarship and a Rural Industries Research and Development Corporation top-up scholarship. The authors also wish to thank the anonymous reviewers who provided valuable feedback that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. P. Grassle.
Rights and permissions
About this article
Cite this article
Swanson, R.L., Byrne, M., Prowse, T.A.A. et al. Dissolved histamine: a potential habitat marker promoting settlement and metamorphosis in sea urchin larvae. Mar Biol 159, 915–925 (2012). https://doi.org/10.1007/s00227-011-1869-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1869-2