Abstract
The food web of two intertidal seagrass (Zostera marina and Zostera noltii) beds that may be influenced by the seasonal variation in food source abundance was studied in winter and in summer with δ13C and δ15N analysis. In spite of high relative variation of abundance of main primary producers at the two sites, the food web did not vary between winter and summer. The δ13C range of primary producers was wide. Zostera leaves, the most 13C-enriched source, were not consumed directly by grazers. Deposit and filter feeders have a similar δ13C and could use a mix of suspended and sedimented organic particulate matter, largely composed of detritus from macroalgae to seagrass. This trophic pathway allows the local incorporation of the high biomass produced by seagrasses. The wide δ15N range of predators was linked either to a large variety from omnivore to carnivore predators or to the also wide ranges of δ15N of primary consumers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seagrass beds constitute complex ecosystems in shallow coastal waters in regard to both diversity and abundance of organisms (Heck et al. 1995). Seagrasses only represent one of many primary producers in these ecosystems in which benthic and epiphytic micro- and macroalgae and phytoplankton also significantly contribute to total biomass (McRoy and McMillan 1977). These systems are highly productive (Duarte and Cebrian 1996) but the fate of primary production remains a matter of study. Studies from different parts of the world reported important export of detrital materials from seagrass beds to adjacent areas (Stephenson et al. 1986; Duarte 2002; Schaal et al. 2008), but the intensity of fluxes is difficult to quantify. The transfer of matter from primary producers to higher trophic levels in temperate seagrass bed has been largely investigated in subtidal areas using stable isotopes (e.g. Stephenson et al. 1986; Jaschinski et al. 2008; Jephson et al. 2008). This technique has been shown to be relevant to study several aspects of seagrass ecosystem functioning (see Lepoint et al. 2004 for review). In particular, studies based on this technique revealed a direct consumption of either Zostera fresh leaves (Stephenson et al. 1986; Jephson et al. 2008) or epiphytic algae (e.g. Jaschinski et al. 2008), benthic microalgae (Baeta et al. 2009; Lebreton et al. 2011) and macroalgae through the detrital material pool (e.g. McConnaughey and McRoy 1979; Kharlamenko et al. 2001) while phytoplankton seemed to have a minor trophic contribution (Jaschinski et al. 2008).
The relative abundance of co-occuring primary producers is known to vary temporally and spatially in Zostera beds. Because of the apparent plasticity in benthic consumer diets and their ability to opportunistically make use of food available in their surroundings, the relative density and distribution of different food sources are also likely to be important in determining dietary composition. This challenges conclusions of food web studies based on a single sampling. Considering the potential temporal variability of food web functioning in these ecosystems, it is therefore of paramount importance to understand the significance of each potential food source.
In this context, the aims of this study were to (1) highlight the main trophic pathways characterizing the food webs of two intertidal seagrass (Z. marina and Z. noltii) communities and (2) to study the impact of the seasonal variability of primary producer abundance on the food web structure and functioning of these communities.
Materials and methods
Study site
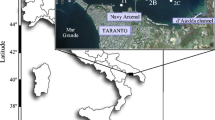
This study was carried out in two temperate intertidal seagrass beds in a mega-tidal system near Roscoff (Western English Channel, France, Fig. 1). The Z. marina (48°N44.299, 3°W58.390) and Z. noltii (48°N41.735, 3°W57.653) beds are located below 1.80 and 3.30 m (above chart datum, lowest astronomical tide), respectively, and can emerge for several hours during low water spring tides. The sediment was sandy (median grain size: 329 and 260 μm in Z. marina and Z. noltii sites, respectively), and the fine grain part (<63 μm) was negligible (<2.1%) in both sites.
Sample collection
The sampling was carried out during spring tide in February and August 2008 in the two Zostera beds. The most abundant food sources (organic matter, algae and seagrass) and the most common consumers (endofauna, epifauna and fishes) were sampled during low tide at each sampling site. Benthic microalgae were not collected because of the lack of collecting methods appropriate to low benthic microalgae biomass in sandy beds. At the Z. noltii site, Ouisse et al. (2010) have measured high Chlorophyll a content (133.70 mg Chla m−2) in sediment but the associated primary production remained very low, highlighting the low benthic microalgae biomass at the site. All samples of macroalgae, seagrass leaves and macrozoobenthic organisms were collected by hand, whereas fishes were collected with a fishnet (mesh size 1 cm) in Zostera beds. In the Z. noltii bed, few fishes have been sampled due to the presence of large drifting macroalgae. 20 L sea water were collected at ca 50 cm depth at each site for suspended particulate organic matter (POM of the site, POM_S) and more than 5 km offshore (in order to avoid the presence of macrophyte detritus in the water column) for marine POM (POM_M). Sedimented Organic Matter (SOM) was collected by scraping the upper two centimetres of sediment at low tide in each site.
Sample processing
In the laboratory, macroalgae and seagrass leaves with epiphytes were cleaned in filtered (0.45 μm) seawater in order to remove detrital fragments and attached animals. Leaves were carefully scraped with a thin brush (Dauby and Poulicek 1995) and removed epiphytes were then cautiously re-suspended in filtered seawater. Both POM and epiphytes were pre-filtered on a 63-μm mesh to remove zooplankton, meiofauna organisms and large detritus. The cleaned POM and epiphyte samples were then filtered on pre-combusted (450°C, 4 h) Whatman GF/F filters. All macrozoobenthic individuals were starved (12 h in 0.45 μm filtered seawater) to clear their guts. The flesh of molluscs and crustaceans was separated from their shell or calcareous external skeletons. Whole animals (small specimens) or muscle tissue (large specimens and fishes) were analysed.
All samples (POM, SOM, Zostera spp. leaves, epiphytes, macroalgae and consumers) were quickly acidified (1 M HCl) in order to remove carbonates, rinsed with distilled water and dried (60°C, 48 h). All samples were then ground to powder using a mortar and pestle before being frozen at −32°C for preservation pending stable isotope analyses (number of replicates for stable isotope analyses was indicated in Tables 1 and 2).
Stable isotope analyses
Carbon and nitrogen isotope ratios were determined using a Flash EA CN analyser coupled with a Finnigan Delta Plus mass spectrometer, via a Finnigan Con-Flo III interface. Data were expressed in conventional δ unit notation
with X is 13C or 15N and R is 13C/12C ratio for carbon or 15N/14N ratio for nitrogen.
The 13C and 15N abundances were calculated in relation to the certified reference materials Vienna-Pee Dee Belemnite-limestone (V-PDB) and atmospheric di-nitrogen (N2). The V-PDB and N2 at air-scales were achieved using in-house protein standards, calibrated against NBS-19 and IAEA N3 reference materials. The standard deviation of repeated measurements of δ13C and δ15N values of the laboratory standard was 0.10% versus V-PDB and 0.05% versus at air, respectively.
The mean isotopic composition of the consumers’ diet can be estimated by assuming a mean trophic enrichment of 1% in δ13C (DeNiro and Epstein 1978; Rau et al. 1983). For δ15N, the trophic enrichment depends on the trophic level of organisms: a mean trophic enrichment of 3.4% was used for carnivores (McCutchan et al. 2003; Vanderklift and Ponsard 2003), whereas 2.5% was preferred for primary consumers (Vander Zanden and Rasmussen 2001).
Primary producer biomass and organic matter in the sediment
In February and August 2008, primary producer biomass in three samples (cores of 0.071 m2) was determined in Z. marina and Z. noltii beds. Rhodophyta, Phaeophyta, Chlorophyta and seagrass (seagrass leaves and epiphytes) were dried separately at 60°C up to a constant weight. On additional samples, Zostera leaves and epiphytes were separated (see above for method) and dried at 60°C up to a constant weight. Epiphyte biomass per area unit was finally calculated from the ratio epiphyte-seagrass biomass and the total seagrass-plus-epiphyte biomass per area unit. Carbon part of Dry Weight (DW) of each primary producer was estimated from CHN analysis and then used to express biomass as gC m−2 using a carbon part of DW of 43.5% for Z. noltii leaves, 40.1% for Z. marina leaves, 33.7% for perennial macroalgae (small red and brown algae) and 35.2% for ephemeral macroalgae (Enteromorpha spp.).
Organic matter content in sediment was estimated in spring and autumn 2008 in Z. marina and Z. noltii beds. At each sampling date, six sediment cores (2.9 cm2, 1 cm depth) were sampled (three at the upper and three at the lower level of the Zostera bed). Samples were dried at 60°C up to a constant weight (W d ), combusted for 6 h (520°C) and weighed again (W c ). The percentage of organic matter in the sediment (OM sed) was calculated using the following formula
Data and statistical analyses
Sixty-three and 52 species at Z. marina site and 24 and 39 species at Z. noltii site have been collected in February and August, respectively. For statistical analyses on isotopic ratios, macrozoobenthic organisms were merged in four trophic groups, grazers, deposit feeders, filter feeders and predators, according to literature.
Any difference on the range of the trophic groups has been highlighted using all consumers rather than only common consumers of the two sampling dates at each site. Only the common sources and consumers of the two sampling dates were then used for further analyses at each site.
As most outputs did not satisfy the conditions of normality, differences between sampling dates for organic matter content in sediment and isotopic ratios of potential primary food sources (organic matter, seagrass leaves, macroalgae and epiphytes) and trophic groups were analysed using non-parametric Mann–Whitney U tests.
The mixed model SIAR v 4.0 (stable isotope analysis in R) of Parnell et al. (2010) was used to estimate the likely contribution of each of the potential food items to the diets of filter feeders in each site. Only SOM and POM of the site (POM_S) were used in the mixed model as an available source for the filter feeders. Mean trophic enrichment factors of 1 ± 1.2% for δ13C and 2.5 ± 2.5% (Vander Zanden and Rasmussen 2001) for δ15N were considered.
Results
Abundance and stable isotope ratios of sources
At the Z. marina site, the biomass (mean ± SD) of the main benthic primary producer, seagrass leaves, was 23.0 ± 6.7 gC m−2 in February and 80.0 ± 29.7 gC m−2 in August (Fig. 2a). Seagrass leaves represented more than 90% of the benthic primary producer biomass in February, while the biomass of red and brown macroalgae fixed on small rocks were low (9%). Epiphyte biomass and ephemeral green macroalgae (Enteromorpha spp.) biomass were negligible. In contrast, Enteromorpha spp. was the most abundant source in August (53%) and Z. marina leaves represented only 39% of the main primary producer biomass. The relative biomass of other macroalgae biomass did not vary (8%). The mean (±SD) organic matter content in the sediment was low and varied between 1.33 ± 0.16% in spring and 1.86 ± 0.51% in autumn.
At the Z. noltii site, the biomass of main benthic primary producers (mean ± SD) was 27.9 ± 13.8 gC m−2 in February and more than twofold higher in August (57.0 ± 22.1 gC m−2, Fig. 2b). Macroalgae were present but scattered on little rocks and their biomass was negligible for both dates. Z. noltii leaves dominated the biomass of primary producers in February (64%) and August 2008 (94%) and epiphytes represented more than one-third of the main benthic primary producer biomass in February (35%). The mean organic matter content in the sediment was low (0.98 ± 0.29% in spring and 1.12 ± 0.19% in autumn).
At both sites, 9 sources were collected for stable isotope analyses in February and August (Tables 1, 2). Among them, Zostera spp. (leaves and old leaves, depending on the site) was the most 13C-enriched whereas, the marine POM (POM_M) was the most 13C-depleted (Figs. 3, 4).
Mean (±SD) δ15N versus δ13C of potential sources and consumers sampled in (a) February and (b) August 2008 in the Z. marina bed. Legends for consumers (closed symbol and number) and sources (open symbol) are given in the Table 1. Loripes lucinalis (16) and L. borealis (17) are not represented on the figure. Symbol shape corresponds to trophic group: grazers (square symbol), filter feeders (circle symbol), deposit feeders (diamond symbol) and predators (triangle symbol)
Mean (±SD) δ15N versus δ13C of potential sources and consumers sampled in (a) February and (b) August 2008 in the Z. noltii bed. Legends for consumers (closed symbol and number) and sources (open symbol) are given in the Table 2. Loripes lucinalis (13) is not represented on the figure. Symbol shape corresponds to trophic group: grazers (square symbol), filter feeders (circle symbol) and predators (triangle symbol)
At the Z. marina site, the δ13C range displayed by primary producers was not different between the two dates (from −21.8 to −10.5% in February and from −18.7 to −10.2% in August, Fig. 3). The range of δ15N displayed by macroalgae and epiphytes was low, their mean δ15N values not varying between the two dates (n = 14, P > 0.05). The SOM was the most 15N-depleted source in February (Table 1).
At the Z. noltii site, the range of δ13C of macroalgae was twofold lower in February (3.0%) than in August (6.4%, Fig. 4). The δ15N range of macroalgae was wide in February (3.6%) and narrow in August (0.8%). Neither δ13C nor δ15N of macroalgae varied between the 2 dates (n = 12, P > 0.05). The SOM was the most 15N-enriched source in August (Table 2).
Finally, the δ13C of epiphytes varied between February and August for both sites and epiphytes were clearly separated from Zostera leaves and macroalgae in either δ13C or δ15N in February but not from macroalgae in August.
Consumers stable isotope ratios
At the two sites, the molluscs Loripes lucinalis and Lucinoma borealis exhibited depleted δ13C and δ15N (Tables 1, 2). This did not reflect an utilization of much depleted sources but the presence of endosymbiontic bacteria in the gills of these bivalves which led to an important depletion in both δ15N and δ13C (Kiyashko et al. 1997). These species were thus excluded from the determination of main trophic pathways in the studied seagrass beds.
At the Z. marina site, δ13C of grazers (5 species of molluscs) varied significantly between the 2 dates (n = 14, P < 0.05). Grazers displayed the widest range of δ13C of consumers for both dates (16.3% in February and 8.4% in August, Fig. 3). Aplysia punctata was the most 13C-depleted consumer in February and August (−23.7 and −20.0%, respectively, see 10 in Fig. 3) and Jujubinus striatus was the most 13C-enriched consumer in February and August (−7.4 and −11.5%, respectively, see 15 in Fig. 3). Mean stable carbon isotope ratios of deposit feeders (4 species of polychaetes, Table 1) varied between −18.6 and −16.4% in February and between −18.0 and −17.3% in August. δ15N of deposit feeders varied significantly between the 2 dates (n = 11, P = 0.004). The mean δ13C of filter feeders (2 species of polychaetes and 2 species of molluscs) varied from −19.1 to −17.9% in February and from −18.7 to −17.9% in August and their δ15N between 7.8 and 10.2% in February and between 7.0 and 8.5% in August. Neither δ13C nor δ15N varied between the two dates (n = 9, P > 0.05). Among all 34 consumers, the predator trophic group was the most diversified (19 species among the six sampled phyla). Predator species displayed a wide range of δ13C and δ15N for both dates (Fig. 3). The fishes Labrus bergylta and Taurulus bubalis were the most N15-enriched consumers in February and August, respectively (see 33 and 34 in Fig. 3).
At the Z. noltii bed, 15 species (2 grazers, 7 filter feeders and 6 predators) and 2 groups of species (nematodes and nemertes which trophic group could not be specified) have been collected in February and August (Table 2). The 2 grazers (the molluscs Gibbula pennanti and Gibbula cineraria) had a similar mean δ13C and δ15N (see 7 and 8 in Fig. 4). Filter feeders (polychaetes and molluscs) displayed a narrow range of δ13C (from −17.5 to −16.8% in February and from −17.6 to −16.1% in August). Their mean δ15N varied between 7.5 and 10.4% in February and between 8.9 and 10.8% in August (Fig. 4). Predators (3 polychaetes, 1 mollusc, 1 crustacean and 1 chordate) displayed a low range of mean δ13C values (1.4% in February and 1.6% in August), whereas the range of mean δ15N value was wide (4.7% in February and 3.0% in August). The mean δ13C and δ15N of each trophic groups did not vary between dates (n = 6, P > 0.05).
Using mixing model, the two sources equally contributed to the diet of the two filter feeders at the Z. marina site (Fig. 5) while the contribution of SOM was higher (about 70%) at the Z. noltii site (Fig. 6).
Contribution of marine particulate organic matter of the site (POM_M) and SOM to the diet of the two deposit feeders: Megalomma vesiculosum (M. vesiculosum) and Lanice conchilega (L. conchilega) at the Z. marina site using mixed model of Parnell et al. (2010) in February and August. The proportions show the credibility intervals plotted at 95–5, 90–10, 75–25 and 50%
Contribution of marine particulate organic matter of the site (POM_M) and SOM to the diet of the two deposit feeders: Megalomma vesiculosum (M. vesiculosum) and Lanice conchilega (L. conchilega) at the Z. noltii site using mixed model of Parnell et al. (2010) in February and August. The proportions show the credibility intervals plotted at 95–5, 90–10, 75–25 and 50%
Discussion
Isotopic characterization of sources in the Zostera food web
The δ13C of Zostera leaves fell in the range reported by Hemminga and Mateo (1996) for seagrass. In addition, the δ13C of old Z. marina leaves was gently more 13C depleted (<1%) compared to fresh tissue for both dates, as previously measured by Kharlamenko et al. (2001) and Hoshika et al. (2006) in the Sea of Japan. The macroalgae were distributed over a wide range of δ13C values, typical of temperate coastal environments (Fredriksen 2003; Bode et al. 2006; Jephson et al. 2008; Kang et al. 2008) and overlapped the δ13C values of epiphytes in August. In contrast, the differences of δ15N among co-occurring macrophytes (seagrass, macroalgae and epiphytes) were low and the values observed here are in accordance with the absence of anthropogenic or freshwater nitrogen sources in both sites which would have resulted in 15N enrichment in primary producers (e.g. Baeta et al. 2009).
Marine POM (POM_M) δ13C is typical of POM dominated by marine phytoplankton, as already measured in the Western English Channel (Schaal et al. 2010). The POM of the site (POM_S) was less 13C depleted, and this might reveal the presence of suspended detritus mixed with phytoplankton (Kaehler et al. 2000).
The organic matter in the sediment (SOM) can derive from various marine sources (mean δ13C of all the primary producers). First, benthic microalgae were not sampled but their δ13C and δ15N presented in the literature are in the range of the ones of the SOM (Riera and Hubas 2003; Jaschinski et al. 2008). In addition, seagrass leaves can greatly modify the hydrodynamic processes enhancing the sedimentation of suspended particulate matter (Kock et al. 2006) and decreasing the export of matter from seagrass bed to neighbouring environment. The SOM in seagrass beds results therefore from a mixture of sources of various origins. Here, the isotopic values found at both sites suggested that the SOM pool was composed of a mixture of benthic microalgae, macroalgae (wide range of values), seagrass detritus (13C-enriched) and sedimented phytoplankton (13C-depleted).
Major trophic pathways in Zostera beds
In the Z. marina bed, the five species of grazer exhibited a wide range of δ13C. Among them, J. striatus seemed to be the only one able to directly use Zostera leaves or epiphytes as a source of carbon. However, its δ15N value rather indicated a preferentially use of epiphytic algae. Seagrass contains a large proportion of indigestible material (lignin) and their phenolic compounds are known to deter herbivory (Harrison 1982). Jujubinus striatus could actually graze epiphytic algae as experimentally shown by Hily et al. (2004). In the Z. noltii bed, the δ13C and δ15N values of the two species of grazer also indicated a use of macroalgae or epiphytes. Then, from these results, the resource of the grazers are likely to derive more from macroalgae or epiphytes than from seagrass, as already concluded in other temperate seagrass beds (e.g. McConnaughey and McRoy 1979; Stephenson et al. 1986; Jaschinski et al. 2008). Therefore, it can be assumed that Z. marina and Z. noltii primarily provide habitat and shelter for grazers, whereas food is mainly supplied indirectly by providing space for attached epiphytes. Grazers play a major role in the food source availability for the others trophic groups (Hily et al. 2004). Some of the material removed from Z. marina or Z. noltii leaves by grazers but not eaten is dropped to the sediment floor (SOM) and thus available for the macrodetritivores and decomposers.
The isotopic values of potential sources for deposit feeders in the Z. marina bed were close to SOM for both dates as expected. However, the more 15N enriched values of deposit feeders in February suggested also the assimilation of a more 15N enriched source than SOM like removed sources dropped to the sediment by grazers (see above). Potential sources for filter feeders were more 13C enriched than POM and more 13C depleted than SOM of both sites, suggesting an assimilation of SOM blended with POM. The proportion of each of them to the diet of the filter feeder species common to the two sites (Megalomma vesiculosum and Lanice conchilega) was estimated via a mixed model (Parnell et al. 2010). The contribution of the SOM and POM_S to the diet of the two filter feeders in both sites may reveal the importance of re-suspension events in the trophic functioning of these seagrass beds, as previously suggested by Lebreton et al. (2011) in the Marennes-Oleron basin. In addition, the δ13C and δ15N range of each trophic group (deposit and filter feeders) suggested that these consumers may display more or less selective ability while foraging in the sediment or in the water column, as already evidenced for filter feeder bivalves for example (Jorgensen 1996).
In the present study, the δ15N range of predators was wide and overlapped those of primary consumers (deposit feeders, filter feeders and grazers) for both sites in February and August. This δ15N range can be linked either to a large variety of predators, from omnivore to carnivore, or to the also wide δ15N ranges of primary consumers and primary producers. The misleading consequences of wide δ15N range of primary producers on the determination of trophic levels have recently been highlighted for rocky shore ecosystems nearby the present study area (Riera et al. 2009). In this context, it was impossible to differentiate each trophic level based on δ15N as it has been currently done in seagrass beds (Lepoint et al. 2004 for review). Z. marina and Z. noltii beds are two complex habitats that can offer to preys protection from predators (Duarte 2002). The high diversity of predators found in this study suggests that this trophic group is likely to exert an important top-down control in the food web associated with the Zostera spp. meadow (Moksnes et al. 2008).
Comparison of summer versus winter situation
At the Z. marina site, the abundance of Enteromorpha spp. was low in winter and its role in the food web may be limited. In summer, the Enteromorpha spp. became the most abundant source, highly palatable, representing a potential source for grazers (Granado and Caballero 2001). Although, the Enteromorpha spp. can probably be consumed directly, this was not highlighted by the isotope analysis. The 13C enrichment observed between February and August both on Enteromorpha spp. and one grazer (A. punctata) suggested the use of that source among others. In addition, the large Enteromorpha spp. development in summer enriched the organic matter pool in sediment (e.g. Riera and Hubas 2003; Ouisse et al. 2011) and likely contributed indirectly to the diet of deposit and filter feeders via the detritus pathway all over the year.
At the Z. noltii site, seagrass epiphytes biomass decreased strongly from winter to summer. In addition, the change in their associated δ13C value suggested a qualitative change of the epiphyte algae community. In the literature, Jaschinski et al. (2008) concluded that epiphytes were one of the major carbon sources for grazers in Z. marina beds. In the present study, the epiphyte biomass strongly varied in Z. noltii bed between winter and summer and their contribution to higher trophic levels remained to be demonstrated during summer since their δ13C and δ15N were not clearly separated from the other macroalgae at this period. In fact, the low abundance of macroalgae and epiphytes and the low palatability of Z. noltii seem rather to confirm the importance of organic matter available in the sediment or in the water column for the benthic food web in the studied Zostera bed.
In conclusion, our results have furthermore emphasized the functional complexity of the food webs associated with seagrass beds in temperate coastal zones. Demersal and benthic compartments were coupled in the same trophic pathway, based on a mixture of sedimented and suspended particulate organic matter, originating from the different food sources of this ecosystem (including seagrass detritus). Although, macroalgae, seagrass and epiphytes varied in biomass and stable isotope ratios over the course of the year, the food webs displayed a striking seasonal stability. This supported the idea that the food web associated with Z. marina, and Z. noltii is mostly based on composite sources rather than pure food sources. The trophic structure of these Zostera beds differ from other Zostera spp. ecosystems previously investigated. As a consequence, it is impossible to generalize the ecosystem functioning to all intertidal Zostera spp. beds. Although, Z. marina and Z. noltii seemed to be of limited importance as fresh food sources for the food web, seagrass created substrate and decreased the flow enhancing the sedimentation of particles which can be used in the food web. In addition, detrital matter of seagrass is incorporated in the SOM pool and integrated in the food web. Finally, the high diversity of generalist predators, in particular in Z. marina bed, suggests an important top-down control of the seagrass community.
References
Baeta A, Valiela I, Rossi F, Pinto R, Richard P, Niquil N, Marques J (2009) Eutrophication and trophic structure in response to the presence of the eelgrass Zostera noltii. Mar Biol 156:2107–2120
Bode A, Alvarez-Ossorio MT, Varela M (2006) Phytoplankton and macrophyte contributions to littoral food webs in the Galician upwelling estimated from stable isotopes. Mar Ecol Prog Ser 318:89–102
Dauby P, Poulicek M (1995) Methods for removing epiphytes from seagrasses: SEM observations on treated leaves. Aquat Bot 52:217–228
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
Duarte CM (2002) The future of seagrass meadows. Environ Conser 29:192–206
Duarte CM, Cebrian J (1996) The fate of marine autotrophic production. Limnol Oceanogr 41:1758–1766
Fredriksen S (2003) Food web studies in a Norwegian kelp forest based on stable isotope (δ13C and δ15N) analysis. Mar Ecol Prog Ser 260:71–81
Granado I, Caballero P (2001) Feeding rates of Littorina striata and Osilinus atratus in relation to nutritional quality and chemical defences of seaweeds. Mar Biol 138:1213–1224
Harrison PG (1982) Control of microbial growth and of amphipod grazing by water-soluble compounds from leaves of Zostera marina. Mar Biol 67:225–230
Heck K, Able K, Roman C, Fahay M (1995) Composition, abundance, biomass, and production of macro fauna in a New England estuary: comparisons among eelgrass meadows and other nursery habitats. Estuar Coasts 18:379–389
Hemminga MA, Mateo MA (1996) Stable carbon isotopes in seagrasses: variability in ratios and use in ecological studies. Mar Ecol Prog Ser 140:285–298
Hily C, Connan S, Raffin C, Wyllie-Echeverria S (2004) In vitro experimental assessment of the grazing pressure of two gastropods on Zostera marina L. ephiphytic algae. Aquat Bot 78:183–195
Hoshika A, Sarker MJ, Ishida S, Mishima Y, Takai N (2006) Food web analysis of an eelgrass (Zostera marina L.) meadow and neighbouring sites in Mitsukushi Bay (Seto Inland Sea, Japan) using carbon and nitrogen stable isotope ratios. Aquat Bot 85:191–197
Jaschinski S, Brepohl DC, Sommer U (2008) Carbon sources and trophic structure in an eelgrass Zostera marina bed, based on stable isotope and fatty acid analyses. Mar Ecol Prog Ser 358:103–114
Jephson T, Nystrom P, Moksnes P-O, Baden SP (2008) Trophic interactions in Zostera marina beds along the Swedish coast. Mar Ecol Prog Ser 369:63–76
Jorgensen CB (1996) Bivalve filter feeding revisited. Mar Ecol Prog Ser 142:287–302
Kaehler S, Pakhomov EA, McQuaid CD (2000) Trophic structure of the marine food web at the prince Edward islands (southern ocean) determined by δ13C and δ15N analysis. Mar Ecol Prog Ser 208:13–20
Kang C-K, Choy E, Son Y, Lee J-Y, Kim J, Kim Y, Lee K-S (2008) Food web structure of a restored macro algal bed in the eastern Korean peninsula determined by C and N stable isotope analyses. Mar Biol 153:1181–1198
Kharlamenko VI, Kiyashko SI, Imbs AB, Vyshkvartzev DI (2001) Identification of food sources of invertebrates from the seagrass Zostera marina community using carbon and sulphur stable isotope ratio and fatty acid analyses. Mar Ecol Prog Ser 220:103–117
Kiyashko SI, Chernyaev MG, Rodionov IA (1997) Mixotrophy in shallow-water bivalve molluscs with bacterial endo symbionts: data on stable carbon isotope ratios. Russ J Aquat Ecol 6:53–57
Kock EW, Ackerman JD, Verduin J, van Keulen M (2006) Fluid dynamics in seagrass ecology—from molecules to ecosystems. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, Dordrecht, pp 193–225
Lebreton B, Richard P, Galois R, Radenac G, Pfléger C, Guillou G, Mornet F, Blanchard GF (2011) Trophic importance of diatoms in an intertidal Zostera noltii seagrass bed: evidence from stable isotope and fatty acid analyses. Estuar Coast Shelf Sci 92:140–153
Lepoint G, Dauby P, Gobert S (2004) Application of C and N stable isotopes to ecological and environmental studies in seagrass ecosystems. Mar Pollut Bull 49:887–891
McConnaughey T, McRoy CP (1979) δ13C label identifies eelgrass (Zostera marina) carbon in an alaskan estuarine food web. Mar Biol 53:263–269
McCutchan JHJ, Lewis WMJ, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulphur. Oikos 102:378–390
McRoy CP, McMillan C (1977) Production ecology and physiology of seagrasses. In: McRoy CP, Helfferich C (eds) Seagrass ecosystems: a scientific perspective. Dekker, New York, pp 53–81
Moksnes P-O, Gullstrom M, Tryman K, Baden S (2008) Trophic cascades in a temperate seagrass community. Oikos 117:763–777
Ouisse V, Migné A, Davoult D (2010) Seasonal variations of community production, respiration and biomass of different primary producers in an intertidal Zostera noltii bed (western English Channel, France). Hydrobiologia 649:3–11
Ouisse V, Riera P, Migne A, Leroux C, Davoult D (2011) Freshwater seepages and ephemeral macroalgae proliferation in an intertidal bay: I effect on benthic community structure and food web. Estuar Coast Shelf Sci 91:272–281
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5:e9672
Rau GH, Mearns AJ, Young DR, Olson RJ, Schafer HA, Kaplan IR (1983) Animals δ13C correlates with trophic level in pelagic food webs. Ecology 64:1314–1318
Riera P, Hubas C (2003) Trophic ecology of nematodes from various microhabitats of the Roscoff Aber Bay (France): importance of stranded macroalgae evidenced through δ13C and δ15N. Mar Ecol Prog Ser 260:151–159
Riera P, Escaravage C, Leroux C (2009) Trophic ecology of the rocky shore community associated with the Ascophyllum nodosum zone (Roscoff, France): a δ13C versus δ15N investigation. Estuar Coast Shelf Sci 81:143–148
Schaal G, Riera P, Leroux C (2008) Trophic coupling between two adjacent benthic food webs within a man-made intertidal area: a stable isotopes evidence. Estuar Coast Shelf Sci 77:523–534
Schaal G, Riera P, Leroux C (2010) Trophic ecology in a northern Brittany (Batz Island, France) kelp (Laminaria digitata) forest, as investigated through stable isotopes and chemical assays. J Sea Res 63:24–35
Stephenson RL, Tan FC, Mann KH (1986) Use of stable carbon isotope ratios to compare plant material and potential consumers in a seagrass bed and a kelp bed in Nova Scotia, Canada. Mar Ecol Prog Ser 30:1–7
Vander Zanden MJ, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066
Vanderklift MA, Ponsard S (2003) Source of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182
Acknowledgments
This study forms part of the Ph.D. thesis of V. Ouisse and was financially supported by the “Ministère de l’Enseignement Supérieur et de la Recherche”. The authors would like to thank G. Schaal for his knowledge of isotope stable analyses, the Service Mer et Observation, R. Michel and L. Oppliger for their help in field sampling. We also would like to thank the two anonymous referees, whose comments greatly contributed to the improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Rights and permissions
About this article
Cite this article
Ouisse, V., Riera, P., Migné, A. et al. Food web analysis in intertidal Zostera marina and Zostera noltii communities in winter and summer. Mar Biol 159, 165–175 (2012). https://doi.org/10.1007/s00227-011-1796-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1796-2