Abstract
The elemental carbon (C), nitrogen (N) and phosphorus (P) compositions of the whole-body and gut content of wild marine fish inhabiting the Bay of Biscay (Northeast Atlantic) were studied. Furthermore, the literature was examined for studies of aquacultured fish, reporting the elemental composition of the whole-body fish, that of their food, and nutrient assimilation and gross growth efficiencies (GGE). In both wild-caught and aquacultured fish, significant differences in C, N and P elemental composition were found between species, with P being the most variable component. Differences among species in terms of C, N and P content could be explained by varying proportions of storage compounds in whole-body fish, and varying degrees of ossification. Aquacultured fish feces were found to be P-rich, because of a lower P assimilation efficiency, compared to C or N assimilation efficiencies. Examination of aquacultured fish literature also revealed that C, N and P GGE and nutrient resupply ratios agreed with basic principles of homeostatic regulation of whole-body fish elemental composition. Extrapolation of the results to broader marine systems indicated that fish may be important for conveying nutrients toward the ocean interior.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ecological stoichiometry of biologically important elements, such as carbon (C), nitrogen (N) and phosphorus (P), is valuable toward understanding how aquatic ecosystems function (Andersen 1997; Hassett et al. 1997; Sterner and Elser 2002). Basically, organisms that function within natural communities often differ with respect to their elemental composition (van de Waal et al. 2010). Such differences arise as a result of variation in the composition and proportion of hard structures such as bones, and biochemicals that constitute all living organisms, such as carbohydrates, proteins and lipids (Sterner and Elser 2002).
In marine systems, differences in the elemental composition of microalgae for example may arise as a result of a species’ evolutionary inheritance (Quigg et al. 2003) and/or environmental conditions such as light and nutrient supply for phytoplankton (Geider and La Roche 2002; Klausmeier et al. 2004). Hence, the elemental stoichiometry of C, N and P within a given taxon of microalgae may be subject to substantial variability (Finkel et al. 2006). The elemental composition of marine and freshwater fauna also differs within and between species groups (Ikeda and Mitchell 1982; Sterner and George 2000; Pertola et al. 2001; Clarke 2008), particularly during ontogeny (Pilati and Vanni 2007). However, unlike phytoplankton, several studies have reported partial or almost complete homeostasis of the elemental composition of multicellular organisms within age classes of different species (Sterner and Elser 2002; Pilati and Vanni 2007).
Existing studies of C, N and P elemental composition of wild fish have mostly focused on freshwater species (Sterner and George 2000; Dantas and Attayde 2007; Pilati and Vanni 2007; Hendrixson et al. 2007), as opposed to marine species (but see Beers 1966; Deegan 1986; Hjerne and Hansson 2002).
In wild freshwater species, interspecific differences in P content are notable when compared to C and N contents (Sterner and George 2000; Dantas and Attayde 2007; Pilati and Vanni 2007; Hendrixson et al. 2007). Differences in whole-body P concentration have some phylogenetic roots and may result from a combination of RNA content and skeletal investment (Sterner and Elser 2002; Hendrixson et al. 2007). Furthermore, whole-body P may reach a relatively constant value during the late stages of development of multicellular organisms, because the fraction of P in RNA decreases relative to the fraction in other pools such as the skeleton (Gillooly et al. 2005). Differences in whole-body C content among marine fish species have been linked to variations in body lipid levels, with deeper-living fish showing lower lipid levels as a result of reduced metabolic rates (e.g., Childress et al. 1990).
For organisms that interact with one another along the food chain, such variability in the requirement of certain elements may influence the fate of nutrients within ecosystems, which in turn feeds back on the organisms themselves. In freshwater systems, the impact of phytoplankton growth being limited by nutrient availability has been shown to travel up the food chain beyond herbivores (Boersma et al. 2008). Conversely, zooplankton and fish have been shown to exert a degree of control on the structure and dynamics of freshwater phytoplankton communities. Such control may arise through (1) appropriating limiting elements of primary production in their tissue (Hjerne and Hansson 2002), (2) consumer-driven nutrient recycling (CNR) of key elements such as N and P (Sterner 1990; Elser and Urabe 1999; Vanni et al. 2006), or (3) the decomposition of dead organisms (Threlkeld 1988).
The ecological and biogeochemical implications of CNR have received relatively less attention in marine systems in comparison to that of freshwaters systems, particularly concerning the role of fish. However, a study in the Baltic Sea revealed that fish may constitute a significant P pool, thus reinforcing the P-limitation of primary production in this system (Hjerne and Hansson 2002). Furthermore, in open oceans, N or P limitations are often found in the subtropical gyres, which account for ~70% of the open ocean surface area (Tyrrell 1999; Wu et al. 2000; Karl et al. 2001; Moutin et al. 2005). Owing to the importance of predation-driven natural mortality in marine pelagic food webs (Hirst and Kiørboe 2002; Landry and Calbet 2004), marine fish may contribute toward controlling nutrient fluxes, as has been recently suggested by Radchenko (2007) in the Bering Sea.
To evaluate the role of fish on nutrient cycling, a better knowledge of the range of variation of body nutrient contents is required, as well as some information on the fate of nutrients after ingestion. The purpose of this study was to obtain insights on the overall variation of C, N and P stoichiometry for a broad taxanomic range of wild-caught marine fish inhabiting the Bay of Biscay (Northeast Atlantic). We processed fish body and fish gut content separately to investigate differences in elemental composition between the fish and its prey items. Owing to the difficulty of measuring the flux of nutrients in and out of fish in natural systems, the literature was also examined for studies of aquacultured fish reporting the elemental composition of the whole-body fish, that of its food, and resulting nutrient assimilation efficiencies and gross growth efficiencies. Wild and aquacultured fish data were combined when possible to search for patterns in the elemental stoichiometry of fish. Finally, we considered the possible impact of the variability in element composition on the distribution and cycles of oceanic nutrients.
Materials and methods
Study location and fish capture
During October–November 2008, specimens of marine teleost fish species were collected onboard the research sea vessel R.V. Thalassa during a survey in the Bay of Biscay (North-East Atlantic Ocean, ESM 1) conducted by the Institut Français de Recherche pour l’Exploitation de la Mer (IFREMER, cruise EVHOE 2008). A total of 10 sites were surveyed. Surveys were conducted using government standard bottom trawl nets (category “34/47 GOV”) which had large vertical openings. Each haul lasted for 30 mn, with an area of 0.068 km2 being swept per trawl. At 1 site (M0881), additional hauls were also carried out using a pelagic trawl 76 × 70 that specifically targeted the capture of bathypelagic species. Once on board, the fish were sorted by total length classes (see next section for details), placed in plastic bags, and frozen at −20°C for storage until processing at the laboratory.
Body length and biomass determination
Total fish length (TL) was measured in millimeters from the tip of the snout to the end of the caudal fin. Wet weight (WW) was measured in milligrams (±0.1 mg), and gut content were separated from the fish and processed separately. For each specimen, the entire fish and its gut content were then separately dried for a minimum of 24 h in a freeze-dryer. Dry weight (DW) was then measured in milligrams (±0.1 mg). Following freeze-drying, fish and gut content were then separately ground into a fine powder, using a pestle and mortar in preparation for elemental analysis.
Elemental analysis
Total whole-body phosphate (Tot-P) was measured in triplicate on sub-samples of each individual fish, using a method similar to that of Sterner and George (2000). Tot-P was measured on subsamples of gut content using the same method, in duplicate when sufficient material was available. Each sample of fish or gut content, typically 5–10 ± 0.1 mg of powder, was placed in Pyrex bottles (100 ml) and incinerated in a muffle furnace at 500°C, for a period of 5 h. After cooling to room temperature, 25 ml of 0.3 M HNO3 (Merck, Germany) was added (i.e., hydrolysis), and the samples were kept tightly sealed (i.e., using Teflon jointed screw caps) at a constant temperature of 80°C overnight. The next day, the samples were again cooled to room temperature, and the volume of each sample was increased to 75 ml, using 5 ml of NaOH 1 M (Merck, Germany) and 45 ml of MilliQ-water (Millipore-Q). Blanks (triplicate) were prepared similarly using empty Pyrex bottles. For each sample, orthophosphate (o-P) was measured, using the acid-molybdate method (Strickland and Parsons 1972), on an aliquot after diluted to 1/50 with MilliQ-water. Each separate assay run was calibrated along a standard curve (range 0–5 μM-P) where a solution of KH2PO4 (Merck, Germany), prepared with 25 ml HNO3 0.3 M, 5 ml NaOH 1 M and 45 ml MilliQ-water, was used as the source for inorganic phosphate. The mean absorbance of the blanks (i.e., controls) was not significantly different from 0 μM-P (o-P = 0.000 ± 0.003 μM-P, ±95% CI, Student t test, P = 0.66). For each assay run, recovery of Tot-P was determined using bovine reference material (NIST 8414), which contains 0.8360 ± 0.0005 g P/g DW. The method used here resulted in a mean recovery of Tot-P of 90 ± 2% (±CI 95%, n = 63), with >70% of the replicates having a recovery of tot-P ≥ 90%.
C and N levels were measured from subsamples of the powder of dry tissue and gut content, using a Carlo Erba 1106 elemental analyzer. Dry tissue and gut content powder samples were typically 5–10 ± 0.1 mg in weight. All samples were run in triplicate. Empty sample vials (or blanks) and acetanilide standards were run for every 5th set of duplicate samples. Elemental composition was calculated based on the mean for all empty sample vials (or blanks) and acetanilide standard runs for each fish species.
Elemental stoichiometry of aquacultured fish
The literature was examined for studies of aquacultured fish reporting the elemental composition of the whole-body fish, that of their food, and nutrient assimilation and gross growth efficiencies (see definitions below). Lists of data sources used in this paper are given in ESM 2. As carbon was seldom measured directly in these studies, total carbon content in whole-body fish and food was estimated from energy content using the conversion factor of 1 g C = 11.4 kcal (Platt and Irwin 1973). Total N content is scaled to protein content, with protein content (% Dry Mass, DM) = total N content (% DM) × 6.25.
Ingested nutrients are either allocated to growth or released as waste products. These waste products include the production of fecal pellets, basal metabolism or release of nutrients in excess to requirements. Also, costs of basal metabolism or growth are additional loss in terms for carbon. Thus, a simplified mass balance equation for a nutrient i ingested by a fish can be written:
where G i is the growth rate, I i is the ingestion rate, F i is the fecal pellets production, and E i is the term of release through either basal metabolism, excretion of nutrients in excess to requirements or costs of biological processes. Equation 1 can be rewritten as follows:
In Eq. 2, β i and a i are the assimilation efficiency and the accumulation efficiency, respectively. The assimilation efficiency is the fraction of ingested nutrients which has passed the gut wall. The accumulation efficiency, defined by Sterner (1990), is actually the fraction of assimilated nutrients allocated to the production of new biomass, when losses from metabolic processes associated with maintenance and growth, and losses required in order to maintain consumer homeostasis have been subtracted (Anderson and Hessen 2005). Consequently, (1 − β i ) represents the fraction of ingested nutrients released as fecal pellets, and (1 − a i ) the fraction of assimilated nutrients (β i I i ) released or excreted in the environment. In aquacultured fish studies, nutrient assimilation efficiencies are deduced from the apparent digestibility coefficient (ADC, e.g., Fernández et al. 1998):
In this equation, β i is corrected for possible biases (leakage of nutrients from fecal pellets before analysis for example) using a passive tracer (here chromium). Nutrient gross growth efficiency (GGE i ) is deduced from simplification of Eq. 2, i.e.,:
Thus, a i can be deduced from β i and GGE i , the two parameters usually reported in aquacultured fish studies (ESM 2).
Stoichiometric model applied to aquacultured fish
Numerous studies have reported partial or almost complete homeostasis of the elemental composition of multicellular organisms within age classes of different species (Sterner and Elser 2002), notably in fish (Pilati and Vanni 2007). When food elemental composition varies, the principles of consumer-driven nutrient recycling (CNR) predict that homeostatic regulation of whole-body elemental composition of the consumer is maintained by releasing elements which are in excess to requirements, while more efficiently retaining limiting elements. In turn, this induces variable nutrient GGE and nutrient resupply ratios (Sterner and Elser 2002). Sterner (1990) formalized a simple stoichiometric model which enabled predictions of nutrient GGE and nutrient resupply ratios using two variables, i.e., the nutrient stoichiometry of the consumer’s whole-body and that of its food, and a fixed coefficient, i.e., the maximum fraction of assimilated nutrients which can be allocated to growth (called the maximum accumulation efficiency, L m). Since Sterner (1990), more realistic stoichiometric models have been developed to account for the various biological processes driving the flux of nutrients in and out of organisms (e.g., Touratier et al. 2001; Anderson et al. 2005; Anderson and Hessen 2005). The aim of this study was not to parameterise a detailed stoichiometric model for aquacultured fish. Rather, we have chosen a simple homeostatic model (Sterner 1990) as supportive material to assess whether variations of nutrient GGE and nutrient resupply ratios in aquacultured fish agreed with basic principles of CNR theory and homeostatic regulation of whole-body elemental composition. Sterner (1990) model calculates the fraction of the assimilated nutrients i which is allocated to growth as a function of the difference between the elemental stoichiometry of the consumer and that of its food. This fraction is called the accumulation efficiency, a i . Conversely, the fraction which is not allocated to growth (1 − a i ) is released in the environment. As said earlier, this term of release is a simplification which encompasses different processes: basal metabolism, excretion of nutrient in excess to requirements, and costs of basal metabolism and of growth (Anderson and Hessen 2005). For example, nitrogen accumulation efficiency, a N, and phosphorus accumulation efficiency, a P, are calculated as followed:When the N/P ratio in the assimilated food (R food × βN/βP) is lower than that of the fish body (R fish), the model predicts that:
In this case, P is in excess to requirement in assimilated food compared to that of fish body. Thus, N is the limiting element and its accumulation efficiency is maximum and equal to L m. Conversely, P accumulation efficiency is lower than L m and proportional to the ratio R food × βN/βP/R fish. Using these parameterisations, strict homeostasis of the N/P ratio in fish biomass is maintained. Indeed, considering a flux of N and P entering the fish pool (I N and I P, respectively, Eq. 2), the N/P ratio in the fraction of nutrient accumulated in fish biomass can be written:
with a N = L m, a P = L m(R food βN/βP)/R fish, and I P = I N/R food, this yields:
Conversely, when R food × βN/βP > R fish
In this case, N is in excess to requirement in assimilated food compared to fish body. Hence, P becomes the limiting element and its accumulation efficiency is maximum. Here again, strict homeostasis of the N/P ratio in fish biomass is also maintained.The N/P resupply or excretion ratio (R resupply) can be deduced from Eqs. 5 and 6:
Conversely, the N/P gross growth efficiency (R GGE) ratio is equal to:
In this model, strict homeostasis of whole-body fish N/P ratio (R fish) implies that R GGE should be equal to R fish. A similar approach can be used to estimate the C/P stoichiometry of GGE and resupply ratios.
Statistical analysis
Linear regression analyses were used to search for global patterns and allometric trends in elemental composition. Kruskall–Wallis tests were used to compare differences in untransformed C, N, P, C/N, C/P and N/P across species. Post hoc multiple comparisons were made using Tukey’s HSD test. All statistical analyses were performed with Sigmaplot 11.0.
Results
Wild fish capture information
Table 1 summarizes the fish species caught at each sampling location during this study (map found in ESM 1). A total of 128 fish, from 8 orders and twelve families, were caught for analysis of whole-body nutrient content (Table 1). Of the 15 fish species captured in this study, nine were typical of the pelagic/neritic domain; A. presbyter, S. sprattus, E. encrasicolus, S. pilchardus, G. argenteus, T. trachurus, S. japonicus, S. Scombrus and L. aurata. Among these species, S. sprattus and L. aurata were caught in the Vilaine Estuary and the Bay of Brest, respectively. The other species caught during EVOHE were mesopelagic / bathypelagic (B. glaciale and A. olfersii) or demersal (A. sphyranea, S. cantharus, C. aper and M. merluccius).
The specimens ranged in size from 4 to 20 cm in length and 1–51 g in wet mass. The moisture content of all species represented 73.6 ± 0.7% (n = 128, ±CI 95%) of wet weight.
Aquacultured fish information
A total of 45 published sources were examined in this study (ESM 2). This represented a total of 26 fish species, which are found in marine, brackish and/or fresh waters. Fish whole-body elemental composition was reported for 16 out of 26 fish species (Table 2). All 45 studies combined, food C/P and N/P elemental stoichiometry ranged from 14.19:1 to 1,195.39:1 (n = 58), and from 3.46:1 to 115.14:1 (n = 117), respectively. Concerning the fate of nutrients after ingestion, a total of n = 170, 245, and 212 data were reported for C, N and P assimilation efficiencies, respectively. Observed C/P and N/P resupply ratios were fairly variables, ranging from 150.02:1 to 1,363.75:1 for C/P (n = 28), and from 3.68:1 to 201.44:1 for N/P (n = 103). Similarly, C, N and P gross growth efficiencies varied by more than one order of magnitude, from 0.05 to 0.88 (with n = 65, 148 and 118 for C, N, and P GGE, respectively).
Predictions from Sterner (1990) homeostatic model were compared to observed nutrient C/P and N/P resupply ratios and GGE ratios. Not all the studies reported simultaneously whole-body fish and food elemental stoichiometry, along with nutrient assimilation efficiencies (these parameters were required as inputs for the model, Eqs. 5–8). These data were reported for 6 out of 26 species for C/P (i.e., D. labrax, G. morhua, O. mykiss, P. maxima, S. salar and S. aurata), and for 9 species for N/P (i.e., C. chanos, D. labrax, G. morhua, O. mykiss, P. major, P. olivaceus, P. maxima, S. salar and S. aurata).
Global patterns in whole-body elemental composition of wild and aquacultured fish
In wild-caught fish, whole-body P content ranged between 0.6 and 6.1% of dry weight (DW; average ± SE: 2.65 ± 0.05%, Fig. 1; Table 2). The C content of fish ranged between 35.6 and 56.4% (43.46 ± 0.29%), while the N content ranged between 5.1 and 16.4% (11.24 ± 0.13%). The elemental stoichiometry (also termed “molar ratios”) was 3.55:1 to 9.21:1 (4.62:1 ± 0.08%) for C/N, 10.82:1 to 190.42:1 (46.52:1 ± 1.23%) for C/P, and 2.31:1 to 39.39:1 (10.61:1 ± 0.26% for N/P) (Table 3).
Covariation in whole-body elemental composition of wild-caught fish (gray circles) and aquacultured fish (black circles). a–b Nitrogen and carbon and c–d phosphorus and carbon (% Dry Weight, DW) for (a), (c) all individuals and (b), (d) species means. Solid lines are linear regression fitted on the combined wild-caught and aquacultured fish data sets. % N versus % C content for all individuals (r 2 = 0.36, slope ± SE = −0.175 ± 0.017, F 1,196 = 107.561, P < 0.001) and species means (r 2 = 0.41, slope ± SE = −0.240 ± 0.053, F 1,22 = 20.212, P < 0.001). % P versus % C content for all individuals (r 2 = 0.48, slope ± SE = −0.083 ± 0.006, F 1,185 = 167.219, P < 0.001), and species means (r 2 = 0.62, slope ± SE = −0.089 ± 0.016, F 1,22 = 31.572, P < 0.001). In a–d, dashed lines represent the 95% CI
In aquacultured fish, whole-body P content ranged between 0.7 and 3.8% (1.8 ± 0.1%) of DW (Fig. 1; Table 2). The C and N content ranged from 42.5 to 62.9% (53.0 ± 1.6%), and from 6.1 to 12.0% (8.8 ± 0.2%), respectively. The C/P and N/P molar ratios ranged from 36.01:1 to 196.00:1 for C/P (85.98:1 ± 9.71), and from 6.22:1 to 26.16:1 for N/P (12.17:1 ± 0.83; Table 3).
Combining wild and aquacultured fish data, it was found that whole-body N content was inversely correlated to whole-body C content (Fig. 1a, P < 0.001). Similarly, P content decreased with increasing C content (Fig. 1c, P < 0.001). These patterns were still significant when wild and aquacultured fish data were processed separately (P < 0.001, not shown), except for %N versus %C in wild-caught fish, for which a slight positive correlation was recorded (slope ± SE = 0.10 ± 0.04, r 2 = 0.05, P = 0.01).
Interspecific stoichiometric patterns
In wild-caught fish, significant differences in elemental composition and stoichiometry were found between species (Kruskall–Wallis test, P < 0.001 in all comparisons, Table 2; results from post hoc multiple comparisons, using Tukey’s HSD test, are found in ESM 3). Whole-body P content varied by about twofold, with C. aper, A. olfersii, L. aurata and S. japonicus having significantly higher P content (≥3.0% of DW) than most other species, except M. merluccius and S. cantharus which also had high P content (2.9 ± 0.2% of DW) (Table 2). While still significant, the variation in whole-body C and N content was relatively less in comparison to that of P content (Table 2). Differences recorded in whole-body C content were attributed to S. japonicus, L. aurata and A. olfersii, which had comparatively lower C content compared to the other species (from 38.8 ± 0.7 to 41 ± 0.1% DW). Whole-body N content, ranged from ~10 to 14% of DW in all species, except C. aper which had significantly lower N content (7.1 ± 0.1% of DW).
Significant variation in the stoichiometric ratios (i.e., C/N, C/P nd N/P) was also recorded between fish (Kruskall–Wallis test, P < 0.001 in all comparisons, Table 3). Higher C/N ratios were recorded in C. aper (C/N = 7.3:1 ± 0.4) in comparison to other species. For example, at the lower range, M. merluccius had a C/N ratio of 3.8:1 ± 0.1 while at the upper range B. glaciale had a C/N ratio of 5.8:1 ± 0.5. This low C/N ratio in C. aper was triggered by the low N content found in this species. Significant interspecific variation in C/P and N/P ratios were also found, with C. aper, L. aurata, A. olfersii, S. japonicus and S. cantharus having significantly lower C/P and N/P ratios than most other species (Table 3).
There was also significant variation in C, N and P elemental composition among aquacultured fish species (Kruskall–Wallis test, P < 0.001 in all comparisons; Table 2). Fish P content varied by about threefold, with P. fulvidraco, O. mykiss and S. salar having lower P content than L. calcarifer, P. maxima, S. aurata, P. major, P. olivaceus and C. chanos. Higher whole-body C content was recorded for S. salar, O. mykiss and D. labrax (from 56.7 ± 0.9 to 58.7 ± 0.7% DW) in comparison to P. maxima and C. chanos (43.7 ± 0.5 and 46.2 ± 0.6% DW, respectively). Differences among species in terms of N content were associated to D. labrax and B. bydianus which had lower N content (6.8 ± 0.1 and 7.6 ± 0.1% DW, respectively) than L. calcarifer, P. maxima, O. kisutch, C. chanos, P. olivaceus and G. morhua (from 9.3 ± 0.1 to 11.3 ± 0.1% DW). S. aurata, S. salar and O. mykiss had also lower N content (from 7.8 ± 0.1 to 8.7 ± 0.1% DW) than P. maxima and L. calcarifer.
Accordingly, there was a significant variation in the stoichiometric ratios among fish (Kruskall–Wallis test, P < 0.001 in all comparisons; Table 3). Fish with low P content such as P. fulvidraco had higher N/P ratios than most of the other species, except the three salmonidae O. mykiss, O. kisutch and S. salar, and G. morhua which had similar N/P ratios. O. mykiss had also lower whole-body C/P ratio in comparison to P. maxima and C. chanos. Finally, fish C/N ratios were higher in D. labrax, S. aurata and O. mykiss in comparison to P. maxima.
Combining wild and aquacultured fish data, it was found that species with higher whole-body C content had lower N and P content and vice versa (Fig. 1b, d, P < 0.001). Figure 2 shows how species mean C, N and P contents were related to body lipid levels or bony matter (using calcium content as a proxy of bony matter) in wild-caught and aquacultured fish. We were not able to measure body lipid and calcium contents in the wild fish examined here. However, for body lipid content, we used data from Spitz et al. (2010) who measured this parameter in the same wild fish species (except L. aurata and S. japonicus), collected at the same location (the bay of Biscay) and the same period (autumn, from 2002 to 2008 during EVOHE cruises, Table 2). Thus, higher whole-body C content was associated with higher lipid content in wild and aquacultured fish (Fig. 2a, P < 0.001). Conversely, N and P content decreased with increasing body lipid content (Fig. 2b, c, P < 0.001). Figure 2d shows that P was correlated to non-storage compounds, especially whole-body Ca content, with a mean slope (or Ca/P ratio) of 2.26 ± 0.24 (±SE, P < 0.001). This Ca/P ratio was statistically indistinguishable (one-sample t test, P = 0.656) from the mean Ca/P ratio of pure hydroxyapatite (Ca/P = 2.15:1), a major component of fish bones (Hua and Bureau 2006; Pilati and Vanni 2007). The intercept with the x-axis on Fig. 2c gave an estimate of the percentage of whole-body P which was not bounded to Ca. Here, we found a value of 0.65%, which represented from ~20% in L. calcarifer to ~82% in juveniles of P fulvidraco of total whole-body P.
Species means whole-body a carbon b nitrogen and c phosphorus content plotted against whole-body lipid content (% of Dry Weight, DW) in wild-caught (gray circles) and aquacultured (black circles) fish. d Whole-body Ca content plotted against whole-body P content (% DW) in aquacultured fish for all individuals (black circles) and species means (white circles). In a–c, solid lines are linear regression fitted on the combined wild and aquacultured fish data sets for % C versus % lipids (r 2 = 0.55, slope ± SE = 0.31 ± 0.06, F 1,21 = 27.478, P < 0.001), % N versus % lipids (r 2 = 0.27, slope ± SE = −0.08 ± 0.02, F 1,27 = 11.356, P = 0.002), % P versus % lipids (r 2 = 0.39, slope ± SE = −0.04 ± 0.01, F 1,26 = 18.308, P < 0.001). In d, solid line represents linear regression fitted on all data (r 2 = 0.72, slope ± SE = 2.26 ± 0.24, intercept ± SE = −1.46 ± 0.40, F 1,34 = 89.974, P < 0.001). In a–d, dashed lines represent the 95% CI. The body lipid content data of wild-caught marine fish came from Spitz et al. (2010)
C, N and P gut content in wild fish
The gut contents of 62 fish were examined for elemental composition. Direct observation indicated that gut content were composed primarily of crustaceans and/or fish remains. Almost completely intact preys were found, including an individual of a bathypelagic fish (serpentiformes) in the stomach of an A. olfersii.
Gut content C, N and P levels were subject to more variability than that of whole-body fish nutrient content. Gut content dry weight ranges of 7–56% for C, 1–10% for N and 1–5% for P were recorded (Table 2). This variability may have reflected either (1) a large diversity in the elemental composition of prey matter, (2) different states of degradation of ingested preys, or (3) differences in nutrient assimilation efficiencies. A significant correlation was found between C and N gut content (r 2 = 0.15, P = 0.002; Fig. 3), but not between either C and P or N and P gut content (P > 0.05).
The stoichiometric ratios C/N, C/P and N/P for fish gut content were also more variable than that recorded for fish whole-body ratios (Table 3). This variability appeared to be a direct consequence of the variability observed in C, N and P gut content (Table 2). Thus, C/N ranged from 5.0:1 to 13.6:1, C/P from 11.6:1 to 291.2:1, and N/P ratios from 2.5:1 to 27.4:1 (Table 3). The global average C/N, C/P and N/P ratios were 7.1 ± 0.3, 78.7 ± 12.6 and 11.5 ± 2.0 (±SE), respectively (Table 4).
Elemental stoichiometry of waste products in aquacultured fish
Nutrient assimilation efficiencies and feces elemental composition
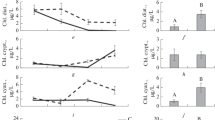
P assimilation efficiency was fairly variable, in the range 0.24–0.93 (ESM 2), and strongly correlated to food quality (Fig. 4a, b). Food quality was evaluated by the ratio between the elemental stoichiometry of the whole-body fish and that of its food, R fish/ R food (Eqs. 5–8). When R fish/ R food increased, this was an indication of P excess in food compared to fish requirement and vice versa. Thus, P assimilation efficiencies decreased when \( R_{\text{fish}}^{{{\text{C}}:{\text{P}}}} :R_{\text{food}}^{{{\text{C}}:{\text{P}}}} \) increased (Fig. 4a, P < 0.001), or when \( R_{\text{fish}}^{{{\text{N}}:{\text{P}}}} :R_{\text{food}}^{{{\text{N}}:{\text{P}}}} \) increased (Fig. 4b, P < 0.001). Conversely, higher C assimilation efficiencies were associated to higher \( R_{\text{fish}}^{{{\text{C}}:{\text{P}}}} :R_{\text{food}}^{{{\text{C}}:{\text{P}}}} \) (Fig. 4a, P < 0.001), but N assimilation efficiencies did not increased when \( R_{\text{fish}}^{{{\text{N}}:{\text{P}}}} :R_{\text{food}}^{{{\text{N}}:{\text{P}}}} \) increased (Fig. 4b, P = 0.77).
Aquacultured fish: the assimilation efficiency of energy (βE, white circles), nitrogen (β N , gray circles) and phosphorus (β P , black circles) plotted against the ratio between whole-body fish and food a C/P and b N/P stoichiometric ratios (published sources are found in supplementary information). The curves in a–b were fitted by linear regression for βp versus C/P(fish)/C/P(food) (r 2 = 0.67, slope ± SE = −0.26 ± 0.03, F 1,29 = 62.596, P < 0.001), βE versus C/P(fish)/C/P(food) (r 2 = 0.11, slope ± SE = 0.06 ± 0.02, F 1,43 = 6.668, P = 0.013), and βp versus N/P(fish)/N/P(food) (r 2 = 0.49, slope ± SE = −0.19 ± 0.02, F 1,67 = 66.378, P < 0.001). d Box plots of assimilation efficiencies (all data combined)
Mean P assimilation efficiency was lower than C or N assimilation efficiencies (Fig. 4c). Thus, the global mean C, N and P assimilation efficiencies were βE = 0.87 ± 0.01 (±SE, n = 170), βN = 0.90 ± 0.01 (n = 245), and βP = 0.58 ± 0.01 (n = 212) (Fig. 4c). βE, βN and βP were significantly different from each other (Kruskall–Wallis test, P < 0.001), with βN > βC > βP (Tuckey HSD test, P < 0.05 for all comparisons). Lower P assimilation efficiencies may result from a high proportion of non-digestible P in artificial food, notably P bounded in phytate (Hua and Bureau 2006). However, in a restricted data set, gathering studies where fish were fed with food containing >80% of a natural diet (fish meal), βE and βN remained significantly higher than βP (Kruskall–Wallis test, P < 0.05), with a mean of βE = 0.89 ± 0.01 (n = 5, ±SE), βN = 0.89 ± 0.01 (n = 10), and βP = 0.51 ± 0.01 (n = 10).
Lower P assimilation efficiencies had consequences on feces C/P and N/P elemental stoichiometry (R feces). R feces was calculated using food C/P and N/P elemental stoichiometry, and nutrient assimilation efficiencies, β i :
It was found that aquacultured fish produced P-rich feces, with a mean C/P ratio of 46.9:1 ± 6.8 (±SE, n = 148), and a mean N/P ratio of 4.6:1 ± 0.3 (±SE, n = 219) (Table 4).
Nutrient gross growth efficiencies (GGE) and nutrient resupply ratios
The model of Sterner (1990) was used to assess whether observed C/P and N/P GGE and resupply ratios could be predicted using three variables: the C/P and N/P elemental stoichiometry of whole-body fish and assimilated food, and a maximum accumulation efficiency, L m (see “Materials and methods”, Eqs. 5–8). The standard value for L m was set at 0.75 for C, N and P. This value felt within the range of observed maximum accumulation efficiencies, which varied from 0.61 to 0.99 (calculated from data given in ESM 2; i.e., for a nutrient i, maximum accumulation efficiency (a i = L m) was derived from Eq. 4 with \( L_{\text{m}} = {{{\text{GGE}}_{\max }^{i} } \mathord{\left/ {\vphantom {{{\text{GGE}}_{\max }^{i} } {\beta_{i} }}} \right. \kern-\nulldelimiterspace} {\beta_{i} }} \)). Given that metabolic costs of carbon are generally thought to be higher than for nutrient elements (Sterner and Elser 2002; Mayor et al. 2009), a sensitivity analysis was performed using a lower value for the maximum accumulation efficiency of carbon (i.e., L m = 0.64). This value represented the highest value recorded in the aquacultured fish literature examined in this study.
Observed C/P and N/P GGE ratios (R GGE) were similar to C/P and N/P ratios in whole-body fish (R fish) (Fig. 5a, b). Linear regression fitted on R GGE versus R fish gave a slope of 1.19 ± 0.34 (±SE, r 2 = 0.33, P = 0.002) for C/P, and 1.04 ± 0.06 (r 2 = 0.88, P < 0.001) for N/P. These slopes were not statistically different from a mean slope of 1.0 (one-sample t test, P = 0.59 for C/P and P = 0.48 for N/P), suggesting a homeostatic regulation of whole-body fish elemental composition, with R GGE = R fish (see “Materials and methods”).
Observed a C/P and b N/P Gross growth efficiency ratios (R GGE) plotted against whole-body fish C/P and N/P ratios (R fish). The curves in a–b were fitted by linear regression for C/P (r 2 = 0.33, slope ± SE = 1.19 ± 0.34, F 1,21 = 11.976, P = 0.002), and N/P (r 2 = 0.88, slope ± SE = 1.04 ± 0.06, F 1,43 = 328.073, P < 0.001) ratios. c Predicted plotted against observed C/P resupply molar ratios. For the C/P resupply ratio, two different maximum accumulation efficiencies were used for carbon, i.e., L m = 0.75 (black circles; linear regression, r 2 = 0.91, slope ± SE = 0.854 ± 0.057, F 1,21 = 221.198, P < 0.001), or L m = 0.64 (gray circles; linear regression, r 2 = 0.91, slope ± SE = 0.860 ± 0.059, F 1,21 = 213.904, P < 0.001). d Predicted plotted against observed N/P resupply molar ratios (linear regression, r 2 = 0.45, slope ± SE = 0.538 ± 0.085, F 1,48 = 40.268, P < 0.001). In a–d, the dashed lines represent y = x
Accordingly, Sterner (1990) homeostatic model predicted C/P resupply ratios which were in agreement with observations, with ranges from ~150:1 to ~1,400:1 (Fig. 5c, r 2 = 0.91, P < 0.001, using either L m = 0.75 or L m = 0.64 for the maximum accumulation efficiency of carbon). Note that, a lower maximum accumulation efficiency for carbon induced slightly higher C/P resupply ratios (Fig. 5c). However, the slopes of the two regressions (using L m = 0.75 or L m = 0.64) were not statistically different (t test, P = 0.942).
Similarly, the ranges of observed and predicted N/P resupply ratios were similar, from 3.7:1 to 201.4:1 and 8.8:1 to 203.3:1, respectively. Predicted and observed N/P resupply ratios were also significantly correlated (Fig. 5d, r 2 = 0.45, P < 0.001).
Discussion
Fish C, N and P content
The C, N and P content values of the wild fish examined here were similar to that obtained for wild freshwater fish (Sterner and George 2000; Dantas and Attayde 2007; Pilati and Vanni 2007; Hendrixson et al. 2007). Several studies have also reported the chemical composition of wild marine fish in terms of lipids, proteins or energy contents (e.g., Childress and Nygaard 1973; Childress et al. 1990; Spitz et al. 2010). However, studies investigating simultaneously the C, N and P elemental composition of wild marine fish are more limited. C, N and P content of fish larvae collected off Bermuda (North Atlantic; Beers 1966) was found to represent 37.9, 9.7 and 1.4% of DW, respectively. Deegan (1986) studied changes in body composition and morphology of wild juvenile gulf menhaden (Brevoortia patronus), which had P content ranges of 2.27–2.97% DW. Hjerne and Hansson (2002) also reported mean values of <10% DW for N and <2% DW for P in both herring and sprat collected in the Baltic Sea. Aguado et al. (2004) fed Atlantic bluefin tuna (Thunnus thynnus) with a mixed diet of wild-caught preys (mackerel, herring, pilchard and giltsardine) which contained 8.3–10.7% N and 1.5–1.8% P. Overall, the C, N and P whole-body content recorded for wild-caught marine fish in our study had also similar ranges.
In this study, differences of C, N and P content in whole-body fish were associated to differences among species in terms of body lipid levels and bony matter. Higher C content, and lower N and P content, were recorded in fish which had high body lipid levels such as the salmonidae (Fig. 2; Table 2). In a study of mid-water fish inhabiting off the Hawaiian Islands, Childress et al. (1990) also reported that higher whole-body C content and lower N content were associated with higher lipid levels in 42 fish species. Furthermore, Childress et al. (1990) found that lower lipid levels were mainly recorded in the deeper-living fish species. It was suggested that reduced metabolic rates of these deeper-living species explained the lower body lipid content. In our study, A. olfersii was also a deep-living fish species which contained lower C and lipid levels in comparison to the other species (Table 2).
With respects to nitrogen, lower N content in lipid-rich species (Fig. 2) was consistent with the fact that lipids are usually N-poor compared to other bio-chemicals (Sterner and Elser 2002). For example, glycerol and fatty acids do not contain N, hence the average C/ N/ P ratios of phospholipids is 39:0.8:1 (Sterner and Elser 2002). For P content, differences among species could be explained by varying degrees of ossification, as revealed by the correlation found between Ca and P whole-body content (Fig. 2d). This pattern has been already reported in freshwater fish (Pilati and Vanni 2007; Hendrixson et al. 2007). These authors suggested that stoichiometric variation in P content between different fish species had a structural, mineralogical basis related to different degrees of ossification in skeleton and integument. In Hendrixson et al. (2007) study, this conclusion was based on the correlation found between Ca and P, with a mean Ca/P in bone of 2.14 which closely matched the Ca/P ratio for pure hydroxyapatite. Here we also found a mean whole-body Ca/P ratio of 2.26:1 ± 0.24 which closely matched the Ca/P ratio of pure hydroxyapatite. Thus, P bounded to Ca represented from ~26% in S. salar (salmoniforme) to 82% in L. calcarifer (perciforme) of the total whole-body P. Although we were not able to measure Ca content in the wild-caught fish, we speculate that high P content found in species such as C. aper, A. olfersii, L. aurata and S. japonicus (Table 2) may be also explained by skeletal investment. Indeed, the perciformes, such as C. aper or S. japonicus, have usually high whole-body P content in comparison to other orders such as salmonifomes (Hendrixson et al. 2007).
Variation in the elemental stoichiometry of fish has been related to ontogeny (Pilati and Vanni 2007), or differences among fish species in terms of allometry (Hendrixson et al. 2007), habitat specificity (Childress et al. 1990; Sterner and George 2000; Hendrixson et al. 2007), or feeding guild (Pilati and Vanni 2007; Hendrixson et al. 2007). Hendrixson et al. (2007) found however that fish size, habitat specificity or feeding type are usually rooted in the phylogeny, and species with similar traits usually share a common evolutionary history. Whole-body P content is such as trait, and the results of this study support the observations of Hendrixson et al. (2007) showing that differing P concentrations among fish species may be tightly coupled to phylogeny.
C, N and P gut content in wild and farmed fish and nutrient resupply ratios
Several mechanisms may account for the difference in wild and aquacultured fish gut or feces content versus whole-body C, N and P content recorded in this study. The state of prey degradation is one such mechanism, as illustrated by the correlation found between C and N gut content (Fig. 3). Examination of aquacultured fish studies has shown that mean P assimilation efficiency was lower than that of N or C (Fig. 4). Accordingly, aquacultured fish released feces with low C/P and N/P stoichiometric ratios (Table 4). Similarly, gut contents of wild-caught fish were fairly P-rich (Table 4). These results are consistent with previous studies on wild marine damselfish (Pinnegar and Polunin 2006; Pinnegar et al. 2007). Indeed, these authors found that Chromis chromis, a planktivorous damselfish living in Mediteranean reefs, released P-rich feces with a low N/P ratio (in the range 0.8–1.0:1) in comparison to that of their planktonic preys, which had a N/P ratio ranges of 8.7–12.0:1. Aguado et al. (2004) also reported low P assimilation efficiency, with ranges of 0.46–0.66, in Atlantic bluefin tuna (Thunnus thynnus thynnus Linnaeus) fed with wild-caught fish preys (mackerel, herring, pilchard and gilt sardine). So, the results of this study and recent works on damselfish suggest a lower P assimilation efficiency in comparison to C or N assimilation efficiencies. A high proportion of poorly digestible P bounded to skeleton and integument in the fish preys could partly explain this lower P assimilation efficiency in piscivorous fish. Indeed, aquacultured fish studies examined here have shown that fish fed with food containing >80% of a fish meal had lower P assimilation efficiency in comparison to C or N assimilation efficiencies.
Of further importance, examination of aquacultured fish studies also revealed that P and C assimilation efficiencies were a function of food quality (Fig. 4). This suggested an adjustment of P and C absorption as a function of nutrient content of ingested food. In comparison, N assimilation efficiency was not correlated to food quality (Fig. 4). Sterner and George (2000) also reported an adjustment of nutrient absorption as a function of nutrient content in food in cyprinid fish. However, Sterner and George found that fish with a high N diet assimilate N more efficiently than those with N-poor diet, suggesting that maintenance of fish homeostasis must be occurring at the level of physiological processes occurring once nutrients have passed the gut wall. Aquacultured fish data presented in this study suggested that maintenance of fish homeostasis may occur both during the digestion process (Fig. 4) and after assimilation, i.e., once nutrients have passed the gut wall (Fig. 5).
In natural marine systems, the fact that marine invertebrates tend to be comparatively P-poor compared to marine fish (Table 4), and that P assimilation efficiency tends to be lower than that of N or C, would result in an increased discrepancy between the elemental stoichiometry of digested food and hence that of the fish body. In turn, this would be expected to result in variables GGE and nutrient resupply ratios (Sterner 1990; Anderson and Hessen 2005). Accordingly, examination of fish farming literature has shown that observed C/P and N/P resupply ratios were extremely variables, with ranges of ~1,400:1 and ~200:1, respectively. In the wild, this situation may arise when diet changes during ontogeny or season. Pilati and Vanni (2007) have shown for example that N/P resupply ratios from larvae and juveniles of the gizzard shad, Dorosoma cepedianum, were extremely variables, in the range <10:1 to ~80:1. These authors suggested that ontogenic variations in N/P resupply ratios were mostly explained by changes in diet N/P ratios, and could be reasonably predicted using a stoichiometric model with homeostatic regulation of whole-body elemental composition. Similar diet changes during ontogeny are recorded in marine fish species (e.g., Mahe et al. 2007), and this should also affect nutrient resupply ratios. Our study has also shown that observed C/P and N/P resupply ratios in aquacultured fish agreed with basic principles of CNR theory and homeostatic regulation of whole-body elemental composition in various marine, brackish and freshwater fish species.
Marine fish elemental stoichiometry and nutrient recycling
Wild and aquacultured fish species examined in this study were found to be P-rich organisms. Furthermore, as discussed earlier, data on wild fish gut content and examination of aquacultured fish literature suggested that P assimilation efficiency was comparatively lower to that of C or N. Consequently, fish fecal pellets would be expected to be P-rich in comparison to C or N. This pattern could potentially enhance the export of P toward the deep ocean through sinking of P-rich fish caracases and fecal pellets. High sinking rates of fish fecal pellets have been recorded at speeds of up to >1,000 m day−1 (Robinson and Bailey 1981), which corresponds to a residence time of these particles within the upper euphotic zone (~100 m) of ~0.1 day. Marine fish may therefore reinforce the P-limitation of marine productivity by sequestering phosphorus in their tissues or by exporting it toward the aphotic zone (Bartell and Kitchell 1978; Kraft 1992). Hjerne and Hansson (2002) found for example that fish biomass increases during the summer months, and may explain up to one third of the summer decrease in ‘‘total phosphorus’’ in the upper 40 m of the water column in the Baltic Sea. Hence, this may reinforce P-limitation of cyanobacteria in the Baltic Sea (Larsson et al. 2001).
This study also revealed that the N/P resupply ratio of fish was extremely variable, depending on food quality, which may have top-down consequences on ecosystem functioning. Indeed, nutrient recycling from fish has been shown to influence primary producers in various freshwater ecosystems (Vanni et al. 1997, 2002, 2006). Such top-down effects have been also evidenced in marine reefs communities (Geesey et al. 1984; Pinnegar and Polunin 2006, 2007), and would deserve more attention in the pelagic ocean, owing to the importance of some fish groups. For example, a recent estimate of the global biomass of marine mesopelagic fish biomass found that a major group of planktivorous fish (i.e., the lanternfish family or Myctophidae) is of the order of ~106 millions tons (Lam and Pauly 2005). If we apply the mean water content of 74% and the P content of. 2–4% recorded in our study, we could expect yields to a phosphorus pool from this single fish group of 0.17–0.34 Tmol P. This predicted pool would remain at a similar, or possibly higher level (if other fish families were accounted for), to the phosphorus pool sequestered in marine phytoplankton (~0.50 Tmol P using C/N/P ratio of 106:16:1, Tyrrell 1999) or in marine zooplankton (~0.50 Tmol P using C/N/P = 147:25:1; Gasol et al. 1997).
Of further significance, the Myctophidae family is known to perform diel vertical migrations, feeding at night in the upper oceanic layers and moving back into the mesopelagic zone (200–1,000 m) during the daytime. Radchenko (2007) found that Myctophidae are important for conveying particulate organic matter toward the ocean interior. This occurs as a result of fecal pellets being released during the daytime, while the fish occupy the mesopelagic zone. Considering the apparently low P assimilation efficiency of marine fish, these Myctophidae may favorably contribute to the vertical transport of this element toward the ocean interior.
All in all, this study highlights the need to improve our understanding of the role of marine fish in controlling nutrient inventories and cycling in the ocean.
Conclusion
In conclusion, the present study provided insights on the stoichiometric patterns of wild-caught and aquacultured fish. Ranges of the elemental composition of the species examined here were found to be similar to that obtained for freshwater teleost fish, notably with respects to whole-body P content. The differences observed in terms of P content could be largely explained by varying degree of ossification. Gut content analysis also suggested a lower P assimilation efficiency in wild fish, a pattern confirmed by examination of aquacultured fish literature. Our study has also shown that observed C/P and N/P resupply ratios in aquacultured fish agreed with basic principles of CNR theory and homeostatic regulation of whole-body elemental composition in aquacultured fish. Extrapolation of our results to broader marine systems indicated that fish may contribute toward determining the distribution and ultimate fate of nutrients in the ocean.
References
Aguado F, Martinez FJ, Garcia-Garcia B (2004) In vivo total nitrogen and total phosphorous digestibility in Atlantic bluefin tuna (Thunnus thynnus thynnus Linnaeus, 1758) under industrially intensive fattening conditions in Southeast Spain Mediterranean coastal waters. Aquac Nutr 10:413–419
Andersen T (1997) Pelagic nutrient cycles: herbivores as sources and sinks for nutrients. Springer, Berlin
Anderson TR, Hessen DO (2005) Threshold elemental ratios for carbon versus phosphorus limitation in Daphnia. Freshw Biol 50:2063–2075
Anderson TR, Hessen DO, Elser JJ, Urabe J (2005) Metabolic stoichiometry and the fate of excess carbon and nutrients in consumers. Am Nat 165:1–15
Bartell SM, Kitchell JF (1978) Seasonal impact of planktivory on phosphorus release by Lake Wingra zooplankton. Verh Int Ver Limnol 20:466–474
Beers JR (1966) Studies on the chemical composition of the major zooplankton groups in the Sargasso Sea off Bermuda. Limnol Oceanogr 11:520–528
Boersma MN, Aberle F, Hantzsche M, Schoo KL, Wiltshire KH, Malzahn AM (2008) Nutritional limitation travels up the food chain. Int Rev Hydrobiol 93:479–488
Childress JJ, Nygaard MH (1973) The chemical composition of midwater fishes as a function of depth of occurrence off southern California. Deep Sea Res I 20:1093–1109
Childress JJ, Price MH, Favuzzi J, Cowles D (1990) Chemical composition of midwater fishes as a function of depth of occurrence off the Hawaiian Islands: food availability as a selective factor? Mar Biol 105:235–246
Clarke A (2008) Ecological stoichiometry in six species of Antarctic marine benthos. Mar Ecol Prog Ser 369:25–37
Dantas MC, Attayde JL (2007) Nitrogen and phosphorus content of some temperate and tropical freshwater fishes. J Fish Biol 70:100–108
Deegan LA (1986) Changes in body composition and morphology of young-of-the-year gulf menhaden, Brevoortia patronus Goode, in Fourleague Bay, Louisiana. J Fish Biol 29:403–415
Elser JJ, Urabe J (1999) The stoichiometry of consumer-driven nutrient recycling: theory, observations, and consequences. Ecology 80:735–751
Fernández F, Miquel AG, Guinea J, Martinez R (1998) Digestion and digestibility in gilthead sea bream (Sparus aurata): the effect of diet composition and ration size. Aquaculture 166:67–84
Finkel ZV, Quigg A, Raven JA, Reinfelder JR, Schofield OE, Falkowski PG (2006) Irradiance and the elemental stoichiometry of marine phytoplankton. Limnol Oceanogr 51:2690–2701
Gasol JM, del Giorgio PA, Duarte CM (1997) Biomass distribution in marine planktonic communities. Limnol Oceanogr 42:1353–1363
Geesey GG, Alexander GV, Bray RN, Miller AC (1984) Fish fecal pellets are a source of minerals for inshore reef communities. Mar Ecol Prog Ser 15:19–25
Geider RJ, La Roche J (2002) Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. Eur J Phycol 37:1–17
Gillooly JF, Allen AP, Brown H, Elser JJ, Martinez del Rio C, Savage VM, West GB, Woodruff WH, Woods HA (2005) The metabolic basis of whole-organism RNA and phosphorus content. Proc Nat Acad Sci 102:11923–11927
Gismervik I (1997) Stoichiometry of some marine planktonic crustaceans. J Plankton Res 19:279–285
Hassett RP, Cardinale B, Stabler LB, Elser JJ (1997) Ecological stoichiometry of N and P in pelagic ecosystems: comparison of lakes and oceans with emphasis on the zooplankton-phytoplankton interaction. Limnol Oceanogr 42:648–662
Hendrixson HA, Sterner RW, Kay AD (2007) Elemental composition of fresh water fishes in relation to phylogeny, allometry and ecology. J Fish Biol 70:121–140
Hirst AG, Kiørboe T (2002) Mortality of marine planktonic copepods: Global rates and patterns. Mar Ecol Prog Ser 230:195–209
Hjerne O, Hansson S (2002) The role of fish and fisheries in Baltic Sea nutrient dynamics. Limnol Oceanogr 47:1023–1032
Hua K, Bureau DP (2006) Modelling digestible phosphorus content of salmonid fish feeds. Aquaculture 254:455–465
Igushi N, Ikeda T (2004) Metabolism and elemental composition of aggregate and solitary forms of Salpa thompsoni (Tunicata: Thaliacea) in waters off the Antartic Peninsula during austral summer 1999. J Plankton Res 26:1025–1037
Ikeda T, Mitchell AW (1982) Oxygen uptake, ammonia excretion and phosphate excretion by krill and other antarctic zooplankton in relation to their body size and chemical composition. Mar Biol 71:283–298
Karl DM, Björkman KM, Dore JE, Fujieki L, Hebel DV, Houlihan T, Letelier RM, Tupas LM (2001) Ecological nitrogen-to-phosphorus stoichiometry at station ALOHA. Deep Sea Res II 48:1529–1566
Klausmeier CA, Litchman E, Levin SA (2004) Phytoplankton growth and stoichiometry under multiple nutrient limitation. Limnol Oceanogr 49:1463–1470
Kraft CE (1992) Estimates of phosphorus and nitrogen cycling by fish using a bioenergetics approach. Can J Fish Aquat Sci 49:2596–2604
Lam V, Pauly D (2005) Mapping the global biomass of mesopelagic fishes. Sea Around US Project Newsletter, July/August, 30:4
Landry MR, Calbet A (2004) Microzooplankton production in the oceans. ICES J Mar Sci 61:501–507
Larsson U, Hajdu S, Walve J, Elmgren R (2001) Baltic Sea nitrogen fixation estimated from the summer increase in upper mixed layer total nitrogen. Limnol Oceanogr 46:811–820
Le Borgne R (1982) Zooplankton production in the eastern tropical Atlantic Ocean: net growth efficiency and P:B in terms of carbon, nitrogen and phosphorus. Limnol Oceanogr 27:681–698
Mahe K, Amara R, Bryckaert T, Kacher M, Brylinski JM (2007) Ontogenetic and spatial variation in the diet of hake (Merluccius merluccius) in the Bay of Biscay and the Celtic Sea. ICES J Mar Sci 64:1210–1219
Mayor DJ, Anderson TR, Pond DW, Irigoien X (2009) Egg production and associated losses of carbon, nitrogen and fatty acids from maternal biomass in Calnus finmarchicus before the spring bloom. J Mar Syst 78:505–510
Moutin T, Van Den Broeck N, Beker B, Dupouy C, Rimmelin P, Le Bouteiller A (2005) Phosphate availability controls Trichodesmium spp. biomass in the SW Pacific Ocean. Mar Ecol Prog Ser 297:15–21
Nugraha A, Pondaven P, Tréguer P (2010) Influence of consumer-driven nutrient recycling on primary production and the distribution of N and P in the ocean. Biogeosciences 7(4):1285–1305
Pertola S, Koski M, Viitasalo M (2001) Stoichiometry of mesozooplankton in N- and P-limited areas of the Baltic Sea. Mar Biol 140:425–434
Pilati A, Vanni MJ (2007) Ontogeny, diet shifts, and nutrient stoichiometry in fish. Oikos 116:1663–1674
Pinnegar JK, Polunin NVC (2006) Planktivorous damselfish support significant nitrogen and phosphorus fluxes to Mediterranean reefs. Mar Biol 148:1089–1099
Pinnegar JK, Polunin NVC, Videler JJ, de Wiljes JJ (2007) Daily carbon, nitrogen and phosphorus budgets for the Mediterranean planktivorous damselfish Chromis chromis. J Exp Mar Biol Ecol 352:378–391
Platt T, Irwin B (1973) Caloric content of phytoplankton. Limnol Oceanogr 18:306–310
Quigg A, Finkel ZV, Irwin AJ, Rosenthal Y, Ho TY, Reinfelder JR, Schofield O, Morel FMM, Falkowski PG (2003) The evolutionary inheritance of elemental stoichiometry in marine phytoplankton. Nature 425:291–294
Radchenko VI (2007) Mesopelagic fish community supplies “biological pump”. Raffles Bull Zool 14:265–271
Robinson BH, Bailey TG (1981) Sinking rates and dissolution of midwater fish fecal matter. Mar Biol 65:135–142
Roger C (1978) Azote et phosphore chez un crustacé macroplanctonique, Meganyctiphanes norvegica (M. Sars) (Euphausiacea): excrtion minrale et constitution. J Exp Mar Biol Ecol 33:57–83
Spitz J, Mourocq E, Schoen V, Ridoux V (2010) Proximate composition and energy content of forage species from the Bay of Biscay: high- or low-quality food? ICES J Mar Sci 67:909–915
Sterner RW (1990) The ratio of nitrogen to phosphorus resupplied by herbivores: zooplankton and the algal competitive arena. Am Nat 136:209–229
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Sterner RW, George NB (2000) Carbon, nitrogen, and phosphorus stoichiometry of cyprinid fishes. Ecology 81:127–140
Strickland JDH, Parsons TR (1972) A practical handbook of sea-water analysis, 2nd edn. J Fish Res Bd Canada, vol 167, p 311
Threlkeld ST (1988) Planktivory and planktivore biomass effects on zooplankton, phytoplankton, and the trophic cascade. Limnol Oceanogr 33:1362–1375
Touratier F, Field JG, Moloney CL (2001) A stoichiometric model relating growth substrate quality (C:N:P ratios) to N:P ratios in the products of heterotrophic release and excretion. Ecol Model 139:265–291
Tyrrell T (1999) The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 400:525–531
Uye S, Matsuda O (1988) Phosphorus content of zooplancton from the Inland Sea of Japan. J Oceanogr 44:280–286
Van de Waal DB, Verschoor AM, Verspagen JMH, van Donk E, Huisman J (2010) Climate-driven changes in the ecological stoichiometry of aquatic ecosystems. Front Ecol 8:145–152
Vanni MJ, Layne CD, Arnott SE (1997) “Top-down” trophic interactions in lakes: effects of fish on nutrient dynamics. Ecology 78:1–20
Vanni MJ, Flecker AS, Hood JM, Headworth JL (2002) Stoichiometry of nutrient cycling by vertebrates in a tropical stream: linking species identity and ecosystem processes. Ecol Lett 5:285–293
Vanni MJ, Bowling AM, Dickman EM, Hale RS, Higgins KA, Horgan MJ, Knoll LB, Renwick WH, Stein RA (2006) Nutrient cycling by fish supports relatively more primary production as lake productivity increases. Ecology 87:1696–1709
Walve J, Larsson U (1999) Carbon, nitrogen and phosphorus stoichiometry of crustacean zooplankton in the Baltic Sea: implications for nutrient recycling. J Plankt Res 21:2309–2321
Wu J, Sunda W, Boyle EA, Karl DM (2000) Phosphate depletion in the western North Atlantic Ocean. Science 289:759–762
Acknowledgments
This research was funded by the EU Marie Curie EST project METAOCEANS (MEST-CT-2005-019678) and supported by INSU/CNRS. The writers would like to thank Herwig Stibor and three anonymous reviewers for constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by X. Irigoien.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Czamanski, M., Nugraha, A., Pondaven, P. et al. Carbon, nitrogen and phosphorus elemental stoichiometry in aquacultured and wild-caught fish and consequences for pelagic nutrient dynamics. Mar Biol 158, 2847–2862 (2011). https://doi.org/10.1007/s00227-011-1783-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1783-7