Abstract
This study determined whether the acoustic roughness of Caribbean reef habitats is an accurate proxy for their topographic complexity and a significant predictor of their fish abundance. Fish abundance was measured in 25 sites along the forereef of Glovers Atoll (Belize). At each site, in situ rugosity (ISR) was estimated using the “chain and tape” method, and acoustic roughness (E1) was acquired using RoxAnn. The relationships between E1 and ISR, and between both E1 and ISR and the abundance of 17 common species and the presence of 10 uncommon species were tested. E1 was a significant predictor of the topographic complexity (r 2 = 0.66), the abundance of 10 common species of surgeonfishes, pomacentrids, scarids, grunts and serranids and the presence of 4 uncommon species of pomacentrids and snappers. Small differences in E1 (i.e. ∆0.05–0.07) reflected in subtle but significant differences in fish abundance (~1 individual 200 m−2 and 116 g 200 m−2) among sites. Although we required the use of IKONOS data to obtain a large number of echoes per site, future studies will be able to utilise RoxAnn data alone to detect spatial patterns in substrate complexity and fish abundance, provided that a minimum of 50 RoxAnn echoes are collected per site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reef fish communities depend fundamentally on the three-dimensional structure of their habitat (Sale 1991; Chabanet et al. 1997). A structurally complex habitat does not only provide transient and permanent refuges from predation to fishes of a variety of sizes and shapes (Caley and St John 1996) but also a variety of foraging, spawning and nesting sites (Robertson and Sheldon 1979) and help to maintain themselves in high-flow environments (Johansen et al. 2008).
Sites with higher topographic complexity have higher surface area and a greater diversity and availability of shelter and/or foraging sites (Luckhurst and Luckhurst 1978a; Bell and Galzin 1984). As a result, topographic complexity and species richness, diversity, total biomass and abundance are positively correlated (Luckhurst and Luckhurst 1978a; Carpenter et al. 1981; Sale and Douglas 1984; Nanami and Nishihira 2002; Friedlander et al. 2003; Gratwicke and Speight 2005a, b). It seems reasonable to expect that different fish species in a community would also be positively correlated with topographic complexity, thus facilitating the prediction of their spatial patterns of abundance. However, the nature of species-specific relationships with topographic complexity has rarely been described (but see Ebersole 1985; Mumby and Wabnitz 2002) and needs further investigation. Different degrees of association with habitat complexity may exist depending on fish size (Choat and Bellwood 1991) mobility, home range and feeding habits (Chabanet et al. 1997) and territoriality (Mumby and Wabnitz 2002). Small fishes may have a stronger need of shelter because of their increased vulnerability to predators (Mumby and Wabnitz 2002) and strongly site-attached fish, or fishes with obligate associations, tend to have higher correlations with the substratum than more widely ranging species or life stages (McCormick 1994). Species with small home ranges may yield strong relationships with habitat variables, as a result of the scale at which these variables are measured (Jennings et al. 1996). Understanding the relationship between topographic complexity and fish abundance at a species level would allow more detailed and useful predictions of spatial and temporal patterns of fish abundance. If such predictions were both readily available for species with critical functional roles or commercial importance and applicable at large spatial scales, these would provide ecologically meaningful information to support stock assessments and the design of management strategies. By conducting a detailed evaluation of the relationships of fish species and life phases with their physical environment, we generated models for large-scale predictions of the spatial patterns of fish abundance. These results contribute directly with the mission of the National Fisheries Department of Belize and the related fishery conservation projects. Moreover, in a broader context, the results generated here contribute with the essential fish habitat (EFH) mandates in the primary laws regulating marine fishery management (NOAA 1996). Furthermore, although species density and biomass may respond similarly to changes in topographic complexity, modelling the response of both metrics separately would allow us to obtain predictions that are useful in different ecological contexts. Understanding spatial patterns of density, for example, may be of importance to understand density dependence and other important aspects of population dynamics, whereas describing spatial patterns of biomass may be relevant in understanding the stock’s availability from a fisheries perspective.
Topographic complexity has traditionally been measured as the ratio of the actual contour of the reef relative to linear distance (Luckhurst and Luckhurst 1978a) using what is known as the “chain and tape” method. Although a fine-scale proxy of the vertical relief is obtained using this method, it involves considerable underwater effort and therefore is generally unsuitable for use across wide spatial scales. The need for alternative large-scale and continuous measurements of topographic complexity that allow a) predictive models and b) the possibility to map topographic complexity has been recognised and recently addressed using remote sensing techniques. A number of recent studies have addressed this by using optical data from the NASA Experimental Advanced Airborne Research Lidar (EAARL) (e.g. Wedding et al. 2008; Pittman et al. 2009; Walker et al. 2009), whereas others have used bathymetry data either measured directly with acoustic instruments or derived from optical remote sensing images and geographical information systems (GIS) (e.g. Lundblad et al. 2006; Pittman et al. 2007; Purkis et al. 2008; Su et al. 2008; Knudby et al. 2010), and in one case optical and acoustic data have been used with comparative purposes (i.e. Costa et al. 2009). However, the study of the correlation between remotely sensed rugosity and in situ rugosity is still in its infancy and would benefit if the spectrum of viable instruments is broadened.
The acoustic ground discrimination system RoxAnn provides a cost-effective approach to measure the acoustic roughness (E1) of the sea bottom and discriminate benthic coral reef habitats with different topographic complexity with relative detail (Hamilton et al. 1999; White et al. 2003). However, the beam width of RoxAnn causes each measurement of E1 to be recorded from a two-dimensional patch of reef surface (beam footprint), which differs from the one-dimensional nature of the “chain and tape” method. Thus, each method measures a different aspect of reef complexity but a comparison between them is warranted because of the widespread use of the “chain and tape” approach. There is no a priori reason why one metric of complexity will be superior to the other, and the relationship between each metric and the fish abundance parameters might differ and vary further according to species or body size.
The capacity of satellite remote sensing to predict habitat complexity at a scale relevant to fish has been recently demonstrated (Purkis et al. 2008). Fish community parameters such as species richness (Kuffner et al. 2007; Pittman et al. 2007; Wedding et al. 2008; Walker et al. 2009), diversity (Purkis et al. 2008) as well as the total fish abundance (Wedding et al. 2008; Walker et al. 2009) and the abundance of fish at a family level (Kuffner et al. 2007) within size, trophic and mobility guilds (Purkis et al. 2008) can be predicted over large spatial scales. Predictions of fish abundance at a species level are only available for a single species of damselfish species (Pittman et al. 2009). The present study aimed to broaden these predictions to a larger number of ecologically and commercially important species and, in the case of parrotfishes, to take these predictions to a finer (i.e. life-phase) level. Within families, species may display differences in behaviour, degrees of territoriality and food requirements which may affect their use of refugia and their degree of association with the topographic complexity of reefs. Furthermore, although certain trophic guilds (e.g. grazers) play a crucial ecological role in the reduction of macroalgal biomass (Mumby et al. 2006), the reduction of coral mortality (Hughes et al. 2007) and the increase in coral recruitment (Mumby et al. 2007a), species or life phases within the guild may contribute differently to these functions (Bruggemann et al. 1994). In addition, some of these species (or life phases) may be preferentially targeted by fishers and therefore more susceptible to overfishing than others. In such cases, prioritisation of management strategies may be required. Mapping fish diversity and richness at large scales provides managers with novel tools to improve ecosystem-based management (Pittman et al. 2007) and identify potential sites for marine-protected areas (Purkis et al. 2008). By extending spatial predictions to a further level of detail, we aim to support targeted management measures oriented to the protection of key species and their habitats. Abundance predictions for certain grazers that play a crucial ecological role could be used to orient conservation efforts to the enhancement of resilience of reefs. Biomass predictions of commercially important families (e.g. groupers and snappers) could be used to direct conservation efforts to maintain desirable levels of fish stocks.
In summary, the present study aimed to determine whether the RoxAnn’s acoustic roughness (E1) is as a reasonable predictor of the topographic complexity of Caribbean reef habitats and therefore the abundance of reef fishes they support. Relationships were analysed at the level of species and life phase and were used to quantify the capacity of acoustic remote sensing to predict the patterns of spatial variability in the abundance of key fish species. The following hypotheses were tested:
-
1.
Despite the differences in scale between the E1 and the in situ rugosity (ISR), E1 is a good predictor of the topographic complexity of a reef.
-
2.
The density and biomass of most reef fish species are positively correlated with ISR and E1.
-
3.
When the density and/or biomass of a species are significantly related with the ISR, these parameters will correlate similarly with E1.
-
4.
Juveniles, small-bodied species and territorial species have the strongest relationship with the topographic complexity.
-
5.
Species that according to the literature are strongly territorial or have small home ranges will be strongly related to the small-scale measure of topographic complexity (ISR), whereas species with larger home ranges will be strongly related with the large-scale measure of topographic complexity (E1).
Materials and methods
Study area
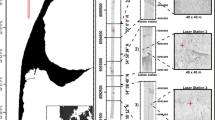
The study was carried out on the forereef of Glovers Atoll (16°44′N, 87°50′W) in Belize (Fig. 1a). This forereef comprises a gently sloping calcareous terrace descending from the emergent reef crest to an escarpment at a depth ranging from approximately 10–20 m where Montastraea spp. are the major reef-building corals. Surveys were conducted at 25 sites (Fig. 1b) within the same forereef zone and at comparable depths (~10 m) including a range of typical forereef habitats such as consolidated Montastraea reefs, unconsolidated Montastraea reefs, dense and sparse gorgonian communities established on hard bottom plains with very few hard coral colonies and spurs and grooves with predominance of hard bottom with small hard coral colonies and a medium relief.
Field surveys
Acquisition of acoustic data
Acoustic data were acquired using the RoxAnn signal processor along a track encompassing ~1.3 miles of the forereef of Glovers Atoll during the last week of March 2005 (Fig. 1b). RoxAnn is a single-beam acoustic system that uses multiple echoes to obtain parameters that are useful for seabed classification (i.e. roughness, hardness and depth) (Schlagintweit 1993). However, only the measure of roughness (E1) or backscatter of the seabed, which is derived from the energy of the tail of the first echo (Hamilton 2001), was considered relevant for the objectives of this particular study.
Although RoxAnn uses a dual-frequency echosounder (Furuno FCV-600L, 50 and 200 kHz), it was operated at 200 kHz during our survey. The depth range of the echosounder was set at 40 m, and therefore, it operated with a pulse repetition rate (PRR) of approximately 1,300–1,400 pulses min−1. RoxAnn recorded data every 2–5 m when the vessel was travelling at a constant speed of 8 km h−1 and then averaged sets of 40–80 pulses to obtain 1 echo return (E1 value). Beam width at 200 kHz was set at 10°, which gave a footprint of approximately 17% of water depth. Since depth logged by RoxAnn was between 3 and 22 m, the diameter of the footprint ranged from approximately 0.51 m in the shallowest areas to 3.74 m in the deepest areas. Distance between the tracks was approximately 100 m (Fig. 1c, d), and geographical co-ordinates were provided by an integrated GPS receiver (Furuno GP-37/GP-32). During data acquisition, the sea surface was calm with waves not exceeding a height of 0.8 m.
Fish census and topographic complexity
Underwater visual censuses were conducted a few days after the acquisition of acoustic data. The abundance of 30 species of reef fish including the families Scaridae, Acanthuridae, Haemulidae, Chaetodontidae, Serranidae, Lutjanidae and Khyposidae (Table 1) was quantified using standard belt transect surveys (Brock 1954). One diver completed 10 30 × 4-m transects at each site swimming along the centre of them at a constant speed. Total length (TL) was visually estimated to the nearest centimetre for every fish encountered in the transects, and life phase was recorded for every parrotfish.
Simultaneously, another diver surveyed six additional species including the smaller, more abundant and site-attached damselfishes (i.e. Stegastes planifrons, S. partitus, S. leucostictus, S. diencaeus and S. adustus) and the commonest squirrelfish (i.e. Holocentrus rufus). Belt transects of a narrower area (30 × 2 m) were used for these species to ensure accurate estimates of their abundance. TL of these species was also visually estimated to the nearest centimetre. Two of these transects were surveyed per site. The choice of species aimed to include the most common, the important functional groups, and commercially important species.
Fish censuses spanned an area of >150 m within which a third surveyor evaluated the in situ topographic complexity using a modification of the “chain and tape” method (Risk 1972). A chain of 4 metres long was moulded to the contour of the substratum at randomly selected starting positions (n = 10–14 per site). Care was taken to orient the chain in a perpendicular direction to the slope of the reef in all sites except for those located on the spur-and-grove zone. Because the “chain and tape” method was used to measure the fine-scale vertical relief of the study sites, we ensured that at spur-and-grove zone the chain was laid along the spurs. In situ rugosity (ISR) was calculated as the ratio of the length of the chain to the linear distance between its start and end point (McCormick 1994).
Data analysis
Acoustic roughness of Glovers forereef
Although a rugosity measurement can be calculated from acoustic bathymetry data using a triangulation method or examining the variance within various kernel sizes (see Purkis et al. 2008; Pittman et al. 2009), the present study focused in the direct use of the roughness parameter (E1) acquired by RoxAnn. E1 was considered to be a potentially useful surrogate of the topographic complexity of the study area because it conveys information on the backscatter of the bottom (Hamilton 2001).
All E1 data within the track above the 95th and below the 5th percentile were discarded as outliers possibly originated by problematic echo acquisition (primarily resulting of the pitch and roll of the boat). The remaining E1 data were normalised to the 95th percentile, resulting in a range of values between 0 and 1. Rough or highly complex surfaces would produce a strong backscatter and the highest E1 values, whereas more smooth ones would generate a weak backscatter and the lowest E1 values.
The mean acoustic roughness for each of our survey sites was calculated by overlaying the geo-referenced acoustic track (previously filtered and normalised) on an IKONOS image of our study area (acquired in March 2005) (Fig. 1c, d). Approximately 10 echo returns containing E1 data were available on the immediate vicinity of our survey sites. To obtain a larger and more representative number of E1 measurements per site, we overlaid the geo-referenced acoustic track on an IKONOS image of our study area (acquired in March 2005) (Fig. 1b–d). An unsupervised classification of this image was conducted to generate 15 spectral classes (Mumby and Edwards 2002; Andréfouët et al. 2003) that could be used as guides to locate pixel areas that were spectrally similar to the fish survey sites and that were also encompassed by the acoustic track. Mean E1 per site was calculated using the echo returns available on the immediate vicinity of the central fish census location, but also inside the pixel areas selected within less than a 100 m (n > 50).
None of the additional pixel areas selected on the satellite image could be visually inspected in situ. However, to ensure that these areas encompassed the same habitat types as those we surveyed and had a similar topographic complexity, they were selected along the same depth contour of our survey sites. Moreover, the selection of these areas was completed by one of the authors (PJM) who has conducted >1,000 h of underwater benthic surveys along the 12-km stretch of the forereef of Glovers Atoll, working on the delimitation of boundaries of different habitat types.
Given that the replicate fish transects at a site spanned an area exceeding this range (typically >150 m2 along the reef contour), the selected RoxAnn echoes can be considered representative of the area surveyed for reef fish (even allowing for 5-m positional uncertainty in the GPS).
Because E1 data were acquired across a sloping forereef (of approximately 15–30°) and therefore over a wide depth range (3–22 m), the performance of the RoxAnn system could have been significantly influenced by the slope (von Szalay and McConnaughey 2002) and depth of the ensonified area (Collins and Voulgaris 1993; Greenstreet et al. 1997; Hutin et al. 2005). Although the RoxAnn system normalises the echosounder waveforms to a reference depth (Hamilton et al. 1999), there is a chance that E1 returns correlate with depth for reasons other than genuine changes in reef habitat complexity. To test for the existence of such depth contamination in our E1 data, we extracted data from sand patches located at different depths (i.e. from 2 to 13 m) and conducted a linear regression between their depth and E1 returns. The E1 of a sand patch was unaffected by its depth (Linear regression, r 2 = 0.0, F 1,66 = 0.10, P = 0.76), therefore implying that RoxAnn data were not systematically biased by depth.
Although acoustic data may also be affected by the different types of sediment (Greenstreet et al. 1997), this was not a major concern in the present study, because the forereef of Glovers Atoll is comprised predominantly of hard bottom.
Fish density and biomass
Biomass for each species was estimated using TL of individual fish and the published allometric scaling relationships between length and weight (Bohnsack and Harper 1998). Mean density (individuals 200 m−2) and biomass (g 200 m−2) were calculated separately for each species and within each species separately for each life phase (in the case of parrotfishes) and for juvenile damselfishes (which were very abundant).
Utility of RoxAnn’s E1 to predict topographic complexity
To determine whether the acoustic roughness (E1) of Caribbean reef habitats can be used as a proxy for their topographic complexity, a linear model was fitted to our benthic data. The model tested whether there was a significant relationship between the ISR and E1. Model adequacy was evaluated by examining (a) the plot of residuals vs. fitted values to look for heteroscedasticity (i.e. non-constant variance) and (b) the normality Q–Q plot to test for the normality of errors (Crawley 2002).
Species relationships with topographic complexity and E1
Fish species were classified in three groups: (1) including common species and/or life phases that were observed in >16 sites (17 species), (2) including uncommon species and/or life phases that were observed in 5–16 sites and therefore contained a substantial proportion of zeros in their data sets (10 species) and (3) including extremely rare species (i.e. 1 individual observed at no more than 4 sites) for which no relationship with ISR or E1 could be tested (9 species).
(a) Common species
For each of the common species, simple linear regression models were fitted to test for the relationships between (1) density and ISR, (2) density and E1, (3) biomass and ISR and (4) biomass and E1. The adequacy of the models was evaluated by examining (a) the plot of residuals vs. fitted values to look for heteroscedasticity and (b) the normality Q–Q plot and the Shapiro test to assess the normality of errors (Faraway 2005). In cases of non-constant variance, non-normality of errors or evidence of non-linearity, the response variable was transformed using either √y, log (y) or log (y + 1) as appropriate. In the case of parrotfishes, adequate models were fitted separately for juveniles, initial-phase (IP’s) and terminal-phase (TP’s) individuals.
In cases where the relationship between variables could not be adequately represented by a straight line and evidence of non-linearity was found in the plot of the residuals vs. the fitted values (Crawley 2002), one or more polynomial terms were added to the linear model and retained subject to their significance and the adequacy of the model’s diagnostics (Faraway 2005). Because the addition of polynomial terms causes collinearity among the monomials and affects the accuracy of the regression models severely (Shacham and Brauner 1997), ISR and E1 were transformed in such cases using:
to yield values between 0 and 1 as recommended by Shacham and Brauner (1997). Nx corresponded then to the normalised value of ISR (NISR) or E1(NE1), X max was the maximum value of ISR or E1, and X min was the minimum value of ISR or E1 observed. A substantial reduction in the variance inflation factor (VIF) of the monomials in the model (i.e. 90%) indicated that collinearity was alleviated (see Mason 1987; O’Brien 2007).
(b) Uncommon species
Uncommon species were treated as having skewed and zero-inflated datasets with an excess of true zeros (Martin et al. 2005). The relationships of each of these species with our two measurements of topographic complexity were evaluated using ZANB or hurdle models that involved two steps (see Zuur et al. 2009 for details). First, the presence/absence of each species was modelled separately as a function of ISR and E1 using logistic regression and second, the abundance if present (total number of individuals of each species per site) was modelled separately as a function of ISR and E1 using negative binomial general linear models (GLMs) with zero-truncated Poisson distributions (Fletcher et al. 2005; Fletcher and Faddy 2007; Zuur et al. 2009).
More than 100 parametric tests were conducted to assess the relationships between pairs of variables for common and uncommon species. Arguably, the significance of such high number of multiple tests should be tested using a Bonferroni-adjusted alpha value of 0.0003 (Šidák 1968; Simes 1986). However, the interpretation of the significance of tests using strictly adjusted α values has been strongly contested as these reduce the probability of Type I error only at the cost of inflating the probability of the equally deleterious Type II error (see Rothman 1990; Perneger 1998). Alpha adjustments are only recommended when a study is concerned with “the universal null hypothesis”, when the same test is applied in different subsamples without an a priori hypothesis that the primary association should differ between these subsamples (Perneger 1998) or when sequential tests are conducted in the same set of subjects (Perneger 1998; Garcia 2004). Alpha adjustments were discarded in this study, and therefore, significance of the relationships examined here was tested using an alpha of 0.05 for a number of reasons. First, this study was not concerned with testing that relationships exist between all reef fish species and the measurements of substrate complexity, and secondly because although we applied the same test (linear regression) in a set of subgroups (life phases and stages), such tests were conducted with a number of a priori hypothesis (see introduction). Lastly, although sequential tests (effect of E1 and SRI) were conducted in the same set of subjects (a group of fish of the same species), we advocate that the interpretation of each test in this study should not depend on the number of other tests included in this paper (see Perneger 1998; Garcia 2004).
Utility of RoxAnn’s E1 to predict patterns of fish abundance
The minimum inter-site difference in fish abundance (density and biomass) that is detectable using RoxAnn’s E1 was determined in 3 steps for an example species (i.e. TP Sparisoma aurofrenatum) (Fig. 2). In step 1, a point “P1” along the regression line representing the relationship between E1 (x axis) and density (or biomass) of TP S. aurofrenatum (y axis) was randomly selected. In step 2, the location of a second point “P2” just outside the upper limit established by the 95% confidence intervals of the regression line was determined. In step 3, the difference between the values corresponding to P1 and P2 projected on the y axis (density or biomass) was calculated and comprised the minimum difference in fish density or biomass (ΔD or ΔB) that can be detected between two sites of different E1 (ΔE1).
Example plot of a linear regression curve between the acoustic roughness (E1) in the x axis and the fish density in the y axis. Steps 1 to 3 illustrate the procedure to determine the minimum difference that has to exist between two sites in fish abundance (e.g. ∆D) for it to be detected using RoxAnn’s E1. See methods for details
The number of acoustic echoes (n) required to detect an inter-site significant difference in E1 of size ΔE1 was calculated using sample size power calculations for differences between two independent means available in the software G*Power (Faul et al. 2007). n was calculated by setting the significance level to 0.05, the power to 0.80 and the Cohen’s (1988) effect size measure (d) to a value calculated as a function of the means of two of our survey sites with a difference in mean of size ΔE1 (μ 1 and μ 2) and their common standard deviation (σ):
Results
Utility of E1 to predict topographic complexity
The ISR of our study sites was a relatively good predictor of their E1 (Fig. 3). The high fit of the linear model including polynomial terms (Linear regression, r 2 = 0.66) indicates that E1 could be used as a comparable proxy for the topographic complexity of Caribbean reefs to that obtained with widely used in situ methods.
Species relationships with topographic complexity and E1
Nine of the species targeted within our censuses were very rarely observed (i.e. 1 individual at no more than 4 sites), and therefore, their relationship with ISR and E1 could not be tested. These species were the large groupers: Epinephelus striatus, E. adscensionis, Mycteroperca venenosa, M. tigris, M. bonaci, the parrotfishes Scarus coelestinus, S. vetula, S. taeniopterus and the Bermuda sea chub (Kyphosus sectator).
Predicting the abundance of common species
The abundance of Chaetodon striatus, C. capistratus, E. guttatus, Sparisoma chrysopterum, Cephalopholis fulva, Ocyurus chrysurus and Holocentrus rufus was not significantly correlated with ISR or E1. However, the density and biomass of the remaining 10 common species found in our study were significantly related to the ISR (Fig. 4). Significant relationships occurred between E1 and both abundance parameters of most of these species, but not between E1 and the density of Haemulon plumierii or between E1 and the biomass of Acanthurus coeruleus and Stegastes partitus. The strongest relationships between fish abundance and the ISR (Linear regression, r 2 > 0.60) occurred in strongly territorial species (i.e. S. iserti, S. planifrons, S. aurofrenatum and S. viride). The r2 values indicated that the relationship between the abundance of a species and the ISR was generally stronger than its relationship with E1. Usually, both measures of topographic complexity had a relatively stronger effect (higher r 2) on the density than on biomass of the species observed.
Strength of the relationship (i.e. linear model’s r 2) a between density and ISR (black bars) and between density and E1 (grey bars) and b between biomass and ISR (black bars) and between biomass and E1 (grey bars) for ten species significantly correlated with at least one of these measurements of topographic complexity. Note that significance of the paired relationships was assessed without an adjustment for multiple tests (i.e. when α < 0.05). Fish diagrams reproduced from Humann and Deloach (2000) with prior publisher's consent
The density and biomass of 5 species, namely A. coeruleus, S. planifrons, S. viride, S. iserti and C. cruentata, increased linearly as ISR increased (Online Resource 1). Except for the biomass of A. coeruleus, both abundance parameters of all these species also increased linearly with E1 (Online Resource 1).
The density and biomass of the adults of H. flavolineatum, H. plumierii and adult S. partitus exhibited a hump-shaped relationship with ISR such that it was relatively low in less structurally complex reefs and reefs with the highest ISR (Online Resource 1); the highest abundance of these species occurred on reefs with intermediate ISR (~1.6–1.7). However, only the abundance of S. partitus responded in the same way to changes in E1 (Online Resource 1). Interestingly, both the density and biomass of H. flavolineatum and only biomass of H. plumierii increased linearly with the E1 rather than with the quadratic (hump-shaped) relationship found for ISR. Further, E1 was not a significant predictor of the density of H. plumierii (Online Resource 1).
The abundance of the Redband parrotfish S. aurofrenatum increased steeply from flat reefs towards reefs with low ISR (~1.4), remained relatively stable throughout reefs of intermediate ISR (1.4–1.9) and increased again in reefs with the highest ISR (Online Resource 1). The response of S. aurofrenatum abundance to changes in E1 was slightly different. Biomass of S. aurofrenatum increased linearly with E1, whereas increases in its density described a polynomial-shaped curve (Online Resource 1).
An unusual response to the increase of ISR and E1 was the consistent linear decrease of A. bahianus in density and biomass. Unlike most reef fish species, large numbers of individuals, and consequently a higher biomass of A. bahianus, were recorded in less structurally complex reefs (Online Resource 1).
In the case of parrotfishes, the nature and strength of the relationships between abundance and reef complexity differed among life phases. The abundance of juveniles of S. iserti was strongly related with both ISR and E1 (Linear regression, r 2 = 0.43−0.89, Online Resource 2), whereas juveniles of S. aurofrenatum were not as strongly affected by reef complexity (Linear regression, r 2 = 0.10–0.29, Online Resource 2). Both density and biomass of terminal-phase individuals (TP’s) of these 3 species of parrotfishes increased linearly with ISR and E1, and these relationships were strong (Linear regression, r 2 = 0.44–0.86, Online Resource 2). r 2 values indicated that the relationships between the initial-phase individuals (IP’s) and both measurements of topographic complexity were generally weaker than those of TP’s (Online Resource 2).
Both the abundance and biomass of the IP S. iserti increased linearly with ISR and E1, whereas only the density but not the biomass of IP S. viride increased linearly with ISR and E1 (Online Resource 2). Specific trends of the abundance of IP S. aurofrenatum were virtually identical to those described for the overall abundance of this species. Both density and biomass increased in a steep curve from flat reefs towards reefs with low ISR (~1.4), density relatively stable throughout reefs of intermediate ISR (1.4–1.9) and then increased again in reefs with the highest ISR. Only the density of IP S. aurofrenatum was also related to E1 (by a polynomial-shaped curve, Online Resource 2).
Predicting the abundance of uncommon species
A total of 10 species of the families Acanthuridae, Pomacentridae, Scaridae, Haemulidae, Lutjanidae and Chaetodontidae were classified and analysed as being uncommon in our study (Fig. 5). Additionally, some life phases and stages of common species were uncommon when counted separately. Such was the case of adult and juvenile S. planifrons and juvenile S. viride.
Bar plot indicating those uncommon species that were significantly correlated with ISR and/or E1 (upper clear bars) and those that were unaffected by ISR and/or E1 (lower grey bars). Correlations were tested using hurdle models, and their significance was assessed without an adjustment for multiple tests (i.e. when α < 0.05) to access P values please refer to Online Resource 3
The occurrence of A. chirurgus and C. ocellatus and the abundance of these species (when present) were unaffected by either measure of topographic complexity. Both the occurrence and abundance (if present) of S. diencaeus, S. leucostictus, adult S. planifrons and Lutjanus apodus were significantly and positively affected by ISR (Fig. 5, Online Resource 3). E1 was a significant predictor for the occurrence of all these species but was not significantly related to the abundance (when present) of any of them.
The occurrence but not the abundance of L. mahogoni, S. atomarium, H. sciurus, juvenile S. viride and juvenile S. planifrons was also significantly and positively related to ISR. However, only the occurrence of L. mahogoni was also related with E1 (Fig. 5 Online Resource 3). Conversely, the abundance but not the occurrence of S. adustus and S. rubripinne was significantly and positively related to ISR, but unaffected by E1.
Utility of E1 to predict patterns of fish abundance
Terminal-phase S. aurofrenatum held the strongest linear relationships with E1 (density-r 2 = 0.69; biomass-r 2 = 0.68) and therefore provided a simple example to demonstrate the application of the models generated in this study (Fig. 6). Using the RoxAnn’s E1, a minimum difference of 0.9 ind 200 m−2 and 116 g 200 m−2 could be predicted for TP S. aurofrenatum (Fig. 6) between two sites. Two sites were likely to support significantly different densities of TP S. aurofrenatum if their differed at least 0.07 units in E1 and supported significantly different biomass of the same species if they differed at least 0.05 units in E1.
Linear regression curves between a x: E1 vs. y: transformed density of TP S. aurofrenatum (Ln (density + 1)) and b x: E1 and y: square root of biomass of TP S. aurofrenatum. Regression line fitted in a is used to illustrate that the smallest difference in density of TP S. aurofrenatum that can be predicted with E1 is 0.9 ind 200 m−2 and would occur in sites that are 0.07 units of E1 different from each other. b The smallest difference in biomass of TP S. aurofrenatum that can be predicted with E1 is 116 g 200 m−2 and would occur in sites that are 0.05 units of E1 different from each other
The standard deviation of E1 measurements in the forereef of Glovers Atoll ranged between 0.03 and 0.11. Therefore, it is recommended that under similar characteristics, future studies aiming to detect significant inter-site E1-differences which would reflect in significant inter-site differences in fish density (∆E1 = 0.07) and biomass (∆E1 = 0.05), count with a minimum of 16 and 19 RoxAnn’s echoes site−1, respectively.
The above may only be safely generalised for fish species that hold strong relationships with E1 (r 2 = 0.50). Significant differences in fish density for a species holding the weakest density–E1 relationship (i.e. Acanthurus bahianus, r 2 = 0.14) could only be detected between sites that lied at opposite sides of the E1 spectrum (∆E1 = ~0.3). Although in such cases, a small number of echoes (i.e. n = 3) would suffice to detect inter-site differences in E1 of enough significance to affect fish density, results indicate that the capacity of E1 to predict differences in fish density among typical forereef habitats is severely limited for species weakly correlated with E1.
Discussion
This study demonstrates that despite the differences in scale between the in situ measurement of ISR and RoxAnn’s E1, the latter can be used as a reasonably good proxy for the topographic complexity of Caribbean reefs. The curvilinear shape of the relationship between ISR and E1 indicates that E1 could sense differences among reefs with lower to intermediate topographic complexity and between reefs with very low and very high topographic complexity relatively easily. However, E1’s capacity to reflect the differences among reefs with medium to high complexity is rather limited. Such limitation may arise from the large footprint over which E1 is measured on the bottom (typically at least 30 cm in diameter). Highly complex reefs may differ subtly in their availability of small refuges or other fine-scale attributes that fail to be detected by the sonar.
We expected that the abundance or presence of most reef fish species would be correlated with ISR. Fifty percent of our species (10 common and 8 uncommon) had significant relationships with ISR in forereef habitats of Glovers Atoll (Figs. 4, 5). These included all the surveyed species of damselfishes and grunts, 67% of the species of surgeonfish and snappers, 56% of the species of parrotfishes and one serranid species. Given the significant relationship observed between ISR and E1, we expected that those species that were significantly correlated with ISR would also be significantly affected by E1. However, the significant relationship of a species with ISR did not always imply a significant relationship with E1 (Figs. 4, 5). E1 was a significant predictor of the abundance patterns for all of the common species affected by ISR, but only for 50% of the uncommon species affected by ISR. Such results indicate that the utility of E1 to predict fish abundance may be limited to common species. The utility of RoxAnn’s E1 could be enhanced if it could yield abundance predictions for rare species, because these are often the focus of management strategies and species recovery plans. This may be achieved in future studies if the detection of rare species is maximised using alternative fish survey methods such as timed swims or census within larger areas of reef.
Fish families included in our surveys held close relationships with the substratum for a number of reasons. Nocturnal foragers such as Haemulids and Lutjanids use physical refuges during the day (Ehrlich 1975; Burke 1995; Ménard et al. 2008), whereas individuals of those families that are active during the day such as Scaridae, Acanthuridae and Pomacentridae seek refuges during the night (Robertson and Sheldon 1979). Some are prominent stationary predators that spend a large amount of time hiding in refuges such as Serranidae (Shpigel and Fishelson 1991). And among these, families of grazers (i.e. Scaridae, Acanthuridae and Pomacentridae) also hold permanent territories and/or feed on the bottom continuously and intensively (Robertson et al. 1976; Thresher 1976; Mumby and Wabnitz 2002).
Furthermore, given that it has been suggested that the strongest relationships between fish and topographic complexity may occur in those species that have smaller home ranges or territories and/or are more vulnerable to predation due to their small body size (Roberts and Ormond 1987; Choat and Bellwood 1991; Mumby and Wabnitz 2002), we expected this to be the case in our study. The fact that Scarus iserti and Stegastes planifrons held the strongest relationships with ISR (Linear regression, r 2 = 0.93 and 0.73, Fig. 4) was consistent with our expectations. Both these grazing species are small bodied. S. iserti has the smallest territory size among species of parrotfish (i.e. 41–120 m2) (Mumby and Wabnitz 2002), and territories of S. planifrons are only about a meter in diameter (Hixon 1996) or 0.25 m3 (Luckhurst and Luckhurst 1978b). Moreover, the relationships of other small territorial species with ISR were significant despite them being relatively uncommon (i.e. Sparisoma atomarium, S. diencaeus and S. leucostictus) (Fig. 5).
Because individual fish are likely to utilise the habitat characteristics on a scale proportional to their home range or foraging area (Roberts and Ormond 1987), it seemed reasonable to expect that the larger-scale measure of topographic complexity E1 would be a stronger predictor of the abundance of species with larger home ranges, whereas ISR will be a stronger predictor for site-attached species or species with smaller home ranges or territories. However, ISR was always a stronger predictor than E1 for all species except for TP S. aurofrenatum and C. cruentata. S. aurofrenatum did not have particularly large home ranges or territory sizes compared to other parrotfish species in Glovers Atoll or in other Caribbean areas (Muñoz and Motta 2000; Mumby and Wabnitz 2002), whereas the mean home range of C. cruentata was 2,120 m2 in St. Lucia (Popple and Hunte 2005). The fact that S. aurofrenatum and C. cruentata held the strongest relationships with the large-scale E1 suggests that these species may be affected by the presence of attributes of the topographic complexity such as mounds or gullies that are not captured with a 4-m chain but may be captured within the footprint of a RoxAnn echo. Our results agree with observations by Pittman et al. (2009) who found that groupers, piscivores and specifically C. cruentata seemed to respond to topographic complexity at relatively broader spatial scales in La Parguera (Puerto Rico).
The size or the site attachment of a species alone did not seem to play a primary role in determining the strength of its relationship with topographic complexity. A number of small-bodied species, some of which are known to be highly site attached such as Holocentrus rufus (Luckhurst and Luckhurst 1978b; Chapman and Kramer 2000) or with at least some individuals holding territories such as Chaetodon capistratus, C. ocellatus and C. striatus (Gore 1983; Bonaldo et al. 2005), were unaffected by ISR. The lack of a relationship between abundance and ISR also occurred with the juveniles of the highly site-attached S. partitus. Two possible reasons may account for the lack of a relationship between the abundance of these small site-attached species and ISR. In some cases, abundance may be determined by aspects of the topographic complexity that are inadequately reflected by the ISR. Despite it being a commonly used method, the “chain and tape” method provides a measure of the vertical relief but fails to provide important information such as the number, size and distribution of holes and crevices. The number and diversity of shapes and sizes of holes plays an important role in determining the species richness and abundance of some families of fish (respectively) (Roberts and Ormond 1987). Similarly, the availability of holes matching the size of individual fish are key determinants of a species’ choice for refuges (Robertson and Sheldon 1979; Hixon and Beets 1993). A larger number of refuges matching the shape and size of some of the small species or juveniles may not necessarily occur in the most topographically complex reefs. The lack of relationships between abundance and ISR may also have occurred because of an overriding influence of other demographic processes such larval supply, predator abundance, food availability and interspecific competition. The distribution and abundance of Chaetodontids for example may be more strongly affected by the abundance of their preferred food resources (i.e. the octocorals Gorgonia ventalina and G. flabellum, and Zoanthus) (Gore 1983; Bonaldo et al. 2005) than by the availability of refuge holes. Moreover, the need for physical refuges may not only be a consequence of restricted home ranges or site attachment but of the species’ behaviour in response to predator attacks. When threatened Caribbean Chaetodontids have been observed to undertake a flight response, swimming long distances, rather than seeking shelter (Clarke 1977), which may explain why shelter does not seem to be of prime importance to certain species (Gore 1983).
Not all species that were significantly correlated with substrate complexity increased linearly with ISR and E1. The abundance of some species seemed to be favoured by attributes of low or intermediate-complexity reefs (see Online Resources 1, 2). Surprisingly, the density and biomass of the ocean surgeonfish Acanthurus bahianus decreased linearly with increasing ISR and E1. Our results contrast with findings of Gratwicke and Speight (2005a) which indicate that A. bahianus was characteristic of highly rugose sites in Tortola. Ocean surgeonfishes differ from other species of surgeonfish in that they consume large amounts of inorganic sediment along with algal material (Randall 1967), though their distribution has been reported to follow the availability of algae (Longley and Hildebrand 1941). It is possible that the daily abundance of A. bahianus at the forereef of Glovers Atoll was determined by the presence of inorganic sediment on algal turfs which tends to occur on or in the proximity of less rugose reefs (pers obs). In fact, high levels of wave-induced resuspension of sediments are likely to contribute to the low structural complexity at some sites on the forereef of Glovers Atoll, because the presence of sediments can inhibit coral settlement (Birrell et al. 2005). Furthermore, by schooling, A. bahianus gains the necessary protection from predators that allows it to utilise such low complexity habitats (Wolf 1987). Finding appropriate refuges for the night might be possible for A. bahianus because it has a documented ability to travel long distances (Chapman and Kramer 2000). Haemulon flavolineatum, H. plumierii and adult S. partitus appeared to have reached a maximum density and/or biomass in reefs of intermediate ISR. For these grunts, such observation is consistent with findings of Gratwicke and Speight (2005b) who found these species to be characteristic species of moderately complex reefs.
Interestingly among the observed parrotfish species, only S. aurofrenatum exhibited a curvilinear relationship with ISR, starting with a rapid increase in density and biomass from low to medium complexity (~1.4). This relationship was driven by the IP individuals because TP’s increased linearly in density with ISR. Why females exhibit this pattern and not TP (males) is unclear, as is the reason why it occurs only in this species of parrotfish, considering that all parrotfish species exhibit territoriality at Glovers Atoll (Mumby and Wabnitz 2002).
In this study, the significance of >100 fish abundance–rugosity relationships was assessed without a Bonferroni alpha adjustment. We chose not to conduct a Bonferroni alpha adjustment in the light of the reported inconsistencies of this procedure (see Perneger 1998) and aiming to avoid the Type II error. Diminishing the chances of incurring in a Type II error is imperative in this study because fish census data are notoriously imprecise (Andrew and Mapstone 1987), and the ample inter-census variability often impairs the detection of significant patterns or relationships. Arguably, the proportion of reef fish species that are significantly correlated with ISR and E1 was possibly overestimated from our data analysis, because of the feasibility of Type I errors given repeated testing. Such overestimation was not serious for common species given that 85% of the significant results at an alpha of 0.05 were significant at a smaller alpha of 0.01. A more serious overestimation could have been made when analysing the relationships of uncommon species with topographic complexity because only 25% of significant results at an alpha of 0.05 were also significant at an alpha of 0.01.
Utility of RoxAnn’s E1 to predict spatial patterns of ISR
Interestingly, only a few studies have explored the relationship between the remotely sensed rugosity and the in situ rugosity determined with manual methods (i.e. Kuffner et al. 2007; Wedding et al. 2008; Pittman et al. 2009; Walker et al. 2009). The relatively strong fit we obtained when modelling the relationship between ISR and E1 (Linear regression, r 2 = 0.66) highlights the value of the RoxAnn system as a tool to measure and predict patterns of topographic complexity over large scales in Caribbean reefs and was comparable to that found by Wedding et al. (2008) between the ISR and the Lidar rugosity determined at a 4-m grid size in Hawaii (r 2 = 0.61). The strength of the RoxAnn’s E1–ISR relationship was considerably higher than that found between the Lidar rugosity and ISR in La Parguera (r 2 = 0.31) (Pittman et al. 2009), in Biscayne National Park (r 2 = 0.15) (Kuffner et al. 2007) and in Broward County reef (r 2 = 0.19) (Walker et al. 2009). Findings of Kuffner et al. (2007) indicate that the utility of Lidar rugosity to predict the patterns of reef complexity can be hindered by the choice of study environment. In patch reef areas, Lidar rugosity was strongly correlated with distance from the edge of the patch reef rather than with topographic complexity. Moreover, inter-patch reef variability in the Biscayne Natural Park was responsible for most of the variability of species richness and diversity rather than the manually or remotely sensed rugosity (Kuffner et al. 2007). Although this remains untested, we can anticipate that the utility of RoxAnn to predict the patterns of ISR could also decline considerably if in hind sight the variability among survey sites is likely to be a strong determinant of the patterns of habitat structure and fish community.
Utility of RoxAnn’s E1 to predict patterns of fish abundance
Relatively few studies have examined the relationship between attributes of fish communities and measures of topographic complexity derived from remote sensing tools (see Mellin et al. 2009 for a review). As a result, sites of high fish species richness were identified in the US Virgin Islands using GIS and a spatial layer representing fine-scale topographic complexity differences among habitat types (Pittman et al. 2007). Spatial patterns of species richness and total fish abundance were predicted in the Diego Garcia Atoll (Chagos Archipelago) using the rugosity determined with DEMs derived from an IKONOS image (Purkis et al. 2008), and the same variables were predicted in Oahu (Hawaii) using Lidar rugosity derived at a 25-m grid size (Wedding et al. 2008). Lidar rugosity also proved to be useful when predicting patterns of abundance at a species level by correlating strongly with the density and biomass of Stegastes planifrons in La Parguera (Puerto Rico) (Pittman et al. 2009). However, Lidar rugosity was relatively less strongly related with species richness (Kuffner et al. 2007) and with both species richness and abundance (Walker et al. 2009) in other areas of the Wider Caribbean.
In most species, both ISR and E1 had stronger relationships with fish density than with biomass. It is possible that such measurements of topographic complexity conveyed more information on the aspects of rugosity that affect fish density (e.g. reef surface area and availability of refuges) than on those that control fish size spectrum and therefore biomass (size of the refuges, availability of refuges of different sizes).
When considering the abundance parameters of uncommon species: presence and abundance when present, the latter was uncorrelated with E1 even when it correlated significantly with ISR. It is unclear why this lack of relationship occurred and it was observed indistinctively in small as well as in relatively large fish species. In future studies, the use of presence instead of abundance when present is recommended for similar objectives with uncommon species.
By assessing the relationships between RoxAnn’s E1 and the occurrence, density and biomass of fish at the species and life-phase level, our study explored the ability of acoustic remote sensing to predict the spatial patterns of fish abundance at a finer level of detail than previously investigated. Our study demonstrated that RoxAnn’s E1 is a significant predictor of the abundance of 15 common species and presence of 4 uncommon reef fish species in the Caribbean. E1 was a relatively stronger predictor of the abundance of parrotfishes in general but also for Stegastes planifrons and Cephalopholis cruentata (r 2 > 0.40) and weaker but also useful for certain species of surgeonfish, damselfish, grunts and snappers.
For S. aurofrenatum, a species that was strongly related with E1, small differences in E1 between sites (i.e. 0.07) resulted in subtle but significant differences in abundance of TP individuals (i.e. of minimum 1 individual 200 m−2 or 116 g 200 m−2). It is necessary to count with a minimum of 16–19 RoxAnn echoes per site to detect significant differences in the density and biomass of species that are highly sensitive to topographic complexity. Detecting differences in fish abundance between sites that are only subtly different in E1 may prove challenging if possible for species that hold weak relationships with E1.
Because the quality of the RoxAnn’s signal is affected by the movements of the boat during data acquisition and several echoes need to be eliminated during the filtration of data, in general a larger number of echoes (i.e. 50 per site) should be collected or even a larger number (i.e. ~100) if high swell conditions occur. Given that the collection of RoxAnn data along a ~1.3 miles stretch of the forereef of Glovers Atoll took 4 days and data processing could be completed within a few hours, its unlikely that collecting up to 100 RoxAnn pings site−1 would cause a significant increase in data collection and processing times.
In our study, the Furuno FCV-600L was operated with a pulse repetition rate of approximately 1,300–1,400 pulses min−1. However, RoxAnn recorded data every 2–5 m and then averaged sets of 40–80 pulses to obtain 1 echo return (E1 value). In these conditions, approximately ~10 echoes could be collected in the immediate vicinity of each fish survey site. Therefore, it was justifiable to collect several more echoes from surrounding areas with a similar spectral signature. However, for future studies, it is recommended to adjust the depth range of the echosounder to obtain maximum pings per second.
In this particular study, IKONOS data were required to assist the collection of large numbers of acoustic echoes per site. Future studies aiming to use RoxAnn data to examine spatial patterns of reef rugosity and the correlation of E1 with the fish community may use an IKONOS image or the interpolation of acoustic data to extrapolate acoustic roughness to broader regions. However, such procedures are unlikely to be required if the recommended minimum number of acoustic pings is collected per site.
Conclusions
The results of our study highlight the utility of a relatively cost-effective acoustic instrument (see White et al. 2003) to predict the variations of topographic complexity and fish abundance within a typical Caribbean coral reef. The use of higher-resolution airborne instruments such as Lidar, or the use of IKONOS-derived DEMs (see Purkis et al. 2008; Wedding et al. 2008; Pittman et al. 2009), may result in similarly useful spatial predictions of fish abundance. However, the acquisition of Lidar data is expensive and takes considerable processing time (Joyce et al. 2009). Moreover, although high-resolution DEM’s can be obtained even in the absence of Lidar data, DEM creation software is not standard in image processing packages, therefore implying extra costs (Joyce et al. 2009). Contrastingly, the present study demonstrates that RoxAnn is a viable instrument to predict the spatial patterns of abundance at a fish species level, at the cost of very simple data processing steps (i.e. filtration and standardisation of E1 data).
Models predicting species-specific spatial patterns of density and biomass facilitate mapping functional attributes of fish populations rather than aggregate attributes of fish communities. Accurate predictions of species richness and diversity (Kuffner et al. 2007; Pittman et al. 2007) have provided tools to aid the design of marine-protected areas sensible to fish richness and diversity. We show that the density of some functionally and commercially important species (e.g. large-bodied parrotfishes and small groupers, respectively) can also be mapped using acoustic remote sensing. We anticipate that such maps will contribute towards the generation of GIS datasets representing the potential resilience of reefs (Mumby et al. 2007b) and their commercial value to fisheries or tourism. Such maps may then be integrated with other relevant physical, biological, social and economic datasets to help identify appropriate zoning for multiple uses and conservation of the coastal zone.
References
Andréfouët S, Kramer P, Torres-Pulliza D, Joyce KE, Hochberg EJ, Garza-Perez R, Mumby PJ, Riegl B, Yamano H, White WH, Zubia M, Brock JC, Phinn SR, Naseer A, Hatcher BG, Muller-Karger FE (2003) Multi-site evaluation of IKONOS data for classification of tropical coral reef environments. Remote Sens Environ 88:128–143. doi:110.1016/j.rse.2003.1004.1005
Andrew NL, Mapstone BD (1987) Sampling and the description of spatial pattern in marine ecology. Oceanogr Mar Biol Annu Rev 25:39–90
Bell JD, Galzin R (1984) Influence of live coral cover on coral-reef fish communities. Mar Ecol Prog Ser 15:265–274. doi:210.3354/meps015265
Birrell CL, McCook LJ, Willis BL (2005) Effects of algal turfs and sediment on coral settlement. Mar Poll Bull 51:408–414. doi:410.1016/j.marpolbul.2004.1010.1022
Bohnsack JA, Harper DE (1998) Length-weight relationships of selected marine reef fishes from the southeastern United States and the Caribbean. Technical Memorandum NMFS-SEFC-215 (National Oceanic and Atmospheric Administration, Miami, Florida, 1988)
Bonaldo RM, Krajewski JP, Sazima I (2005) Meals for two: foraging activity of the Butterflyfish Chaetodon striatus (Perciformes) in Southeast Brazil. Braz J Biol 65:211–215
Brock VE (1954) A preliminary report on a method of estimating reef fish populations. J Wildl Manag 18:297–308. doi:210.2307/3797016
Bruggemann JH, Kuyper MWM, Breeman AM (1994) Comparative analysis of foraging and habitat use by the sympatric Caribbean parrotfish Scarus vetula and Sparisoma viride (Scaridae). Mar Ecol Prog Ser 112:51–66. doi:10.3354/meps112051
Burke NC (1995) Nocturnal foraging habitats of French and bluestriped grunts, Haemulon flavolineatum and H. sciurus, at Tobacco Caye, Belize. Environ Biol Fish 42:365–374. doi:310.1007/BF00001467
Caley MJ, St John J (1996) Refuge availability structures assemblages of tropical reef fishes. J Anim Ecol 65:414–428. doi:410.2307/5777
Carpenter KE, Miclat RI, Albaladejo VD, Corpuz VT (1981) The influence of substrate structure on the local abundance and diversity of Philippine reef fishes. In: Proceedings of 4th international coral reef symposium, Manila, pp 497–502
Chabanet P, Ralambondrainy H, Amanieu M, Faure G, Galzin R (1997) Relationships between coral reef substrata and fish. Coral Reefs 16:93–102. doi:110.1007/s003380050063
Chapman MR, Kramer DL (2000) Movements of fishes within and among fringing coral reefs in Barbados. Environ Biol Fish 57:11–24. doi:10.1023/A:1004545724503
Choat JH, Bellwood DR (1991) Reef fishes: their history and evolution. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic Press, Inc., London, pp 39–66
Clarke RD (1977) Habitat distribution and species diversity of Chaetodontid and Pomacentrid fishes near Bimini, Bahamas. Mar Biol 40:277–289. doi:210.1007/BF00390882
Cohen J (1988) Statistical power analysis for the behavioral sciences. Erlbaum, Hillsdale
Collins MB, Voulgaris G (1993) Empirical field and laboratory evaluation of a real-time acoustic sea-bed surveying system. In: Proceedings of Institutional Acoustics, 15, Part 2, pp 343–351
Costa BM, Battista TA, Pittman SJ (2009) Comparative evaluation of airborne LiDAR and ship-based multibeam SoNAR bathymetry and intensity for mapping coral reef ecosystems. Remote Sens Environ 113:1082–1100. doi:1010.1016/j.rse.2009.1001.1015
Crawley MJ (2002) Statistical computing, an introduction to data analysis using S-Plus. Wiley, West Sussex
Ebersole JP (1985) Niche separation of two damselfish species by aggression and differential microhabitat utilization. Ecology 66:14–20. doi:10.2307/1941302
Ehrlich PR (1975) The population biology of coral reef fishes. Annu Rev Ecol Syst 6:211–247
Faraway JJ (2005) Linear models with R. Chapman & Hall, London
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Fletcher D, Faddy M (2007) Confidence intervals for expected abundance of rare species. J Agric Biol Environ Stat 12:315–324. doi:310.1198/108571107X108229322
Fletcher D, Mackenzie D, Villouta E (2005) Modelling skewed data with many zeros: a simple approach combining ordinary and logistic regression. Environ Ecol Stat 12:45–54. doi:10.1007/s10651-10005-16817-10651
Friedlander AM, Brown EK, Jokiel PL, Smith WR, Rodgers KS (2003) Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian archipelago. Coral Reefs 22:291–305. doi:210.1007/s00338-00003-00317-00332
Garcia LV (2004) Escaping the Bonferroni iron claw in ecological studies. Oikos 105:657–663. doi:610.1111/j.0030-1299.2004.13046.x
Gore MA (1983) The effect of a flexible spacing system on the social organization of a coral reef fish, Chaetodon capistratus. Behaviour 85:118–145. doi:110.1163/156853983X156800066
Gratwicke B, Speight MR (2005a) Effects of habitat complexity on Caribbean marine fish assemblages. Mar Ecol Prog Ser 292:301–310. doi:310.3354/meps292301
Gratwicke B, Speight MR (2005b) The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J Fish Biol 66:650–667. doi:610.1111/j.0022-1112.2005.00629.x
Greenstreet SPR, Tuck ID, Grewar GN, Armstrong E, Reid DG, Wright PJ (1997) An assessment of the acoustic survey technique, RoxAnn, as a means of mapping seabed habitat. ICES. J Mar Sci 54:939–959. doi:910.1006/jmsc.1997.0220
Hamilton LJ (2001) Acoustic seabed classification systems. DSTO Aeronautical and Maritime Research Laboratory, Australia
Hamilton LJ, Mulhearn PJ, Poeckert R (1999) Comparison of RoxAnn and QTC-view acoustic bottom classification system performance for the Cairns area, Great Barrier Reef, Australia. Cont Shelf Res 19:1577–1597. doi:1510.1016/S0278-4343(1599)00020-00025
Hixon MA (1996) Effects of reef fishes on corals and algae. In: Birkeland C (ed) Life and death of the coral reefs. Chapman and Hall, New York, pp 230–248
Hixon MA, Beets JP (1993) Predation, prey refuges and the structure of coral-reef fish assemblages. Ecol Monogr 63:77–101. doi:110.2307/2937124
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365. doi:310.1016/j.cub.2006.1012.1049
Humann P, Deloach N (2000) Reef fish identification: Florida Caribbean Bahamas. New World Publications, Inc., Jacksonville
Hutin E, Simard Y, Archambault P (2005) Acoustic detection of a scallop bed from a single-beam echosounder in the St. Lawrence. ICES. J Mar Sci 62:966–983. doi:910.1016/j.icesjms.2005.1003.1007
Jennings S, Boulle DP, Polunin NVC (1996) Habitat correlates of the distribution and biomass of Seychelles’ reef fishes. Environ Biol Fish 46:15–25. doi:10.1007/BF00001693
Johansen JL, Bellwood DR, Fulton CJ (2008) Coral reef fishes exploit flow refuges in high-flow habitats. Mar Ecol Prog Ser 360:219–226. doi:210.3354/meps07482
Joyce KE, Belliss SE, Samsonov SV, McNeill SJ, Glassey PJ (2009) A review of the status of satellite remote sensing and image processing techniques for mapping natural hazards and disasters. Prog Phys Geogr 33:183–207. doi:110.1177/0309133309339563
Knudby A, LeDrew E, Brenning A (2010) Predictive mapping of reef fish species richness, diversity and biomass in Zanzibar using IKONOS imagery and machine-learning techniques. Remote Sens Environ 114:1230–1241. doi:1210.1016/j.rse.2010.1201.1007
Kuffner IB, Brock JC, Grober-Dunsmore R, Bonito VE, Hickey TD, Wright CW (2007) Relationships between reef fish communities and remotely sensed rugosity measurements in Biscayne National Park, Florida, USA. Environ Biol Fish 78:71–82. doi:10.1007/s10641-10006-19078-10644
Longley WH, Hildebrand SF (1941) Systematic catalogue of the fishes of Tortugas, Florida with observations on color, habits, and local distribution. Pap Tortugas Lab 34 (Canergie Inst. Wash. Pub. 535): xiii + 331, 334 pls
Luckhurst BE, Luckhurst K (1978a) Analysis of influence of substrate variables on coral-reef fish communities. Mar Biol 49:317–323. doi:310.1007/BF00455026
Luckhurst BE, Luckhurst K (1978b) Diurnal space utilization in coral-reef fish communities. Mar Biol 49:325–332. doi:310.1007/BF00455027
Lundblad ER, Wright DJ, Miller J, Larkin EM, Rinehart R, Naar DF, Donahue BT, Anderson SM, Battista T (2006) A benthic terrain classification scheme for American Samoa. Mar Geod 29:89–111. doi:110.1080/01490410600738021
Martin TG, Wintle BA, Rhodes JR, Kuhnert PM, Field SA, Low-Choy SJ, Tyre AJ, Possingham HP (2005) Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecol Lett 8:1235–1246. doi:1210.1111/j.1461-0248.2005.00826.x
Mason G (1987) Coping with collinearity. Can J Program Eval 2:87–95
McCormick MI (1994) Comparison of field methods for measuring surface topography and their associations with a tropical reef fish assemblage. Mar Ecol Prog Ser 112:87–96. doi:10.3354/meps112087
Mellin C, Andréfouët S, Kulbicki M, Dalleau M, Vigliola L (2009) Remote sensing and fish-habitat relationships in coral reef ecosystems: review and pathways for systematic multi-scale hierarchical research. Mar Poll Bull 58:11–19. doi:10.1016/j.marpolbul.2008.1010.1010
Ménard A, Turgeon K, Kramer DL (2008) Selection of diurnal refuges by the nocturnal squirrelfish, Holocentrus rufus. Environ Biol Fish 82:59–70. doi:10.1007/s10641-10007-19253-10642
Mumby PJ, Edwards AJ (2002) Mapping marine environments with IKONOS imagery: enhanced spatial resolution can deliver greater thematic accuracy. Remote Sens Environ 82:248–257. doi:210.1016/S0034-4257(1002)00041-X
Mumby PJ, Wabnitz C (2002) Spatial patterns of aggression, territory size, and harem size in five sympatric Caribbean parrotfish species. Environ Biol Fish 63:265–279. doi:210.1023/A:1014359403167
Mumby PJ, Dahlgren CP, Harborne AR, Kappel CV, Micheli F, Brumbaugh DR, Holmes KE, Mendes JM, Broad K, Sanchirico JN, Buch K, Box S, Stoffle RW, Gill AB (2006) Fishing, trophic cascades, and the process of grazing on coral reefs. Science 311:98–101. doi:110.1126/science.1121129
Mumby PJ, Harborne AR, Williams J, Kappel CV, Brumbaugh DR, Micheli F, Holmes KE, Dahlgren CP, Paris CB, Blackwell PG (2007a) Trophic cascade facilitates coral recruitment in a marine reserve. Proc Natl Acad Sci USA 104:8362–8367. doi:8310.1073/pnas.0702602104
Mumby PJ, Hastings A, Edwards HJ (2007b) Thresholds and the resilience of Caribbean coral reefs. Nature 450:98–101. doi:110.1038/nature06252
Muñoz RC, Motta PJ (2000) Interspecific aggression between two parrotfishes (Sparisoma, Scaridae) in the Florida Keys. Copeia 3:674–683
Nanami A, Nishihira M (2002) The structures and dynamics of fish communities in an Okinawan coral reef: effects of coral-based habitat structures at sites with rocky and sandy sea bottoms. Environ Biol Fish 63:353–372. doi:310.1023/A:1014952932694
NOAA (1996) Manguso-Stevens fishery conservation and management act, as ammended through October 11, 1996. NOAA Tech. Memo. NMFS-F/SPO-23, pp 121
O’Brien RM (2007) A caution regarding rules of thumb for variance inflation factors. Qual Quant 41:673–690. doi:610.1007/s11135-11006-19018-11136
Perneger TV (1998) What’s wrong with the Bonferroni adjustments. Brit Med J 316:1236–1238
Pittman SJ, Christensen JD, Caldow C, Menza C, Monaco ME (2007) Predictive mapping of fish species richness across shallow-water seascapes in the Caribbean. Ecol Model 204:9–21. doi:10.1016/j.ecolmodel.2006.1012.1017
Pittman SJ, Costa BM, Battista TA (2009) Using lidar bathymetry and boosted regression trees to predict the diversity and abundance of fish and corals. J Coast Res 53:27–38. doi:10.2112/SI2153-2004.2111
Popple ID, Hunte W (2005) Movement patterns of Cephalopholis cruentata in a marine reserve in St Lucia, WI, obtained from ultrasonic telemetry. J Fish Biol 67:981–992. doi:910.1111/j.0022-1112.2005.00797.x
Purkis SJ, Graham NAJ, Riegl BM (2008) Predictability of reef fish diversity and abundance using remote sensing data in Diego Garcia (Chagos Archipelago). Coral Reefs 27:167–178. doi:110.1007/s00338-00007-00306-y
Randall JE (1967) Food habits of reef fishes of the West Indies. Stud Trop Oceanogr Miami 5:665–847
Risk MJ (1972) Fish diversity on a coral reef in the Virgin Islands. Atoll Res Bull 153:1–6
Roberts CM, Ormond RFG (1987) Habitat complexity and coral reef fish diversity and abundance on Red Sea fringing reefs. Mar Ecol Prog Ser 41:1–8. doi:10.3354/meps041001
Robertson DR, Sheldon JM (1979) Competitive interactions and the availability of sleeping sites for a diurnal coral reef fish. J Exp Mar Biol Ecol 40:285–298. doi:210.1016/0022-0981(1079)90057-90051
Robertson DR, Sweatman HPA, Fletcher EA, Cleland MG (1976) Schooling as a mechanism for circumventing the territoriality of competitors. Ecology 57:1208–1220. doi:1210.2307/1935045
Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1:43–46. doi:10.1097/00001648-199001000-199000010
Sale PF (1991) The ecology of fishes on coral reefs. Academic Press, Inc, UK
Sale PF, Douglas WA (1984) Temporal variability in the community structure of fish on coral patch reefs and the relation of community structure to reef structure. Ecology 65:409–422. doi:410.2307/1941404
Schlagintweit GEO (1993) Real-time acoustic bottom classification for hydrography: a field evaluation of RoxAnn Oceans 93. In: Proceedings, pp C214–C219
Shacham M, Brauner N (1997) Minimizing the effects of collinearity in polynomial regression. Ind Eng Chem Res 36:4405–4412. doi:4410.1021/ie970236k
Shpigel M, Fishelson L (1991) Territoriality and associated behaviour in three species of the genus Cephalopholis (Pisces: Serranidae) in the Gulf of Aqaba, Red Sea. J Fish Biol 38:887–897. doi:810.1111/j.1095-8649.1991.tb03628.x
Šidák Z (1968) On multivariate normal probabilities of rectangles: their dependence on correlations. Ann Math Stat 39:1425–1434
Simes RJ (1986) An improved Bonferroni procedure for multiple tests of significance. Biometrika 73:751–754. doi:710.2307/2336545
Su H, Liu H, Heyman WD (2008) Automated derivation of bathymetric information from multi-spectral satellite imagery using a non-linear inversion model. Mar Geod 31:281–298. doi:210.1080/01490410802466652
Thresher RE (1976) Field analysis of the territoriality of the Threespot Damselfish, Pomacentrus planifrons (Pomacentridae). Copeia 1976:266–276. doi:210.2307/1443946
von Szalay PG, McConnaughey RA (2002) The effect of slope and vessel speed on the performace of a single beam acoustic seabed classification system. Fish Res 99–112. doi:110.1016/S0165-7836(1002)00020-00026
Walker BK, Jordan LKB, Spieler RE (2009) Relationship of reef fish assemblages and topographic complexity on southeastern Florida coral reef habitats. J Coast Res 25:39–48. doi:10.2112/SI2153-2005.2111
Wedding LM, Friedlander AM, McGranaghan M, Yost RS, Monaco ME (2008) Using bathymetric lidar to define nearshore benthic habitat complexity: Implications for management of reef fish assemblages in Hawaii. Remote Sens Environ 112:4159–4165. doi:4110.1016/j.rse.2008.4101.4025
White WH, Harborne AR, Sotheran IS, Walton R, Foster-Smith RL (2003) Using an acoustic ground discrimination system to map coral reef benthic classes. Int J Remote Sens 24:2641–2660. doi:2610.1080/0143116031000066981
Wolf NG (1987) Schooling tendency and foraging benefit in the Ocean Surgeonfish. Behav Ecol Sociobiol 21:59–63. doi:10.1007/BF00324436
Zuur AF, Leno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York. doi:10.1007/978-0-387-87458-6
Acknowledgments
This study was funded by the World Bank/Global Environmental Facility Coral Reef Targeted Research Project (CRTR). We thank Victor Ticzon, Dr. Colette Wabnitz, Carlos Zapata and Gilberto Acosta for assistance in the field and 3 anonymous reviewers for comments that improved this manuscript considerably.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Goulet.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bejarano, S., Mumby, P.J. & Sotheran, I. Predicting structural complexity of reefs and fish abundance using acoustic remote sensing (RoxAnn). Mar Biol 158, 489–504 (2011). https://doi.org/10.1007/s00227-010-1575-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1575-5